Abstract
Unique characteristics such as biocompatibility, degradation capability, and mechanical properties have positioned magnesium alloys as highly favorable choices for use in various medical devices and implants. However, their rapid degradation and associated challenges have limited their widespread use. This study conducts a thorough analysis into the corrosion behavior of magnesium alloys when open to various coatings, using both in vitro and in vivo environments. The review focuses on understanding the degradation mechanisms, factors influencing corrosion, and the resulting consequences. Additionally, it explores the composition of coatings and metals as effective means to control degradation, along with surface treatment and corrosion management methods. To enhance the degrading behavior, bioactivity, and biocompatibility of magnesium alloys, a multistep approach involving coating techniques such as HA coating, LDH, CaP, and titanium dioxide coating is recommended. These coatings have shown significant potential in improving the exterior properties of Mg alloys. Furthermore, using multifunctional coatings is extremely effective in creating secure and bioactive substrates for the application of biodegradable implants, demonstrating significant potential in the field of biomedical engineering.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
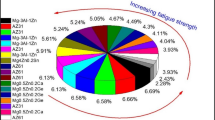
Recently, there has been a developing curiosity in biodegradable metals as alternatives to traditional materials, viz. titanium alloy, steel grade alloys and cobalt alloys. Three types of biodegradable metal materials have emerged: zinc alloy, ferroalloy and magnesium alloy [1]. Magnesium (Mg), a naturally occurring lightweight metal found in bone tissue, plays a vital role in human metabolism. Mg ions are deemed a vital component in the human body, with their toxicity levels falling within acceptable limits [21]. Magnesium alloys are considered as a demanding material for implants due to the special features in terms of biocompatibility and degradability [2,3,4]. Their remarkable mechanical capabilities, high compatibility with biological systems, and optimal decay rate have sparked significant interest in the field of biometal materials. One notable aspect is that the magnesium alloys have a density of around 1.74 g/cm3, which closely resemble human bones that are compact with a density of 1.8 g/cm3. However, while the yield point of uncontaminated magnesium is 45 GPa, human bone has a yield point range of 40–57 GPa. This suggests that magnesium can greatly improve the yield stress effect in comparison with other metal materials like titanium and its alloys. As a result, alloys made from magnesium have emerged as an attractive option for implant materials [5, 6]. Currently, magnesium alloys like AZ91, AZ31, AM50, WE43, ZK60, are considered as deep rooted material for various biomedical applications (Table 1).
These applications include the fixation of bone fractures using osteosynthetic muscle-skeletal fracture fixation and the treatment of heart muscles and peripheral artery diseases through cardiovascular implants such as stents. These alloys are appealing to researchers due to their excellent biocompatibility, mechanical properties and proven ability to induce bone development. Nevertheless, the presence of aluminum in magnesium alloys may render them inappropriate for a long time implantation, as the release of aluminum ions, which are thought to be neurotoxic, has been related to Alzheimer's disease and other neurological disorders. Elevated levels of aluminum have also been associated with dialysis osteomalacia, encephalopathy, and certain types of anemia [7, 8]. Furthermore, the deterioration of Mg alloys leads to the generation of hydroxyl ions, elevating the pH in the vicinity and potentially impeding bone tissue formation. Moreover, magnesium exhibits susceptibility to localized corrosion [9]. Unfortunately, the significant corrosion that occurs to magnesium alloys in the body of humans limits their long-term usefulness, resulting in a rapid degradation of mechanical strength and limiting tissue regeneration [10,11,12,13,14,15,16,17].
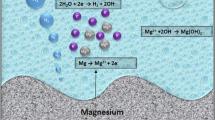
The vital chemical characteristics of magnesium alloys make them susceptible to corrosion in physiological conditions. As a result, hydrogen and magnesium hydroxide are formed. The corrosion by products of magnesium alloys are thought to be non-toxic, and the body can get rid of them through metabolism, unlike other types of metal implantation. However, the body's high rate of magnesium corrosion might lead to premature deterioration, mechanical failure, and rejection of the implant before the healing process can take place. This poses significant challenges in developing magnesium alloys for biomedical applications. Surface modification methods, on the other hand, provide a quick and easy way to control the deterioration rate for magnesium alloys. These technologies include micro-arc oxidation, electrodeposition, physical vapor deposition, chemical vapor deposition, anodic oxidation, electrospinning and ion implantation. These coatings exhibit similar crystalline structures, excellent bonding strength, resistance to wear, repeatability, and easy control of thickness [18].
To address this issue, several ways were developed to enhance the anti-corrosion properties of Mg alloys. The techniques include, coating technology and modifying the alloy composition through rigorous purification processes [19, 20]. Surface modification methods, such as mechanical approaches, have also been explored [21]. The advancement of surface treatment technology for biomedical magnesium alloys has classified into non-metallic inorganic, composite and metallic polymer coatings [22]. Major focus of this review is to provide insights into different methods to adapt for Mg alloys in order improve the corrosion properties and their compatibility with living organisms. To achieve this goal, we have suggested employing hybrid coatings that include bioactive, bioinert, and biomimetic features. Figure 1 shows that these coatings were specially developed to elicit favorable responses from bone tissue, making them appropriate for a variety of biological applications. Furthermore, by improving the creep resistance, fatigue, corrosion and wear of important engineering components, the lifespan of these components can be extended. This is made possible through the implementation of surface finishing techniques such as ball and roller burnishing. These techniques have been proven to enhance wear resistance, micro hardness, surface roughness, corrosion resistance and fatigue strength while also generating compressive residual stresses. Based on the observed outcomes, ball and roller burnishing techniques have been extensively employed across diverse industries such as aerospace, automotive, medical and marine sectors [23].
2 Utilizing Magnesium Alloy Coatings in Biomedical Settings
2.1 Hydroxyapatite Coating on Magnesium Alloy
Hydroxyapatite coating on magnesium alloy has been found to possess similar compositions to actual bone hard tissue. This coating shows impressed results such as enhanced resistance to corrosion, improved bioactivity, and increased biocompatibility [22]. When utilized as biomaterials, metallic implants with a hydroxyapatite (HA) coating exhibit superior bioactivity, osteoconductivity and more rapid integration with the supporting bone tissue. [24]. According to Jamesh et al. research, HA coating was successfully adapted to a pure Magnesium sample through an electrodeposition process. The process involved using a combination of electrolytes consisting of 0.1 MCa(NO3)2, 0.06 M (NH4)3PO4 and 10 ml/l of 30% Vol, H2O2, at 27 °C, pH value of 4. The process and its results are shown in Fig. 3 [25]. Alizadeh-Osgouei et al. investigated hydroxyapatite (HA), an essential inorganic element found in bones from humans. Several studies have found that HA can be used as a biologically active coating for Mg alloys with a focus of biocompatibility and resistance to corrosion [26]. Chaharmahali and colleagues investigated the PEO process to form composite coatings with hydroxyapatite (HAp) to use on magnesium-based materials. [27]. Wang and colleagues were successful in creating a micro-arc oxidation coating infused with HAp on magnesium-based alloys by introducing HAp particles into Ca–P electrolytes. This resulted in the creation of a denser covering with increased durability against degradation [28].
Figure 2 depicts the investigations done to find the bioactivity generated by the HA layer applied to the AZ31 mg alloy. These studies made use of EDS (Energy Dispersive Spectroscopy), FESEM (field emission scanning electron microscopy) and cross-sectional coating analysis. The goal of these tests was to evaluate the effectiveness and performance provided by the HA coatings on the AZ31 Mg alloy [29].
FESEM images (a), (b), (c), EDS spectra (d), (e), (f), and cross-sectional images of EPD coatings (g), (h), (i) as depicted in the study [29]
2.2 Titanium Dioxide Coating on Magnesium Alloy
Establishing a robust mechanical and biological interface between orthopedic implants and the surrounding bone tissue remains a significant challenge. Orthopedic implants, such as joint replacements or fracture fixation devices, need to reliably integrate with the patient's bone in order to provide long-lasting stability and functionality. However, achieving optimal bonding between the implant surface and adjacent bone tissue is inherently difficult. The overarching goal of this research is to develop orthopedic implant materials and surface modifications that can better encourage and facilitate the natural regeneration of bone tissue, resulting in enhanced biological and mechanical fixation of the implant within the body.
The biodegradability of implant materials must be combined with their osteoconductivity, as they need to be absorbed or biodegraded within the body to facilitate the development of new tissue and ensure adequate support. Calcium phosphates, particularly hydroxyapatite (HA), have been suggested as substances that can promote the Osseo integration of metal implants [30, 31]. Samiee, M. et al. used magnetic sputtering to deposit a TiO2/MgO double layer and a thin TiO2 layer on the exterior of the AZ91D alloy. These features encompass improved resistance to corrosion and enhanced biocompatibility, setting them apart from others. The use of XRD techniques and FESEM demonstrated that coating were equally and continually produced on the external layer of the alloy [32]. Hernandez-Montes et al. performed a study to collect data on the formation of titanium coating for increasing the rate of corrosion and biocompatibility of magnesium in biological materials. Additionally, the authors emphasized the importance of sol–gel technologies in the development of biomedical coatings. [33]. Titanium dioxide coatings are widely recognized for their antibacterial features, and Guo et al. studied the biocidal effects of a porous coating combining Ag and TiO2 nanoparticles synthesized using the sol–gel technique [34]. Fu et al. conducted investigations using sol–gel TiO2 nanoparticle coat layers on glass substrates, the biocidal effect of sol–gel TiO2 nanoparticle coatings [35]. Electrophoretic deposition (EPD) was used to apply ceramic coatings comprising organic substances and titanium dioxide nanoparticles such as alginate to the Mg AZ91D alloy, resulting in 3 min to 7 times better corrosion resistance than uncoated magnesium. The researchers used electrochemical impedance spectroscopy (EIS) to get their results [36].
2.3 CaP Coating on Magnesium Alloy
Shadanbaz et al. worked with calcium phosphate (CaP) coatings and observed encounter of naturally occurring substance within a diverse range of biological environments including tissues and bone. Bone is made up of two components: an inorganic part made up of living apatites (CaP) and a biological component made up mostly of water and collagen. Extensive evidence demonstrates that artificial hydroxyapatite closely mimics the inorganic composition of natural CaP found in bone, exhibiting remarkable similarity in properties. As a result, CaP has been extensively researched and used as coatings on orthopedic devices to protect against corrosion, improve compatibility with the body, and promote biocompatibility [37]. The process of applying CaP coatings involves mimicking natural procedures in a laboratory setting, using simulated body fluids (SBF) that closely resemble physiological conditions. In this process, calcium phosphate (CaP) phases are formed from the supersaturated ions present in the simulated body fluid (SBF) solution, which interact with the substrate surface to nucleate and grow the desired CaP phases. The resulting solution is allowed to form on the desired surface [38, 39]. The sol–gel synthesis coatings approach has been widely explored for its efficiency in coating magnesium and alloys in order to prevent corrosion and increase adhesion. Figure 3 shows an illustration of calcium phosphate coatings created using this process. [37].
SEM images of calcium phosphates made with a sol–gel process at high magnification and low magnification [37]
2.4 LDH Coating on Magnesium Alloy
Bioengineering researchers are currently focusing on the captivating subject of biodegradable implants. These implants offer promising prospects for promoting cell growth and facilitating osseointegration. Additionally, they gradually disintegrate over time, eliminating the necessity for a subsequent surgical procedure [40, 41]. In electrochemical studies, through observation, it was noted that the implementation of a hydrothermally synthesized Mg–Al LDH coating significantly enhanced the corrosion resistance of the AZ91D magnesium alloy [42]. Peng et al. conducted further investigations into a novel hydrothermal treatment for Mg alloys [43]. Wu et al. used the contact angle (CA) measurements to evaluate the surface wettability of different materials. Figure 4 depicts the findings for CA. After etching, the metal substrate showed the contact angle of 68.4° ± 5.1°, representing it as hydrophilic. Water CA on the MgAl-LDH layer reduced considerably to 21.4° ± 1.8° due to higher surface roughness from LDH nanosheets of material. After PFOTMS modification of the LDH films, the LDH sample demonstrated superhydrophobic characteristics, with a CA greater than 150°. The 12 h–125 °C-LDHs-M had the greatest water CA of 163.0° ± 1.1° and the roughest surface [44, 45]. Table 2 provides the different coatings following the enhancement process of magnesium alloy.
shows the shapes of water droplets on the surface of several substances, as well as the related water CA: A etched Mg substrate, B MgAl-LDHs, C 6 h–125°C-LDHs-M, D 18 h–125°C-LDHs-M, E 12 h–125°C-LDHs-M, F 12 h–100°C-LDHs-M, and G 12 h–150°C-LDHs-M Ref [44]
3 Absorbable Magnesium Alloy
Magnesium alloys, with a density ranging from 1.75 to 1.85 g/cm3, are remarkably lightweight metals commonly used in structural applications. Their density is 1.75 g/cm3 highly extreme in comparison with the cortical bone in the human body [46]. Compared to iron and zinc alloys, magnesium alloys are considered more suitable for bone implants [47, 48]. As these implants decay, ions of magnesium are released, creating a pH balance that promotes new bone tissue formation (osteogenesis). This method stimulates mineral deposition by osteoblasts while inhibiting osteoclast activity [49,50,51].
4 Coating Methods
This section primarily investigated with multiple techniques in order to improve the exterior of the Mg alloys, with a special emphasis on developing a bioactive coating. There are numerous options for adding bioactive inorganic compounds and organic to Mg alloys using a variety of processes. The techniques described include conversion coatings like micro-arc oxidation, biomimetic protective coatings, hydrothermal and alkali-heat treated coatings, as well as applied coatings like immersion and sol–gel, physical vapor deposition (PVD), and electrodeposition [51]. The chemical-based conversion coating works by dissolving and precipitating substances. In the realm of bioengineering, particularly in orthopedics, calcium phosphate is frequently employed as a type of chemical conversion coating to form biocompatible and osteoconductive HA layers [52]. Alabbasi et al. explored the possibility of applying this coating after the MAO process to enhance its adhesion to the substrates. This helps seal the porous structure of the surfaces and reduces localized corrosion [53]. Kumar et al. used a sol–gel silica-based coating to enclose the porous framework of a magnesium alloy that has undergone MAO treatment. This specific coating proficiently impedes ion diffusion from the solution, augmenting the alloy's corrosion resistance [54]. The fundamental aspects of each coating process are illustrated in Fig. 5 [46].
Schematic illustration of coating process that may be useful for Mg alloys [46]
5 Absorbable Magnesium Implants
Swaminathan undertook research exploring the mechanisms involved in the absorption and elimination of magnesium within the human body. Their findings indicate that the body has the capacity to manage surplus magnesium ions by transporting them through the circulatory system and subsequently eliminating them via urine without detrimental effects [55]. Yang investigated the magnesium, an essential trace element in the human body, is the fourth most prevalent mineral and serves as a cofactor in over 325 enzymatic activities necessary for energy metabolism. Its presence is essential for the proper functioning of the heart, muscles, neurons, bones, and kidney. The WHO recommends a daily consumption of 280–300 mg for adults, 250 mg for children, and 80 mg for babies. The body regulates blood magnesium levels through a dynamic balance of absorption and excretion via the gastrointestinal system and kidneys. Figure 6 visually represents the equilibrium between magnesium absorption and excretion in the human body [14]. Magnesium implants offer various benefits, such as enhanced radiographic imaging, mechanical durability, and absorption capabilities.
Ding (2016) and colleagues investigated the features of JDBM-2, highlighting its excellent ductility and moderate strength, which make it suitable for cardiovascular stents. Comprehensive testing, both in the laboratory and on animals, has demonstrated JDBM's advantages over alternative materials in terms of degradation rate, mechanism, biocompatibility, and bioactivity, particularly in the context of orthopedic implants and cardiovascular stents. Furthermore, many orthopedic implants, such as magnesium-based devices, have been effectively used in clinical settings, as shown in Fig. 7 [56].
The most prominent uses of Mg alloys a stents and b orthopedic implants [56]
6 Distinctive Properties of Magnesium Alloys that Make Them Promising Candidates for Temporary Orthopedic Implants
The biodegradability feature of magnesium alloys makes them suitable for temporary orthopedic implants, in line with medical standards, encompass their biocompatibility, strong mechanical properties to maintain structural integrity during bone healing, degradability, and dynamic corrosion (sometimes referred to as flow rate) [57,58,59]. The efficacy of such implants in a biological setting can solely be ascertained through in vivo experimentation on animals, subsequently followed by clinical trials. Additionally, assessments can be made regarding the development of new bone, the contact between bone and the implant, and inflammatory responses.
7 Magnesium-Based Alloy in Orthopedic Implants
Prakash Rout et al. conducted research to elucidate the significance of magnesium, affirming its essential role for both mammals and humans. The daily requirement for humans is approximately 250–350 mg of Mg, with most of it being excreted through the gastrointestinal (GI) tract and kidneys. However, the human body absorbs around 70–180 mg of magnesium, which contributes to the well-being of the kidneys, cellular fluids, and bones. This element is both biodegradable and biocompatible, supporting molecular and cellular responses within the human body. Orthopedic implants serve as replacements for vanished soft tissue or bone due to infections, cancers, inflammations, amputations and traumas. These implants can be used temporarily or permanently until the patient fully recovers. Each prosthesis can either promote bone generation (osteogenic) or bone removal (osteoclastic) [60]. Magnesium is frequently combined with calcium, zinc, zirconium, manganese, silver, yttrium in the form of binary alloys: Mg–Zr, Mg–Zn, Mg–Ca, Mg–Y, Mg–Ag, and Mg-RE. This technique of alloying is frequently employed to improve the characteristics of pure magnesium. [61].
7.1 Mg–RE-, Zn-, Ca-, Zr-, Ag-, and Y-Based Alloys
This segment presents a summary of the primary magnesium alloys investigated with a focus on orthopedic applications. It explores the biocompatibility, structural, mechanical, biodegradability behavior and chemical compositions of various Mg-based alloys. Magnesium–rare earth (RE) alloys demonstrate promising degradation characteristics. Magnesium–zinc alloys comprise a magnesium matrix with precipitates of MgZn [48]. Gu et al. carried out investigations into the cytotoxicity of several magnesium-based alloys, such as Mg–Y alloys, revealing that the inclusion of Y resulted in adverse effects on various cell lines [62]. Furthermore, Zhang et al. examined impact of zinc (Zn) concentration on the mechanical strength of magnesium, observed that higher levels of Zn content leads to a significant reduction in mechanical strength [63]. In a study, Nanda et al. [64], examined how binary Mg–Zn alloys deteriorate and tested five alloys with varying concentrations of Zn (2, 4, 6, 8, and 10). The results showed a clear connection between the Zn concentration and a higher corrosion potential. The accelerated degradation of certain magnesium–zinc (Mg–Zn) alloys used for temporary orthopedic implants can be attributed to the formation of intermetallic Mg–Zn phases within the alloy microstructure, which hinder ionic transport and reduce the electrochemical interaction between the alloy and the physiological environment, thereby promoting overall corrosion and degradation; however, by carefully controlling the Zn content and solidification conditions during alloy processing, the Mg–Zn phase constitution can be tailored to optimize the degradation kinetics and meet the desired performance requirements for these temporary implant applications. Previous research suggests that Zn concentrations exceeding 6 wt. percent can lead to the development of additional intermetallic segments in Mg–Zn alloys, further exacerbating the corrosion process in these alloys. Conventional methods for bone repair and surgery involve the use of strong metal implants like stainless steel, titanium, or cobalt alloys in the form of bone screws and plates [65,66,67,68,69,70,71,72]. These traditional metallic implants are significantly stiffer than actual bones.
The variance in stiffness between the implants and the bones leads to a phenomenon known as stress shielding, which leads to various complications in clinical practice. These complications include premature loosening of the implant, impaired healing of the implant and surrounding tissues, thickening of the skeleton, and persistent inflammation [73,74,75,76,77,78,79,80,81,82,83,84,85,86]. Feser et al. conducted a study to examine how degradable Mg–Ca alloys with varying amounts of calcium (0.6%, 0.8%, 1.0%, and 1.2% by weight) affect the function of dendritic cells, which play a crucial role in representing the body's cells. Their findings revealed that Mg–Ca alloys are quite compatible with living organisms, as well as the formation of calcium and magnesium ions during the decomposition of these alloys in a laboratory setting did not significantly disturb the activities of dendritic cells [87]. Thomann et al. conducted a study on MgCa0.8 implants and found evidence of pitting corrosion three months after the implants were placed. The corrosion process persisted for a duration of six months, with MgCa0.8 implants demonstrating an average decrease in cross-sectional area exceeding 50% compared to their initial size after 12 months. Additionally, the volume of the implants was observed for 3, 6 and 12 months to understand the percentage decrease, and 11, 31, and 51% of decrease were observed after implantation [88]. The corrosion process is crucial, particularly concerning the biomechanical characteristics of medical implants. Pitting corrosion is a recognized susceptibility of magnesium-based alloys, particularly exposed to chloride ions. On contrary, carbonate ion’s presence exerts a notable preventive effect on corrosion-related concerns. Additionally, the incorporation of calcium into magnesium-based alloys significantly enhances their resistance to both general and pitting corrosion. Mg–Ca alloys consist of up to 0.8 wt% Ca and possess a homogeneous microstructure and uniformly dispersed corrosion. Calcium concentrations over a certain threshold cause irregular and extensive corrosion [89]. Corrosion of the implant components may shorten the lifespan or demand revision surgery. Moreover, an excessive release of metallic ions could pose a potential risk. Extensive research conducted by Tsutsumi [90] delved deep on Zr and its binary alloys to observe the corrosion. Notably, when performing potentiodynamic anodic polarization measurements on commercially pure (CP) Zr immersed in Hanks' solution, the emergence of pitting corrosion was observed. However, Zr demonstrates notably greater resistance to pitting in comparison with 316L stainless steel, and this resistance can be augmented by the incorporation of additional components [91]. In a separate study, Azizi et al. examined the deposition of thin films Zr–2.5Nb on untreated magnesium using magnetron sputtering. This approach effectively controls the degradation rate and enhances the mechanical properties at nano-level. While the Zr–Nb thin films exhibited enhanced corrosion resistance for magnesium in simulated body environments, future research should prioritize assessing the bioactivity of these films. [92]. Li et al. investigated the degradation of Mg–xZr–ySr alloys, with x and y representing concentrations of 1%, 2%, or 5%. When compared all Mg alloy specimens, the specimen with 1% Zr and 5% Sr displayed the icorr value (3 × 10−3 A/cm2) at peak in SBF, whereas the specimen with 2% Sr resulted with very low icorr value 0.5 × 10−3 A/cm2. The alloys with varying Sr concentrations, specifically 2 and 5% Zr, displayed similar fluctuations. For instance, in SBF, the Mg–2Zr–ySr alloy had an icorr value of 2.5 × 10−3 A/cm2, while the Mg–5Zr–ySr alloy had an icorr value of 6 × 10−3 A/cm2, where y represents either 2% or 5% [71]. Zerankeshi M. M. has expressed an intense interest in enhancing the mechanical behavior of degradable magnesium alloys in order to extend their potential applications. The addition of silver (Ag) to these alloys might have a substantial impact on their characteristics, endowing them with extraordinary abilities [93]. The insertion of Ag in biodegradable magnesium-based alloys produces modifications in their microstructure, primarily by reducing the grain size and forming new secondary phases containing Ag. These changes can potentially affect the corrosion and mechanical properties [94]. Incorporating silver (Ag) into pure magnesium has been shown to significantly reduce particle size, resulting in enhanced mechanical characteristics. The ultimate tensile strength of pure magnesium (108.3 MPa) practically doubled to 215.9 MPa when 6% silver was added to the Mg–6Ag alloy [95]. Using antibacterial agents like silver (Ag) to coat degradable Mg-based alloys is an efficient way to improve their biodegradability and biological properties. This coating acts as a protective barrier, effectively limiting the passage of corrosive chemicals while increasing the resistance to corrosion, but also allows for the continuous release of Ag + ions from the coated implant, providing long-term antibacterial effects [96].
8 In vivo Corrosion Rates of Coated Magnesium Alloys
Biomedical research necessitates in vivo studies to comprehend the impact of drugs, therapies, or interventions on biological systems. These investigations provide vital information about the safety, efficacy, and mechanisms of numerous medications or treatments. The rate of corrosion for coated magnesium alloys varies depending on several factors, including coating type, ambient conditions, and alloy composition. Magnesium alloys are prone to corrosion; hence, the application of coatings is commonly employed to improve their corrosion resistance in in vivo studies. Table 3 provides the details of the in vivo studies on corrosion rate of magnesium alloy. Moreover, Dorozhkin investigated the better corrosion behavior in in vivo studies on biodegradable magnesium alloys with various coating methods [97].
9 In vitro Corrosion Rates of Coated Magnesium Alloys
A study conducted by Kumar, Singh et al. examined the application of alumina-doped ceria and alumina coatings on AZ91 alloy that underwent a solution treatment. The team conducted protein adsorption and potentiodynamic corrosion tests to assess the potential suitability of these coatings to use in bioimplants. The addition of CeO2 to Al2O3 improves corrosion resistance by increasing localized melting while decreasing substrate element diffusion into the coating. By using thermal spray method, the in vitro studies on corrosion rate of magnesium alloy AZ91 is Ecorr1.29 V and CR 0.0120 mpy. Figure 8 shows the Tafel plot for Al2O3-coated AZ91 corrosion test [107]. Hanas et al. studied the manufacture of an extremely fine AZ31/HA composite material using frictional stirring processing. The integration of scattered HA inside the fine-grained substrate increases the specimen's bioactivity. By subjecting the composite to acid treatment, the degradation rate is reduced, and biomineralization is fortified. By using anodizing method, the in vitro studies on corrosion rate of magnesium alloy is Icorr 2.2 × 10−6 [108]. Wang et al. investigated the decomposition of magnesium and its AZ31 alloy in simulated bodily fluids. They investigated corrosion behavior with polarization curves and immersion testing. Both methods produced consistent results, indicating that using high-purity magnesium and alloying are effective approaches to reducing magnesium deterioration. Notably, both materials have much lower corrosion current densities than commercially pure magnesium [109]. Bobby Kannan et al. studied the impact of applying a hydroxyapatite coating onto the magnesium alloy AZ91 and its properties. The researchers employed a potentiostatic method and alkaline treatment during the coating procedure. In vitro testing was used to evaluate the coated alloy's mechanical strength. The outcomes demonstrated that the hydroxyapatite coating enhances the mechanical properties on the magnesium alloy in vitro by about 20%. Furthermore, the researchers noted that coatings with high cathodic voltage performed poorly, potentially due to excessive hydrogen evolution, leading to the deterioration of the coating. [110]. Wang et al. studied a combination of cerium-based coatings and hydrophobic properties of SA that were used to create anticorrosive coatings on AZ91D Mg alloy. The process involved applying cerium-based coatings to the surface and immersing it in an SA bath. The concentration of the SA bath affected the hydrophobicity of the surfaces, with an optimum concentration of 0.75 mmol/L SA resulting in a contact angle (CA) greater than 90°. This concentration also improved the corrosion resistance of the substrate [111]. Barberi, J and Saqib et al. studied the TA coating which failed to protect the AZ31 substrates from deterioration due to the Mg alloy's highly reactive nature, resulting in a fractured and barely adhered TA-Mg oxide layer. Various coating protocols did not help the problem. Additionally, electrochemical experiments demonstrated that the coating exacerbated the behavior of corrosion in both AZ31 and AZ91 metals. The result was due to the creation of galvanic cells between the coated scales and the exposed metallic substrate, which caused an increase in corrosion current and rate [112]. The study of Qiang Wang et al. examined the dynamic characteristics of an AZ31B magnesium alloy coated with Ca–P. The evaluation involved both in vivo and in vitro degradation tests, including assessments of mechanical loading capacity, hemolysis and animal implantation. Based on the findings, it was illustrated that AZ31B alloys had improved resistance toward degradation due to Ca–P coating in simulated body fluid, which otherwise would gradually deteriorate in laboratory conditions. The polarization curves demonstrate that the formation of Ca–P on the surface greatly increases the corrosion resistance of the AZ31B alloy. The rate of corrosion is Icorr 1.18 × 10−6 and Ecorr − 1.548 [111]. In their study, Makkar et al. explored the efficacy of Ca–Sr–P coatings on ZK60 alloy in both laboratory and in vivo settings for bone-related applications. The laboratory experiments revealed that the corrosion resistance of the Mg alloys was meaningfully bolstered by the coating process and also enhanced its biointeractivity with living tissues. These findings indicated that the coating of Ca–Sr–P, with its degradation reduction and rise in compatibility with biological systems, holds promise for potential utilization in magnesium-based orthopedic implants [113]. Zohra Benzarti et al. investigated how Zr was added to the Mg matrix to improve the corrosion resistance and mechanical properties of DCMS-deposited Mg coatings. Furthermore, they discovered that when submerged in plasma SBF, Mg–1.0Zr, Mg–2.0Zr, and Mg–3.4Zr coatings deteriorated rapidly. This was owing to optimal Zr doping and improved microstructure [114].
Tafel plot for Al2O3-coated AZ91 corrosion test [107]
10 Conclusions and Future Directions
Research has been extensively carried out in the realm of orthopedic implants to investigate the prospective benefits of magnesium and its related alloys. These materials have demonstrated the ability to promote bone growth while gradually degrading under load-bearing conditions. However, a thorough assessment of the benefits and limitations associated with magnesium ions is imperative. It is imperative to attain a thorough comprehension of the degradation mechanisms of magnesium alloys under varying conditions and stressors in order to advance our understanding in this domain. Moreover, attaining a delicate balance among the deterioration rate of magnesium alloys and their biocompatibility and safety is of utmost importance for better control. In recent developments, the incorporation of surface coating technology has emerged as a highly promising approach for enhancing both the corrosion resistance and mechanical properties of magnesium alloys. Coating applications on magnesium alloys hold significant potential for improving surface characteristics, particularly by creating stable and bioactive surfaces suitable for biodegradable implants. The development of efficient techniques to apply biocompatible coatings onto magnesium-based alloys has the potential to create biodegradable implant components suitable for the biomedical field. By devising suitable methods of fabricating biocompatible anti-corrosion coatings on Mg-based alloys, considerable progress can be achieved in the medical sector concerning the utilization of biodegradable implants. This presents a significant opportunity for improving the medical utility of these materials. The discovery of biodegradable magnesium implantation has changed our knowledge of metal-based biomaterials. The prospective use of coated Mg alloys for biodegradable implants shows promise in enhancing the performance of implants for orthopedic and cardiovascular purposes in biomedical sectors.
References
Wang H, Bai M, Yuan H, Hou Y, Liu Y, Fang Z, and Guan S, J Magnes Alloys 9 (2021) 2145.
Hampp C, Angrisani N, Reifenrath J, Bormann D, Seitz J M, and Meyer-Lindenberg A, Mater Sci Eng: C 33 (2013) 317.
Zhang S, Zheng Y, Zhang L, Bi Y, Li J, Liu J, and Li Y, Mater Sci Eng: C 68 (2016) 414.
Amerstorfer F, Fischerauer S F, Fischer L, Eichler J, Draxler J, Zitek A, and Prohaska T, Actabiomaterialia 42 (2016) 440.
Tian P, and Liu X, Regen Biomater 2 (2015) 135.
Wang C, Cui Y J, and Liu H Y, Mater Rep 6 (2015) 55.
Alfrey A C, LeGendre G R, and Kaehny W D, N Engl J Med 294 (1976) 184.
Parkinson I S, Ward M K, and Kerr D, J Clin Pathol 34 (1981) 1285.
Ghali E, Dietzel W, and Kainer K U, J Mater Eng Perform 13 (2004) 7.
Zhang Y, Chen F, Zhang Y, and Du C, Tribol Int 146 (2020) 106135
Agarwal S, Curtin J, Duffy B, and Jaiswal S, Mater Sci Eng C 68 (2016) 948.
Hornberger H, Virtanen S, and Boccaccini A R, Actabiomaterialia 8 (2012) 2442.
Guo Y, Su Y, Gu R, Zhang Z, Li G, Lian J, and Ren L, Surf Coat Technol 401 (2020) 126318
Yang Y, He C, Dianyu E, Yang W, Qi F, Xie D, and Shuai C, Mater Des 185 (2020) 108259
Saris N E L, Mervaala E, Karppanen H, Khawaja J A, and Lewenstam A, Clin Chim Acta 294 (2000) 1.
Yamasaki Y, Yoshida Y, Okazaki M, Shimazu A, Kubo T, Akagawa Y, and Uchida T, Biomaterials 24 (2003) 4913.
Zreiqat H, Howlett C R, Zannettino A, Evans P, Schulze-Tanzil G, Knabe C, and Shakibaei M, J Biomed Mater Res: Off J Society Biomater, Jpn Soc Biomater, Aust Soc Biomater Korean Soc Biomater 62 (2002) 175.
Singh N, Batra U, Kumar K, Ahuja N, and Mahapatro A, Bioact Mater 19 (2023) 717.
Munir K, Lin J, Wen C, Wright P F, and Li Y, Actabiomaterialia 102 (2020) 493.
Mohamed S R, Friedrich S, and Friedrich B, Metals 9 (2019) 85.
Yin Z Z, Qi W C, Zeng R C, Chen X B, Gu C D, Guan S K, and Zheng Y F, J Magnes Alloys 8 (2020) 42.
Tong P, Sheng Y, Hou R, Iqbal M, Chen L, Li J, Smart Mater Med (2021).
Jagadeesh G V, and GangiSetti S, Adv Mater Process Technol 8 (2022) 4499.
Rahman M, Dutta N K, and Roy Choudhury N, Front Bioeng Biotechnol 8 (2020) 564.
Jamesh M, Kumar S, and Sankara Narayanan T S N, J Coat Technol Res 9 (2012) 495.
Alizadeh-Osgouei M, Li Y, and Wen C, Bioact Mater 4 (2019) 22.
Chaharmahali R, Fattah-alhosseini A, and Babaei K, J Magnes Alloys 9 (2021) 21.
Wang Z, Ye F, Chen L, Lv W, Zhang Z, Zang Q, and Lu S, Coatings 11 (2021) 667.
Jafari Z, Pishbin F, Ghambari M, and Dehghanian C, J Alloys Compd 976 (2024) 172961
Rahman M, Li Y, and Wen C, J Magnes Alloys 8 (2020) 929.
Narayanan R, Seshadri S K, Kwon T Y, and Kim K H, J Biomed Mater Res: Off J Society Biomater, Jpn Soc Biomater, Aust Soc Biomater Korean Soc Biomater 85 (2008) 279.
Samiee M, Hanachi M, Seyedraoufi Z S, Eshraghi M J, and Shajari Y, Ceram Int 47 (2021) 6179.
Hernández-Montes V, Betancur-Henao C P, and Santa-Marín J F, Dyna 84 (2017) 261.
Guo L, Feng W, Liu X, Lin C, Li B, and Qiang Y, Mater Lett 160 (2015) 448.
Fu G, Vary P S, and Lin C T, J Phys Chem B 109 (2005) 8889.
Alaei M, Atapour M, and Labbaf S, Prog Org Coat 147 (2020) 105803
Shadanbaz S, and Dias G J, Actabiomaterialia 8 (2012) 20.
Liu G Y, Tang S W, Chuan W A N G, Jin H U, and Li D C, Trans Nonferrous Met Soc China 23 (2013) 2294.
Zhou H, Li J, Li J, Ruan Q, Jin W, Yu Z, and Chu P K, Surf Coat Technol 401 (2020) 126248
Zhang F, Liu Z G, Zeng R C, Li S Q, Cui H Z, Song L, and Han E H, Surf Coat Technol 258 (2014) 1152.
Chen J, Lin W, Liang S, Zou L, Wang C, Wang B, and Cui X, Appl Surf Sci 463 (2019) 535.
Shao Z, Li P, Zhang C, Wu B, Tang C, and Gao M, J Magnes Alloys (2022).
Peng F, Li H, Wang D, Tian P, Tian Y, Yuan G, and Liu X, ACS Appl Mater Interfaces 8 (2016) 35033.
Han X, Hu J, Wang Y Q, Xiao T B, Xia W, Chen Y N, and Wu L, Front Mater 8 (2021) 743112
Peng F, Wang D, Zhang D, Yan B, Cao H, Qiao Y, and Liu X, ACS Biomater Sci Eng 4 (2018) 4112.
Sarian M N, Iqbal N, Sotoudehbagha P, Razavi M, Ahmed Q U, Sukotjo C, and Hermawan H, Bioact Mater 12 (2022) 42.
Kong L, Heydari Z, Lami G H, Saberi A, Baltatu M S, and Vizureanu P, Materials 16 (2023) 4797.
Antoniac I, Miculescu M, Mănescu V, Stere A, Quan P H, Păltânea G, and Earar K, Materials 15 (2022) 1148.
Zhou H, Liang B, Jiang H, Deng Z, and Yu K, J Magnes Alloys 9 (2021) 779.
Uppal G, Thakur A, Chauhan A, and Bala S, J Magnes Alloys 10 (2022) 356.
Tong P, Sheng Y, Hou R, Iqbal M, Chen L, and Li J, Smart Mater Med 3 (2022) 104.
Alias J, Alang N A, Ahmad A H, and Abd Razak N A, J Adhes Sci Technol 38 (2024) 1125.
Alabbasi A, Kannan M B, and Blawert C, Mater Lett 124 (2014) 188.
Kumar K P P, Subasri R, Mater Perform Charact 11 (2021).
Swaminathan R, Clini Biochem Rev 24 (2003) 47.
Ding W, Regen Biomater 3 (2016) 79.
Kamrani S, and Fleck C, Biometals 32 (2019) 185.
Han H S, Loffredo S, Jun I, Edwards J, Kim Y C, Seok H K, and Glyn-Jones S, Mater Today 23 (2019) 57.
Zhao D, Witte F, Lu F, Wang J, Li J, and Qin L, Biomaterials 112 (2017) 287.
Bismay P R, Using Magnesium-Based Alloys in Orthopedic Implants. AZoM. Retrieved on August 08, 2022 from https://www.azom.com/news.aspx?newsID=58124 (2022).
Hermawan H, Biodegradable metals: From Concept to Applications, Springer Science & Business Media, Berlin (2012).
Gu X, Zheng Y, Cheng Y, Zhong S, and Xi T, Biomaterials 30 (2009) 484.
Zhang S, Zhang X, Zhao C, Li J, Song Y, Xie C, and Bian Y, Acta Biomater 6 (2010) 626.
Nanda I P, Hassim M H, Idris M H, Jahare M H, Abdulmalik S S, and Arafat A, IOP Conf Ser: Mater Sci Eng 602 (2019) 012094.
Lotfabadi A F, Bakhsheshi-Rad H R, Idris M H, Hamzah E, and Kasiri-Asgarani M, Can Metalll Q 55 (2016) 53.
Yu L, Zhao Z, Tang C, Li W, You C, and Chen M, J Mater Res Technol 9 (2020) 10863.
Kavyani M, Ebrahimi G R, Ezatpour H R, and Jahazi M, J Magnes Alloys 10 (2022) 1640.
Sun Y, Zhang B, Wang Y, Geng L, and Jiao X, Mater Des 34 (2012) 58.
Kawamura Y, Hayashi K, Inoue A, and Masumoto T, Mater Trans 42 (2001) 1172.
Huan Z G, Leeflang M A, Zhou J, Fratila-Apachitei L E, and Duszczyk J, J Mater Sci: Mater Med 21 (2010) 2623.
Li Y, Wen C, Mushahary D, Sravanthi R, Harishankar N, Pande G, and Hodgson P, Acta Biomater 8 (2012) 3177.
Abdel-Gawad S A, and Shoeib M A, Surf Interfaces 14 (2019) 108.
Antoniac I V, Antoniac A, Vasile E, Tecu C, Fosca M, Yankova V G, and Rau J V, Bioact Mater 6 (2021) 3383.
Imwinkelried T, Beck S, Iizuka T, and Schaller B, Acta Biomater 9 (2013) 8643.
Witte F, Kaese V, Haferkamp H, Switzer E, Meyer-Lindenberg A, Wirth C J, and Windhagen H, Biomaterials 26 (2005) 3557.
Cheng M Q, Wahafu T, Jiang G F, Liu W, Qiao Y Q, Peng X C, and Liu X Y, Sci Rep 6 (2016) 24134.
Liu Y J, Yang Z Y, Tan L L, Li H, and Zhang Y Z, Braz J Med Biol Res 47 (2014) 715.
Antoniac I, Adam R, Biță A, Miculescu M, Trante O, Petrescu I M, and Pogărășteanu M, Materials 14 (2020) 84.
Chaya A, Yoshizawa S, Verdelis K, Noorani S, Costello B J, and Sfeir C, J Oral Maxillofac Surg 73 (2015) 295.
Kong X, Wang L, Li G, Qu X, Niu J, Tang T, and Hao Y, Mater Sci Eng: C 86 (2018) 42.
Schaller B, Saulacic N, Beck S, Imwinkelried T, Goh B T, Nakahara K, and Iizuka T, Mater Sci Eng: C 69 (2016) 247.
Marukawa E, Tamai M, Takahashi Y, Hatakeyama I, Sato M, Higuchi Y, and Harada H, J Biomed Mater Res Part B: Appl Biomater 104 (2016) 1282.
Windhagen H, Radtke K, Weizbauer A, Diekmann J, Noll Y, Kreimeyer U, and Waizy H, Biomed Eng Online 12 (2013) 1.
Bita A I, Antoniac I, and Ciuca I, UPB Sci Bull Ser B 78 (2016) 173.
Plaass C, von Falck C, Ettinger S, Sonnow L, Calderone F, Weizbauer A, and Windhagen H, J Orthop Sci 23 (2018) 321.
Leonhardt H, Franke A, McLeod N M H, Lauer G, and Nowak A, Br J Oral Maxillofac Surg 55 (2017) 623.
Feser K, Kietzmann M, Bäumer W, Krause C, and Bach F W, J Biomater Appl 25 (2011) 685.
Thomann M, Krause C, Angrisani N, Bormann D, Hassel T, Windhagen H, and Meyer-Lindenberg A, J Biomed Mater Res: Off J Society Biomater, Jpn Soc Biomater, Aust Soc Biomater Korean Soc Biomater 93 (2010) 1609.
Zeng R C, Qi W C, Cui H Z, Zhang F, Li S Q, and Han E H, Corros Sci 96 (2015) 23.
Tsutsumi Y, Zairyo-to-Kankyo 63 (2014) 360.
Zhang H, Han J, Sun Y, Huang Y, and Zhou M, Mater Sci Eng: C 56 (2015) 22.
Azizi S, Ehsani M H, and Zareidoost A, Mater Charact 112179 (2022).
Zerankeshi M M, and Alizadeh R, Materialia 101445 (2022).
Pogorielov M, Husak E, Solodivnik A, and Zhdanov S, Interv Med Appl Sci 9 (2017) 27.
Liu Z, Schade R, Luthringer B, Hort N, Rothe H, Müller S, and Feyerabend F, Oxid Med Cell Longev (2017).
Zhang X, Zhang Y, Lv Y, Dong Z, Yang L, Zhang E, and Zhou X, J Magnes Alloys 11 (2023) 2182.
Dorozhkin S V, Actabiomaterialia 10 (2014) 2919.
Wang P, Liu J, Shen S, Li Q, Luo X, Xiong P, and Xi T, ACS Biomater Sci Eng 5 (2019) 3279.
Jang Y, Tan Z, Jurey C, Xu Z, Dong Z, Collins B, and Sankar J, Mater Sci Eng: C 48 (2015) 28.
Acheson J G, McKillop S, Ward J, Roy A, Xu Z, Boyd A R, and Meenan B J, Surf Coat Technol 421 (2021) 127446
Ostrowski N J, Lee B, Roy A, Ramanathan M, and Kumta P N, J Mater Sci: Mater Med 24 (2013) 85.
Gu X N, Li N, Zhou W R, Zheng Y F, Zhao X, Cai Q Z, and Ruan L, Acta Biomater 7 (2011) 1880.
Razavi M, Fathi M, Savabi O, Vashaee D, and Tayebi L, Mater Sci Eng: C 48 (2015) 21.
Abdal-hay A, Barakat N A, and Lim J K, Ceram Int 39 (2013) 183.
Tang Y, Zhu L, Zhang P, Zhao K, and Wu Z, Corros Sci 176 (2020) 108939
Yu W, Sun R, Guo Z, Wang Z, He Y, Lu G, and Chen K, Appl Surf Sci 464 (2019) 708.
Kumar S, Singh H, Gaur N, Patil S, Kumar D, and Singh N, Surf Coat Technol 383 (2020) 125231
Hanas T, Kumar T S, Perumal G, Doble M, and Ramakrishna S, J Mater Process Technol 252 (2018) 398.
Wang H, and Shi Z, J Biomed Mater Res Part B: Appl Biomater 98 (2011) 203.
Bobby Kannan M, Singh Raman R K, Witte F, Blawert C, and Dietzel W, J Biomed Mater Res Part B: Appl Biomater 96 (2011) 303.
Wang Q, Tan L, Xu W, Zhang B, and Yang K, Mater Sci Eng: B 176 (2011) 1718.
Barberi J, Saqib M, Dmitruk A, Opitz J, Naplocha K, Beshchasna N, and Ferraris S, Materials 17 (2024) 343.
Makkar P, Kang H J, Padalhin A R, Faruq O, and Lee B, Appl Surf Sci 510 (2020) 145333
Benzarti Z, Itani S, Castro J D, Carvalho S, and Ramos A S, J Magnes Alloys (2024).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vinothkumar, C., Rajyalakshmi, G. An Analytical Review on the Degradation Mechanisms and Magnesium Alloy Protective Coatings in Biomedical Applications. Trans Indian Inst Met (2024). https://doi.org/10.1007/s12666-024-03424-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12666-024-03424-7