Abstract
In this study, the bismuth (III) oxide (Bi2O3) nanoparticles and chromium (III) sulphide/bismuth (III) oxide (Cr2S3–Bi2O3) nanocomposites were prepared hydrothermally by sonochemical assisted methods. The different devices such as Scanning Electron Microscopy, UV–vis spectroscopy, dynamic light scattering and, X-ray analysis were used for evaluation of the morphology and structural data of the prepared catalyst. The photo-degra dation activity of Cr2S3–Bi2O3 was comparing with Bi2O3. It was revealed that the Cr2S3–Bi2O3 could raise their photo-degradation performance for the removal of Malathion as an organophosphate insecticide under visible and UV light irradiation. The result from XRD and UV–vis DRS studies were shown the values of crystallite size and band gap for Bi2O3, and Cr2S3–Bi2O3-1 have obtained from and found 50.12, 58.45 nm and 2.81, 2.54 eV, respectively. The optimal condition of Malathion photo-degradation was found at time: 50 min, and pH: 5 for the Cr2S3–Bi2O3-2 with 90.5, and 97.5% photo-decomposition activity after 50 min under visible and UV light elucidation, respectively. The bactericidal possible of the prepared catalyst was appraised by using the disk diffusion proceeding and determining the lowest inhibitory and bactericidal concentration versus the two various bacteria groups. The results demonstrated that Cr2S3–Bi2O3-2 nanocomposites had high antibacterial properties.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The descriptive development of human crowd and the industrial operations conducted to an ongoing rise in the solicitation for the earth’s limited water reservoir [1, 2].
The removal of insecticide from water is vital for the environmental medium. Therefore, several various water treatment technologies have been created [3, 4]. Photo-decomposition by using the semiconducting oxide/sulphide for removal of insecticide pollutants was great to choose due to pure exploitation, excellent performance, and low cost [5,6,7,8,9,10,11,12,13,14,15]. Chromium (III) sulphide or bismuth (III) oxide-based nano-materials have been broadly applied in the photo-degradation of contamination by their affairs like as cost, chemical stability, environmentally friendly and electronic features [16,17,18,19,20]. The Bi2O3 nanoparticle was synthesized by Chen et al. [21] and investigation of the catalytic activity for decomposition of the antibiotic. The Bi and Bi2O3 nanoparticles were prepared by He et al. [22] for degradation of the organic substrate under source light and evaluation of product-degradation reaction. The spray pyrolysis method was used for the synthesis of ZnO/Bi2O3 for the dye decomposition by Medina et al. [23]. Huang et al. [24] synthesized Cs-doped Bi2O3 for methylene blue degradation performance. Ke et al. [25] prepared the Cu2O on Bi2O3 nanoparticles for water degradation efficiency under solar light irradiation. Hussain et al. [26] synthesized the Cr2S3 nanoparticles for the decomposition of the organic compound under light illumination. The photo-degradation performance of metal oxides nanoparticles was enhanced with combine the metal sulphide nanoparticles due to the band gap was decreased. Preparation of ZnS/SnO2 via Hydrothermal method by Hu et al. [27] showed the highest photo-degradation efficiency of Rhodamine B dye compound. Yuan et al. [28] synthesized SnS2/MgFe2O4/rGO by the solvothermal technique for the photo-decomposition of methylene blue. Park et al. [29] prepared CuS on TiO2/rGO, which demonstrated enhanced photo-degradation performance. Hitkari et al. [30] indicated that the combination of ZnS into ZnO/α-Fe2O3 nanocomposites makes to the excellent photo-degradation reaction. Hong et al. [31] synthesized Bi2S3 on Bi2WO6/WO3 nanocomposites by in situ growth method and reported the photocatalysis of Tetracycline antibiotic compound.
The target of this research is to present an excellent photocatalyst as Bi2O3, Cr2S3–Bi2O3 composite for the decomposition of Malathion under UV and visible light illumination. In addition to that, the antibacterial progress was studied by using the two bacteria groups. It is clear, the bactericidal properties of the Cr2S3–Bi2O3 composite were enhanced. The novelty of this work, synthesis of Cr2S3–Bi2O3 as the hybrid catalyst and used for degradation of the organic substrate. With attention to other same studies, there are not any projects on preparation on the Cr2S3–Bi2O3 nanocomposites.
2 Experimental
All chemical substrate were procured from Sigma Aldrich Co. without further purification.
2.1 Synthesis manner
The sonochemical/hydrothermal manner was used for the preparation of Bi2O3, and Cr2S3–Bi2O3 composites. The 20 mL of Bi(NO3)3·5H2O (0.02 m), 4 mL of nitric acid/citric acid (1:1) and 10 mL of Polyvinylpyrrolidone (0.03 M) was augmented to Teflon tube with 50 mL of distilled water. The ultrasonication instrument (pulse sonicator (Misonix S-4000)) was used for an ultrasonic medium with the 10 s pulse cycle (30 kHz frequency, and 450 W power). Then, the suspension was located to the autoclave for 2 h at 150 °C and dried at 90 °C for 4 h and calcined at 550 °C for 4 h. The Bi2O3 nanoparticles added in 140 mL of doubly distilled water and mixed with 0.50 g Cr(NO3)3·9H2O and 20 mL of Na2S (0.03 M) at 150 °C under nitrogen flow. The ultrasonication instrument (pulse sonicator (Misonix S-4000)) was used for an ultrasonic medium with the 10 s pulse cycle (30 kHz frequency, and 450 W power). Then, the suspension was located to the autoclave for 2 h at 150 °C and dried in the oven at 120 °C for 1 h and calcined at 400 °C for 2 h. The hybrid nano-catalyst in this study was presented as CrSBiO-0, CrBiO-1, and CrBiO-2 nanocomposites.
2.2 Characterization devices
The powder X-ray diffractometer (Philips X’Pert) was operated for evaluation of crystal data. The Scanning electron microscope (SU-800, Hitachi) and Transmission Electron Microscope (JEM-2100FHR) was operated for evaluation of morphology data. The X-ray photoelectron (Kratos Axis Ultra DLD) was operated for investigation of chemical states. The UV–vis (JASCO V-630) and photoluminescence (TEC Avaspec 2048) were operated for evaluation of optical data. The particle size was found by Dynamic light scattering (Nano Series Malvern). The dielectric analysis was performed by using high-frequency analyzer from Alpa-A novo control technology company.
2.3 Photo-degradation test
The photo-decomposition performance of Bi2O3, CrSBiO-0, CrBiO-1, and CrBiO-2 nanocomposites were evaluated by photo-degradation of Malathion [MAL, is an organophosphate insecticide, (Table 1)] under visible (300 W, λ ≥ 420 nm) and UV (100 W, λ = 254 nm) light. A UV cutoff filter was used to cut the separation of the two range wavelengths. The lamps were allowed to warm for 5 min before initiating experiments. Each lamp is placed in the center of the glass cell, yielding an irradiation intensity of 6.0 ± 0.2 mW cm−2 as determined with a Radiometer (VLX, ALYS Technologies). The experiments were prepared by using nano-photocatalyst dispersed into the reactor, including MAL solution (50 mL), and the pH was adjusted by the Hydrochloric acid and sodium hydroxide solution. The MAL residual was determined using a UV–vis spectrophotometer (Shimadzu) (λ = 420 nm). The degradation percent was computed as an equation in the previous study [31,32,33].
2.4 Antimicrobial tests of the nanocomposites
The microbicide activity of the Bi2O3, CrSBiO-0, CrBiO-1, and CrBiO-2 nanocomposites was determined as a disk diffusion method [34]. The Escherichia coli, Pseudomonas aeruginosa (as gram negative), and Staphylococcus epidermidis, Bacillus cereus, (as gram-positive) bacteria were applied for evaluation of the antibacterial study. The concentration of catalyst is 0.1 mg/mL in all tests. The plates were incubated at 35 °C for 24 h. For MIC test, the bacteria colonies content was 106 CFU/mL. The PBS concentrations of the prepared catalyst were 31–1000 μg/mL. These tests were done in three stages.
3 Results and discussion
3.1 Nano-material characterization
The SEM analysis of the Bi2O3 and CrBiO-1 nanocomposites are demonstrated in Fig. 1. It is obvious; the Bi2O3 were created as agglomerated particles with a spherical shape. Figure 1c reveals the particles morphological of CrBiO-1 catalyst composites indicated the Cr2S3 was coated on the Bi2O3 nanoparticles and highest nanoparticles size was formed in compared to Bi2O3 nanoparticles. TEM images shown in Fig. 1b, d, the Bi2O3 nanoparticles were synthesized as the spherical shape with the particle size about 40–80 nm. The TEM image of CrBiO-1 nanocomposites demonstrates the composite particles with high agglomeration. The elemental ratio was investigated with EDS analysis and showed the CrBiO-1 nanocomposites contain 32% Bismuth (Bi), 19% oxygen (C), 31% Chrome (Cr), and 18% sulphur (S), respectively. The average particle sizes were studied with using a DLS analysis (Fig. 1e), and which that demonstrates the mean size of the Bi2O3, nanoparticles and CrBiO-1 nanocomposites were 55.0, and 65.0 nm, respectively.
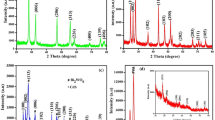
Figure 2 illustrates the XRD pattern of Bi2O3, CrSBiO-0, CrBiO-1, and CrBiO-2 nano-catalyst. The plots in Fig. 3 demonstrates the monoclinic of Bi2O3 phase (JCPDS No. 41-1449) [34] and hexagonal of Cr2S3 phase (JCPDS No. 00-011-0007) with prominent peaks [25]. The pattern in Fig. 3 demonstrates that the intensity of the phase peak was enhanced with the Cr2S3 ratio raised in the Cr2S3–Bi2O3 nano-hybrid photocatalyst. The crystallite size [35] is recognized to be 50.12, 54.54, 58.45 and 62.21 nm for Bi2O3, CrSBiO-0, CrBiO-1, and CrBiO-2 nanocomposites, respectively. To check the recombination status of the Bi2O3, and CrBiO-1, photoluminescence (PL) experiments were analyzed with an excitation λ = 300 nm (Fig. 3). PL spectra show the transmission of the e− and h+. In PL study, the e− are transferred VB to CB at the certain excitation wavelength. These e− may go back to VB giving upraise to PL signal. The photoluminescence intensity was attained with reflecting a high recombination rate of charge carriers [36]. The emission peak was observed at 400–440 nm for Bi2O3 and CrBiO-1 nano-catalyst. The PL intensity of the CrBiO-1 is larger than Bi2O3, and which that the recombination reflects for CrBiO-1 was lower than Bi2O3 nanoparticles.
The spectra of UV–vis diffuse reflectance was used to explore the variation in optical properties of Bi2O3, CrSBiO-0, CrBiO-1, and CrBiO-2 nanocomposites and are shown in Fig. 4. It was seen that the prepared samples absorbed UV and visible light. However, the absorption intensity in UV light is higher than visible light. The absorption intensity for hybrid catalyst increases with raising the concentration ratio of Cr2S3 nanoparticles. The bandgap (Eg) can be computed by the Kabelka–Munk function [36] and presented for the as-prepared Bi2O3, CrSBiO-0, CrBiO-1, and CrBiO-2 are 2.81, 2.63, 2.54 and 2.48 eV, respectively (Fig. 3b). The X-ray photoelectron spectroscopy (XPS) was operated to exploring the chemical states of the CrBiO-1 nanocomposites. From Fig. 5, the Cr 2p3/2 and Cr 2p1/2 was located at 578.18 and 588.01 eV (Fig. 5b) [37]. The doublet energy peaks were located at 159.0 eV and 164.0 eV due to the Bi 4f7/2 and 4f5/2 chemical state, respectively (Fig. 5c) [38]. The binding energy peak of O 1 s and S 2p at 528.5 and 162.0 eV was apperceived in spectra from Fig. 5d, e, respectively [25, 38].
3.2 Photo-degradation studies
The photo-decomposition studies of Bi2O3, CrSBiO-0, CrBiO-1, and CrBiO-2 were evaluated for decomposing of MAL under visible and UV light. Figure 6 demonstrates the photo-decomposition percent vs. illumination time. The photo-decomposition performance appertains on the MAL structural. It is obvious, the photo-degradation performance of MAL by the prepared nano-photocatalyst was completed after 50 min irradiation time (Figs. 6a, b). It is clear that the CrBiO-2 reveals the highest photo-degradation with 87.4%, and 97.5% percent compared to Bi2O3 (50.5% and 45.5%), CrSBiO-0 (78.0% and 74.0%), and CrBiO-1 (90.4% and 85.4%), under visible and UV light, respectively. As can be seen, the degradation amount with UV illumination is higher compared to visible light. Table 2 indicates that the photo-degradation activity of CrBiO-2 was higher than another nano photocatalyst in previous reported. The mechanism for MAL degradation by using Cr2S3–Bi2O3 catalysts. Under light illumination, e− are motivated and conducted from VB to the CB. Therefore, the h+ is produced in the VB. The fraction of photo-generated e−/h+ pairs is important in photo-degradation reaction and leading to reduce of photo-degradation performance of Bi2O3. After combining with Cr2S3, the photo-induced e− are trapped, resulting in the increased e−/h+ separation of Cr2S3–Bi2O3 catalyst. Electrons can decrease the surface adsorbed O2 into ·O2−, which may cause degradation of MAL. Moreover, the h+ can oxidize the H2O or ·OH molecules by ·OH, which are great reactive forms. The h+ may attack MAL molecules by itself to convert to pathways. The h+, ·OH, and ·O2− forms can degrade MAL to other intermediate and ultimate compounds (dioxide carbon and water). The degradation rate of MAL was identified using a Langmuir–Hinshelwood model [39, 40], ln(C/Co) = kt, where, k is the Langmuir–Hinshelwood rate. The rate constant (k) for the MAL removal under UV and visible light by using Bi2O3, CrSBiO-0, CrBiO-1, and CrBiO-2 were found 0.0105, 0.0132, 0.0180, 0.0188, min−1 and 0.085, 0.0112, 0.0151, 0.0157 min−1, respectively.
The effect of pH on the photocatalytic performance of the Bi2O3, CrSBiO-0, CrBiO-1, and CrBiO-2 is substantial for attain to behaviour reaction at various pH [41, 42]. Therefore, the photo-degradation activity was tested at various pH media, as indicated in Fig. 6c. It can be seen, the photo-degradation activity enhances with the reducing of pH, and highest photo-degradation amount occur at pH: 5, this can be demonstrated by the lowest electrostatic attraction force onto the interface of MAL surface and the prepared nano-catalyst [43,44,45,46,47,48].
3.3 Repeatability test
These test demonstrated that CrBiO-2 nanocomposites have excellent stability after recovery and that nano-catalyst reuse is impressive. The photocatalysis process of CrBiO-2 slightly decreased after the five cycles (Fig. 7a). The first cycle and five cycles are 97.5, 90.5% and 94.5, 87.5% under UV and visible light, respectively, which that shows the photocatalysis process of the CrBiO-2 nanocomposites was decreased about 3%.
3.4 Scavenger tests
To identify the effect of scavenger compound, the isopropanol (IPA), ammonium oxalate (AO) and p-benzoquinone (BQ) were applied to quench ·OH, h+ and O2·− generated during the MAL photo-degradation [48,49,50]. Figure 7b demonstrates the MAL photocatalytic degradation was decreased with the addition of 0.1 mM BQ into the suspension of MAL and CrBiO-2 nanocomposites. It can be seen, the 0.1 mM BQ had not effect on the MAL photo-degradation. The results suggested that ·OH and h+ are the dominant oxidative species in the photo-degradation process.
3.5 Antibacterial activity tests
The antimicrobial activity study of Bi2O3, CrSBiO-0, CrBiO-1, and CrBiO-2 nanocomposites was measured by using agar diffusion analysis method (Table 3). These data revealed that the bactericidal progress of Bi2O3 and CrSBiO-0 was the same, demonstrating that they had no considerable bactericidal influences. The CrBiO-2 revealed the highest antimicrobial activity (Table 4). As the data of zone inhibition, the high ratio of Cr2S3 raised the bactericidal effect, as compared to other catalysts. Moreover, the MIC and MBC data of CrBiO-2 nanocomposites indicated the bactericidal influence versus gram-positive and negative bacterial strains (Table 4).
3.6 Dielectric behaviour of prepared Bi2O3 and Bi2O3–Cr2S3 nano-catalyst
Figure 8 demonstrates the change of dielectric constant with frequency range in room temperature. The dielectric constant value reduces with increase in frequency value. This manner may be revealed by polarization progress of Bi2O3, and Cr2S3–Bi2O3 nanomaterials due to the semiconductor nanoparticles contain high defects in the interface, which decreases surface charge distribution.
4 Conclusions
For the photo-degradation of Malathion as an organophosphate insecticide, a novel photocatalytic based Bi2O3, CrSBiO-0, CrBiO-1, and CrBiO-2 was successfully synthesized. The photo-degradation of MAL from water under visible and UV light was studied. The mean particles size of the Bi2O3 and CrBiO-1 nanocomposites were 55.0, and 65.0 nm, respectively. It is clear that the CrBiO-2 reveals the highest photo-degradation with 87.4%, and 97.5% under visible and UV light, respectively. It was observed that time (50 min), pH (5.0) and photocatalyst concentration (0.1 g/L) considerably influence on the photo-degradation activity. The results indicated that CrBiO-2 is the great nano-catalyst for removal of MAL and advanced wastewater treatment. The results data of the antibacterial mechanism indicated that CrBiO-2 could be used as an antibacterial nanomaterial.
Change history
26 March 2024
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1007/s10854-024-12414-w
References
A. Mittal, J. Mittal, A. Malviya, V.K. Gupta, Removal and recovery of Chrysoidine Y from aqueous solutions by waste materials. J. Colloid Interface Sci. 344, 497–507 (2010)
V.K. Gupta, R. Jain, A. Nayak, S. Agarwal, M. Shrivastava, Removal of the hazardous dye—tartrazine by photodegradation on titanium dioxide surface. Mater. Sci. Eng. C 31, 1062–1067 (2011)
T.A. Saleh, V.K. Gupta, Photo-catalyzed degradation of hazardous dye methyl orange by use of a composite catalyst consisting of multi-walled carbon nanotubes and titanium dioxide. J. Colloid Interface Sci. 371, 101–106 (2012)
H. Khani, M.K. Rofouei, P. Arab, V.K. Gupta, Z. Vafaei, Multi-walled carbon nanotubes-ionic liquid-carbon paste electrode as a super selectivity sensor: application to potentiometric monitoring of mercury ion(II). J. Hazard. Mater. 183, 402–409 (2010)
V.K. Gupta, R. Kumar, A. Nayak, T.A. Saleh, M.A. Barakat, Adsorptive removal of dyes from aqueous solution onto carbon nanotubes: a review. Adv. Colloid Interface Sci. 193–194, 24–34 (2013)
R. Saravanan, E. Sacari, F. Gracia, M.M. Khan, V.K. Gupta, Conducting PANI stimulated ZnO system for visible light photocatalytic degradation of coloured dyes. J. Mol. Liq. 221, 1029–1033 (2016)
M. Devaraj, R. Saravanan, R. Deivasigamani, V.K. Gupta, S. Jayadevan, Fabrication of novel shape Cu and Cu/Cu2O nanoparticles modified electrode for the determination of dopamine and paracetamol. J. Mol. Liq. 221, 930–941 (2016)
R. Saravanan, S. Joicy, V.K. Gupta, V. Narayanan, A. Stephen, Visible light induced degradation of methylene blue using CeO2/V2O5 and CeO2/CuO catalysts. Mater. Sci. Eng. C 33, 4725–4731 (2013)
R. Saravanan, S. Karthikeyan, V.K. Gupta, G. Sekaran, A. Stephen, Enhanced photocatalytic activity of ZnO/CuO nanocomposite for the degradation of textile dye on visible light illumination. Mater. Sci. Eng. C 33, 91–98 (2013)
R. Saravanan, E. Thirumal, V.K. Gupta, V. Narayanan, A. Stephen, The photocatalytic activity of ZnO prepared by simple thermal decomposition method at various temperatures. J. Mol. Liq. 177, 394–401 (2013)
N. Mohammadi, H. Khani, V.K. Gupta, E. Amereh, S. Agarwal, Adsorption process of methyl orange dye onto mesoporous carbon material–kinetic and thermodynamic studies. J. Colloid Interface Sci. 362, 457–462 (2011)
T.A. Saleh, V.K. Gupta, Synthesis and characterization of alumina nano-particles polyamide membrane with enhanced flux rejection performance. Sep. Purif. Technol. 89, 245–251 (2012)
R. Saravanan, N. Karthikeyan, V.K. Gupta, E. Thirumal, A. Stephen, ZnO/Ag nanocomposite: an efficient catalyst for degradation studies of textile effluents under visible light. Mater. Sci. Eng. C 33, 2235–2244 (2013)
R. Saravanan, M.M. Khan, V.K. Gupta, E. Mosquera, A. Stephen, ZnO/Ag/CdO nanocomposite for visible light-induced photocatalytic degradation of industrial textile effluents. J. Colloid Interface Sci. 452, 126–133 (2015)
Wei Gao, Razieh Razavi, Ali Fakhri, Preparation and development of FeS2 quantum dots on SiO2 nanostructures immobilized in biopolymers and synthetic polymers as nanoparticles and nanofibers catalyst for antibiotic degradation. Int. J. Biol. Macromol. 114, 357–362 (2018)
X. Huang, W. Zhang, Y. Tan, J. Wu, Y. Gao, B. Tang, Facile synthesis of rod-like Bi2O3 nanoparticles as an electrode material for pseudocapacitors. Ceram. Int. 42, 2099–2105 (2016)
Wei Li, Facile synthesis of monodisperse Bi2O3 nanoparticles. Mater. Chem. Phys. 99, 174–180 (2006)
M. Schlesinger, M. Weber, S. Schulze, M. Hietschold, M. Mehring, Metastable β-Bi2O3 nanoparticles with potential for photocatalytic water purification using visible light irradiation. Chem. Open 2, 146–155 (2013)
OGh Abdullah, D.A. Tahir, D.R. Saber, Optical properties of the synthesized Cr2S3 nanoparticles embedded in polyvinyl alcohol. Sci. J. Koya Univers 1, 5 (2015)
A. Loukanov, S. Emin, Biotinylated vanadium and chromium sulfide nanoparticles as probes for colocalization of membrane proteins. J. Environ. Chem. Eng. 6, 3306–3321 (2018)
T. Chen, Q. Hao, W. Yang, C. Xie, D. Chen, C. Ma, W. Yao, Y. Zhu, A honeycomb multilevel structure Bi2O3 with highly efficient catalytic activity driven by bias voltage and oxygen defect. App. Catal. B Environ 237, 442–448 (2018)
W. He, Y. Sun, G. Jiang, H. Huang, X. Zhang, F. Dong, Activation of amorphous Bi2WO6 with synchronous Bi metal and Bi2O3 coupling: photocatalysis mechanism and reaction pathway. App. Catal. B Environ. 232, 340–347 (2018)
J.C. Medina, N.S. Portillo-Vélez, M. Bizarro, A. Hernández-Gordillo, S.E. Rodil, Synergistic effect of supported ZnO/Bi2O3 heterojunctions for photocatalysis under visible light. Dyes Pigm. 153, 106–116 (2018)
Y. Huang, J. Qin, C. Hu, X. Liu, D. Wei, H.J. Seo, Cs-doped α-Bi2O3 microplates: hydrothermal synthesis and improved photochemical activities. Appl. Surf. Sci. 473, 401–408 (2019)
J. Ke, Ck Zhao, H. Zhou, X. Duan, S. Wang, Enhanced solar light driven activity of p-n heterojunction for water oxidation induced by deposition of Cu2O on Bi2O3 microplates. Sustain. Mater. Technol. 19, 00088 (2019)
W. Hussain, A. Badshah, R.A. Hussain, I. Din, M.A. Aleem, A. Bahadur, S. Iqbal, M.U. Farooq, H. Ali, Photocatalytic applications of Cr2S3 synthesized from single and multi-source precursors. Mater. Chem. Phys. 194, 345–355 (2017)
L. Hu, F. Chen, P. Hu, L. Zou, X. Hu, Hydrothermal synthesis of SnO2/ZnS nanocomposite as a photocatalyst for degradation of Rhodamine B under simulated and natural sunlight. J. Mol. Catal. A Chem. 411, 203–213 (2016)
X. Yuan, H. Wang, Y. Wu, X. Chen, G. Zeng, L. Leng, C. Zhang, A novel SnS2–MgFe2O4/reduced graphene oxide flower-like photocatalyst: solvothermal synthesis, characterization and improved visible-light photocatalytic activity. Catal. Commun. 61, 62–66 (2015)
C.Y. Park, T. Ghosh, Z. Meng, U. Kefayat, N. Vikram, W.C. OH, Preparation of CuS-graphene oxide/TiO2 composites designed for high photonic effect and photocatalytic activity under visible light. Chin. J. Catal. 34, 711–717 (2013)
G. Hitkari, S. Singh, G. Pandey, Photoluminescence behavior and visible light photocatalytic activity of ZnO, ZnO/ZnS and ZnO/ZnS/α-Fe2O3 nanocomposites. Trans. Nonferrous Met. Soc. China 28, 1386–1396 (2018)
H. Liu, H. Zhou, H. Li, X. Liu, C. Ren, Y. Liu, W. Li, M. Zhang, Fabrication of Bi2S3@Bi2WO6/WO3 ternary photocatalyst with enhanced photocatalytic performance: synergistic effect of Z-scheme/traditional heterojunction and oxygen vacancy. J. Taiwan Inst. Chem. E. 95, 94–102 (2019)
W. Hong, L. Wang, K. Liu, X. Han, E. Liu, Asymmetric supercapacitor constructed by self-assembled camellia-like BiOCl and activated carbon microspheres derived from sweet potato starch. J. Alloys Compd. 746, 292–300 (2018)
X. Ma, Y. Xia, L. Ni, L. Song, Z. Wang, Preparation of gold nanoparticles–agarose gel composite and its application in SERS detection. Spectrochim. Acta Part A 121, 657–661 (2014)
M. Hosseini, M.R.R. Kahkha, A. Fakhri, S. Tahami, M.J. Lariche, Degradation of macrolide antibiotics via sono or photo coupled with Fenton methods in the presence of ZnS quantum dots decorated SnO2 nanosheets. J. Photochem. Photobiol. B Biol 185, 24–31 (2018)
V.K. Gupta, A. Fakhri, M. Azad, S. Agarwal, Synthesis of CdSe quantum dots decorated SnO2 nanotubes as anode for photo-assisted electrochemical degradation of Hydrochlorothiazide: kinetic process. J. Colloid Interface Sci. 510, 95–102 (2018)
H. Cheng, B. Huang, J. Lu, Z. Wang, B. Xu, X. Qin, X. Zhang, Y. Dai, Synergistic effect of crystal and electronic structures on the visible-light-driven photocatalytic performances of Bi2O3 polymorphs. Phys. Chem. Chem. Phys. 12, 15468–15475 (2010)
A. Fakhri, M. Azad, L. Fatolahi, S. Tahami, Microwave-assisted photocatalysis of neurotoxin compounds using metal oxides quantum dots/nanosheets composites: photocorrosion inhibition, reusability and antibacterial activity studies. J. Photochem. Photobiol. B Biol. 178, 108–114 (2018)
B.T. Sone, E. Manikandan, A. Gurib-Fakim, M. Maaza, Single-phase α-Cr2O3 nanoparticles’ green synthesis using Callistemon viminalis’ red flower extract. Green Chem. Lett. Rev. 9, 85–90 (2016)
L. Escobar-Alarcón, J.G. Morales-Mendez, D.A. Solís-Casados, S. Romero, M. Fernández, E. Haro-Poniatowski, Preparation and characterization of bismuth nanostructures deposited by pulsed laser ablation. J. Phys: Conf. Ser. 582, 012013 (2015)
K.H. Wu, Y.M. Shin, C.C. Yang, W.D. Ho, J.S. Hsu, Preparation and ferromagnetic properties of Ni0.5Zn0.5Fe2O4/polyaniline core–shell nanocomposites. J. Polym. Sci. Part A Polym. Chem. 44(8), 2657–2664 (2006)
Y. Wang, D. Yang, Y. Shi, Z. Jiang, Bio-inspired synthesis of TiO2 hollow nanospheres in agarose gels. J. Alloys Compd. 560, 42–48 (2013)
Y. Wu, F. Geng, P.R. Chang, J. Yu, X. Ma, Effect of agar on the microstructure and performance of potato starch film. Carbohydr. Polym. 76, 299–304 (2009)
H.A.J.L. Mourão, O.F. Lopes, C. Ribeiro, V.R. Mastelaro, Rapid hydrothermal synthesis and pH-dependent photocatalysis of strontium titanate microspheres. Mater. Sci. Semicond. Process. 30, 651–657 (2015)
A. Fakhri, S. Tahami, P.A. Nejad, Preparation and characterization of Fe3O4-Ag2O quantum dots decorated cellulose nanofibers as a carrier of anticancer drugs for skin cancer. J. Photochem. Photobiol. B Biol. 175, 83–88 (2017)
R Moheimani, M Hasansade, A closed-form model for estimating the effective thermal conductivities of carbon nanotube–polymer nanocomposites, Proceedings of the Institution of Mechanical Engineers, Part C: Journal of Mechanical Engineering Science, 2018
R. Moheimani, R. Sarayloo, H. Dalir. Symmetrical and antisymmetrical sequenced fibers with epoxy resin on rectangular reinforced structures under axial loading, American Society for Composites, (2018)
A. Khavaji, D.D. Ganji, N. Roshan, R. Moheimani, M. Hatami, A. Hasanpour, Slope variation effect on large deflection of compliant beam using analytical approach. Struct. Eng. Mech. 44, 405–416 (2012)
V.K. Gupta, A. Fakhri, S. Agarwal, E. Ahmadi, P.A. Nejad, Synthesis and characterization of MnO2/NiO nanocomposites for photocatalysis of tetracycline antibiotic and modification with guanidine for carriers of Caffeic acid phenethyl ester-an anticancer drug. J. Photochem. Photobiol. B Biol. 174, 235–242 (2017)
V.K. Gupta, N. Atar, M.L. Yola, Z. Üstündağ, L. Uzun, A novel magnetic Fe@Au core–shell nanoparticles anchored graphene oxide recyclable nanocatalyst for the reduction of nitrophenol compounds. Water Res. 48, 210–217 (2014)
A. Asfaram, M. Ghaedi, S. Agarwal, I. Tyagi, V.K. Gupta, Removal of basic dye Auramine-O by ZnS: Cu nanoparticles loaded on activated carbon: optimization of parameters using response surface methodology with central composite design. RSC Adv. 5, 18438–18450 (2015)
A.N. Kadam, R.S. Dhabbe, M.R. Kokate, Y.B. Gaikwad, K.M. Garadkar, Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 133, 669–676 (2014)
Dina M. Fouad, Waleed A. El-Said, Mona B. Mohamed, Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 140, 392–397 (2015)
N.A. Ramos-Delgado, L. Hinojosa-Reyes, I.L. Guzman-Mar, M.A. Gracia-Pinilla, A. Hernández-Ramírez, Catal. Today 209, 35–40 (2013)
Acknowledgements
This project was supported and presented by Islamic Azad University, Science research Branch of Tehran (IRAN) and thanks for it.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1007/s10854-024-12414-w
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, L., Hosseini, M., Fakhri, A. et al. RETRACTED ARTICLE: Synthesis and characterization of Cr2S3–Bi2O3 nanocomposites: photocatalytic, quenching, repeatability, and antibacterial performances. J Mater Sci: Mater Electron 30, 13067–13075 (2019). https://doi.org/10.1007/s10854-019-01668-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-019-01668-4