Abstract
A bismuth vanadate (BiVO4) irregular nanoplates surface decorated with tin disulphide (SnS2) nanoplates is developed as a visible light responsive photocatalyst for the degradation of ciprofloxacin antibiotics. The photocatalyst, named SnS2/BiVO4 is characterized by various physiochemical techniques such as powder X-Ray diffraction, field-emission scanning electron microscopy, high-resolution transmission electron microscopy, X-ray photoelectron spectroscopy, UV–vis diffuse reflectance spectroscopy, electrochemical impedance spectroscopy and photoluminescence. The optimized batch 0.15SnS2/BiVO4 photocatalysts degraded 92% (CIP, 10 mg/L) with a rate constant 0.0184 min−1, which was about 3.75 times and 7.66 times higher than that of the BiVO4 (k = 0.0049 min−1) and SnS2 (k = 0.0024 min−1) photocatalyst respectively. The improved photocatalytic performance of SnS2/BiVO4 nanocomposite is mainly due to (a) strengthened visible light absorption by 0.15SnS2/BiVO4 due to the shifting of absorption edge towards higher wavelength (b) Z-scheme-like band position of BiVO4 and SnS2, (c) efficient inhibition of charge carrier recombination, and (d) in situ generation of reactive oxygen species.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Harmful organic pollutants such as pharmaceutical products, personal care products, dyes, pesticides etc., released from the pharmaceutical, plastic, textile and leather industries into the water bodies are of great concern [1,2,3,4]. Ciprofloxacin drug is one of the broad-spectrum antibiotics, which have found wide applications in treating various bacterial infections in animals and humans [5,6,7]. Their consumption has been tremendously increased worldwide because of their low cost [8]. However, the long persistent nature of these drugs due to their slow metabolized nature in an aqueous medium, make them fatal for the water bodies [9, 10]. They can easily toxify ground and surface water, causing harmful infection in animals and humans due to the development of anti-bacterial resistant strains inside their bodies [11, 12]. Many techniques have been used to date, out of which semiconductor-based photocatalysis has found great potential in removing antibiotics from wastewater [8, 13, 14]. However, majority of the semiconductor photocatalysts suffered from certain limitations such as high charge carrier recombination rate, low redox properties, intrinsic defects, etc. [15,16,17]. Therefore, it is of great interest to design an efficient visible light active photocatalyst with high stability and enhanced photocatalytic activity toward the antibiotic degradation in an aqueous medium under sunlight irradiation.
Bismuth vanadate (BiVO4) is the simplest ternary oxide semiconductor, with monoclinic scheelite phase. Due to its unique structure, together with its non-toxic, environmentally benign nature with excellent chemical stability, BiVO4 has been considered a promising material for photocatalytic applications [18,19,20]. As compared with large band gap TiO2 whose electronic structure consists of only O \(2p\) orbitals, the BiVO4 consists of Bi \(6s\) and O \(2p\) orbitals, the presence of extra Bi \(6s\) orbital minimize the distance of excited electron from the valence band of BiVO4 to the conduction band of V\(3d\) state of VO43−, which results in the reduction of band gap energy, therefore increasing the light absorption region up to visible light [21, 22]. However, the low band gap (2.4–2.5 eV) makes BiVO4 susceptible to rapid recombination of the photoexcited charge carriers, and thus, various strategies have been designed to overcome the intrinsic limitations of pristine BiVO4 [23,24,25]. For instance, the construction of Z-scheme type heterojunctions with semiconductors having matching band edges with respect to that of BiVO4 has proven to be an effective approach to overcome these limitations [26, 27]. The construction of a Z-scheme heterojunction composite is beneficial from a photocatalytic point of view by minimizing the charge carrier recombination rate and possessing strong redox abilities [28,29,30]. This occurs due to the retention of electron and holes in thermodynamic favorable condition i.e., electrons in the conduction band of one semiconductor and holes in the valence band of other in the heterostructure, which then takes part in the redox reactions generating in-situ active species required for the photocatalytic process [31,32,33]. Yong et al. prepared a Z-scheme In2S3/BiVO4 photocatalyst, which shows enhanced photocatalytic activity towards the degradation of glyphosate [26]. Luo et al. synthesized a MoSe2/BiVO4 heterojunction with Z-scheme, which exhibits superior photocatalytic performance toward the photocatalytic removal of glyphosate [34]. Similarly, Chen et al. prepared Z-scheme AgI/BiVO4 heterojunction which show superior photocatalytic activity toward the degradation of tetracycline under visible light irradiation [35].
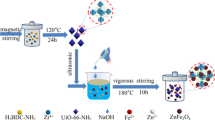
In this regard, the band edges of a small band gap semiconductor, SnS2, match well with that of BiVO4, and hence, using SnS2 as a sensitizer to construct heterostructure with BiVO4 can improve the photocatalytic performance of the photocatalyst [26, 36]. In this work, a novel SnS2/BiVO4 heterostructure was fabricated via a two-step hydrothermal route. The effects of varying mass ratios of SnS2 and BiVO4 on the physicochemical properties of the as-synthesized composites were analysed by their structural, morphological, and optical properties. A possible formation of a Z-scheme heterostructure between BiVO4 and SnS2 has been proposed. The SnS2/BiVO4 nanocomposite exhibited enhanced photocatalytic efficiency towards ciprofloxacin (CIP) degradation under natural sunlight.
2 Experimental Section
2.1 Preparation of SnS2
Firstly, SnS2 nanoplates were synthesized using the hydrothermal method. stannous chloride pentahydrate (4mmol), thioacetamide (12 mmol), were dispersed in 70 mL DI water. The final suspension was transferred to a Teflon-lined stainless autoclave (100 mL) and maintained at 180 °C for 12 h in an oven. Finally, the supernatant liquid was discarded, and the final product was collected, washed with water and ethanol, and dried at 50 °C overnight.
2.2 Preparation of SnS2/BiVO4
The SnS2/BiVO4 nanocomposites were prepared by a hydrothermal method. 2 mmol NH4VO3, and 2 mmol Bi(NO3)3. 5H2O were mixed in 50 mL DI water under stirring for 30 min. The pH of the reaction mixture was kept at about 5–6 using NaOH (10.0 M) solutions. Afterward, the specified amount of SnS2 (wt%) (10 wt%, 15 wt%, and 20 wt%) was added to the above reaction mixture under constant stirring. Finally, the resulting solution was transferred into a Teflon-lined autoclave and kept at 160 °C for 24 h. The obtained batches of SnS2/BiVO4 nanocomposites were centrifuged and washed with water and ethanol several times and dried at 60 °C overnight. The batches of SnS2/BiVO4 nanocomposites were denoted as 0.10SnS2/BiVO4, 0.15SnS2/BiVO4, and 0.20SnS2/BiVO4 according to the amount of SnS2 precursor added. The pure BiVO4 was prepared by the same synthesis protocol without using SnS2.
2.3 Characterization of Photocatalyst
The as-synthesized batches of SnS2/BiVO4 NCs, BiVO4, and SnS2 were characterized by different techniques. All details related to these techniques, including the sample preparation, were discussed in Section S1 of Supporting Information.
2.4 Photocatalytic Activity Test
The photocatalytic test of the SnS2/BiVO4 nanocomposites were conducted by degrading ciprofloxacin (CIP, 10 mg/L, 100 mL) using 0.06 g of photocatalyst in aqueous solution under the exposure of natural sunlight. Before the irradiation, the reaction mixtures were vigorously stirred for 60 min under dark to ensure adsorption/desorption equilibrium and then the reaction mixture were kept under natural sunlight for about 105 min in order to carry out the degradation studies. During photoreaction, 1 mL of aliquots was collected at regular intervals and centrifuged to separate the solid photocatalyst. Finally, the CIP concentration were monitored by UV–vis spectrophotometer. The leached concentrations of Bi, V, and Sn were analysed with the help of ICP-OES following the calibration technique (details were discussed in Supporting Information section S2).
3 Results and Discussion
3.1 XRD Analysis
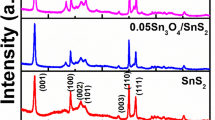
The crystalline structure and phase composition of the as-synthesized heterostructures were analysed through XRD method, as represented in Fig. 1a. All the XRD peaks of BiVO4 and SnS2 are in good agreement with their monoclinic scheelite phase (JCPDS No.- 14-0688) and hexagonal phase (JCPDS No.-83-1705) respectively [37, 38]. The diffraction peak of the SnS2/BiVO4 heterojunction are similar to the BiVO4, which confirm that the coupling of SnS2 does not alter the phase of BiVO4 (Fig. 1a). No distinctive peak of SnS2 were observed in the SnS2/BiVO4 nanocomposite but in the enlarged view (Fig. 1b), reveals some shift in the peak at about 2θ of 29.1° and 30.7° related to the (121) and (040) lattice planes (Fig. 1b), when compared to BiVO4, the XRD peaks of SnS2/BiVO4 shows slightly shift towards the higher 2θ region. This event suggested the strong interaction and intimate contact between SnS2 and BiVO4 [39]. The detailed information about the crystallite size of SnS2/BiVO4 NCs and BiVO4 are discussed in supporting information section S3.
3.2 FE-SEM and HR-TEM Analysis
The microstructure and sizes of pure SnS2, BiVO4, and 0.15SnS2/BiVO4 nanocomposites were investigated by field emission electron microscopy (FE-SEM) (Fig. 2). The FESEM image of SnS2 reveals the formation of a nanoplates of irregular sizes having diameters of 150–250 nm (Fig. 2a). The FESEM image of BiVO4 revealed a smooth irregular nanoplates-like structure with a size ranging between 300 and 700 nm in length (Fig. 2b). The FE-SEM image of 0.15SnS2/BiVO4 reveals the formation of irregular nanoplates of BiVO4 with nanoparticles of SnS2 tightly adhered to the surface of BiVO4 (Fig. 2c), which is substantiated from the XRD data also. Thus, it can be inferred that loading SnS2 nanoplates on BiVO4 irregular nanoplates surface favours charge transmission between the two components; however, the SnS2/BiVO4 composites retain the morphology, size, and hierarchical structure of pristine BiVO4.
The detailed morphology and microstructure of 0.15SnS2/BiVO4 NCs were further investigated by HR-TEM, and it can be observed that the nanoplates of SnS2 in the range of 80–100 nm were evenly dispersed over the 100–300 nm long nanoplates of BiVO4 (Fig. 2d-e). As shown in Fig. 2f, the lattice fringe (0.315 nm) of 0.15SnS2/BiVO4 corresponds to (121) plane of monoclinic BiVO4, while the other interatomic layer spacing of (0.292 nm) corresponds to the (011) lattice phase of hexagonal SnS2. The SAED pattern confirms the polycrystalline nature of 0.15SnS2/BiVO4, and the obtained electron diffractions rings were indexed, which corresponds to the monoclinic phase of BiVO4 (JCPDS No. 14-0688) (Fig. 2g).
3.3 XPS Analysis
XPS analysis was used to investigate the chemical composition of 0.15SnS2/BiVO4, Fig. 3a exhibits the survey spectrum of batch 0.15SnS2/BiVO4 nanocomposite and high-resolution XPS spectra of Bi \(4f\), V \(2p\), Sn\(3d\), S \(2p\), and O \(1s\). The XPS spectra reveal that the 0.15SnS2/BiVO4 nanocomposite consists of Bi, V, Sn, S, and O elements. The XPS spectrum of Bi \(4f\) revealed two major peaks at 164.01 eV and 158.77 eV, which corresponds to Bi \({4f}_{5/2}\) and Bi \({4f}_{7/2 }\), respectively (Fig. 3b). The XPS spectra of V \(2p\) shows two peaks at 523.91 eV and 516.03 eV which corresponds to V\({2p}_{1/2}\)and V\({2p}_{3/2}\), which represents vanadium (V) in the state of \({V}^{5+}\) state as shown in Fig. 3c. The \({Sn}^{4+}\)state in the 0.15SnS2/BiVO4 photocatalyst is reflected from the peaks at 486.49 eV and 494.85 eV (Fig. 3d), which corresponded to Sn \({3d}_{5/2}\)and Sn \({3d}_{3/2}\)peaks, respectively. In the S \(2p\) XPS spectra shows two peaks at 162.40 eV and 161.46 eV, which attributes to Sn\({ 2p}_{1/2}\) and Sn \({2p}_{3/2}\), respectively, that correspond to \({S}^{2-}\) ion in SnS2 (Fig. 3e). The O \(1s\) spectra show peaks at 529.50 eV, which was related to crystal lattice oxygen in 0.15SnS2/BiVO4 nanocomposite and at 530.02 eV which could possibly due to the surface defects and adsorbed oxygen species (Fig. 3f).
3.4 UV–Vis DRS Analysis
The optical properties of SnS2, BiVO4, and SnS2/BiVO4 were explored by UV–vis DRS spectra. Figure 4a reveals that pristine BiVO4 and the SnS2/BiVO4 composites exhibit strong absorbance in the UV region extending to the visible light region, whereas SnS2 absorbs only in the visible region (~ 570 nm) (inset of Fig. 4a). The absorption band edges of BiVO4 lie at around 480 nm and on increasing the SnS2 content, the absorption wavelength of the SnS2/BiVO4 composites exhibit a red shift with respect to pristine BiVO4. Using the Tauc expression, (αhν)1/n =A(hν–Eg) [40], the optical band gaps (Eg) of pristine BiVO4, SnS2, and SnS2/BiVO4 composites were calculated. Here α, h, v, represent absorption coefficient, Planck constant, irradiation frequency, whereas A, Eg and n represent the absorption constant, gap energy and a constant, respectively. Combining SnS2 (Eg =2.2 eV) with BiVO4 (Eg = 2.41 eV) results in a red shift in the values of the band gap to 2.39 eV, 2.36 eV and 2.34 eV for 0.10SnS2/BiVO4, 0.15SnS2/BiVO4, and 0.20SnS2/BiVO4 composites respectively (Fig. 4b). Thus, it can be inferred that optical band-gaps of heterostructure composites can be finely tuned by regulating the weight ratios of the two semiconductors.
3.5 BET Analysis
The textural features of BiVO4, SnS2, and 0.15SnS2/BiVO4 were determined from BET isotherm surface area and Barrett-Joyner-Halenda (BJH) pore size distribution curves and the results are presented in Fig. 5. All isotherm curves (Fig. 5a) are related to type-IV with H3 hysteresis. The specific surface area of BiVO4, SnS2, and 0.15SnS2/BiVO4 are determined as\(21.2 {\text{m}}^{2}{\text{g}}^{-1}\), \(51.4 {\text{m}}^{2}{\text{g}}^{-1}\), and\(34.6 {\text{m}}^{2}{\text{g}}^{-1}\), respectively, while their pore volumes are 0.02 c\({\text{m}}^{3}{\text{g}}^{-1}\), 0.12 c\({\text{m}}^{3}{\text{g}}^{-1}\), and 0.03 c\({\text{m}}^{3}{\text{g}}^{-1}\), respectively (Table. S2). The pore size distribution curves (Fig. 5b) show pore size distribution between 3 and 9 nm, which further substantiates its mesoporous characteristics. Compared to BiVO4, the specific surface area and pore volume of 0.15SnS2/BiVO4 is more, which is attributable to deposition of SnS2 on the nanoplates of BiVO4. Such mesoporous texture can be an advantage for improving its adsorptive photocatalytic efficiency.
3.6 Photocatalytic Measurement
The impact of photocatalyst dose on the photocatalytic performance was investigated by treating the aqueous solution CIP with different amounts of photocatalyst, i.e., 40–80 mg of photocatalyst per 100 mL under the same experimental environment as mentioned above. The degradation rate and removal efficiency were first increased with the increase in the photocatalyst dose from 0.4 mg/mL (k = 0.0044 min−1) to 0.6 mg/mL (k = 0.0184 min−1) and then decreased for the dose 0.8 mg/mL (k = 0.0115 min−1) (Fig.S1). The impact of the change in concentration of CIP solution has been investigated by changing the initial concentration of CIP solution from 10 to 30 mg/L. The decrease in the photocatalytic performance was observed with the increase in CIP concentrations from 10 mg/L (k = 0.0184 min−1) to 30 mg/L (k = 0.0041 min−1) (Fig.S1). A complete photocatalytic degradation study was performed with a photocatalyst dose of 60 mg per 100 mL aqueous solution of CIP (10 mg/L).
Photocatalytic degradation efficacies of pristine BiVO4, SnS2, and batches of SnS2/BiVO4 composites were tested against ciprofloxacin drug (10 mg/L−1). The photocatalytic test was performed under natural sunlight. The degradation profile of CIP under sunlight irradiation by SnS2/BiVO4 NCs, BiVO4 irregular nanoplates, and SnS2 nanoplates are showed in Fig. 6b. Ciprofloxacin shows negligible self-degradation without any catalyst, whereas the pristine SnS2 and BiVO4 displayed poor photocatalytic efficiency toward the CIP degradation after 105 min of sunlight irradiation. From all the batches prepared the optimized batch 0.15SnS2/BiVO4 shows the highest photocatalytic degradation efficiency towards the CIP, which was about 92% after 105 min of sunlight irradiations. The degradation rate and removal efficiency were first increased with the increase in the amount of SnS2 added from 0.10 wt% to 0.15 wt% in SnS2/BiVO4 NCs. The possible reason for this increase may be the better separation and transmission of photogenerated e‾-h+ pairs. When the amount of SnS2 is further increased from 0.15 wt% to 0.20 wt%, the active sites present on the BiVO4 will be occupied, due to which the light absorption tendency has been reduced, leading to a decrease in photocatalytic efficiency.
Moreover, the degradation mechanism was estimated by fitting time-dependent CIP degradation data with a pseudo-first-order: ln(C0/C) = kt, as shown in Fig. 6c, where C0 is the initial concentration of CIP, C is the concentration at a preselected time t, and the k is apparent reaction rate constant (Table 1). The optimized batch 0.15SnS2/BiVO4 exhibited the highest apparent rate constant (k = 0.0184 min−1), which is 3.75 and 7.66 times of BiVO4 (k = 0.0049 min−1) and SnS2 (k = 0.0024 min−1), respectively. Figure 6a shows the corresponding UV–vis absorption spectrum of CIP degraded by the optimized batch 0.15SnS2/BiVO4 under sunlight irradiation. The decrease in the intensity of absorption peak of CIP (276 nm) was observed over 105 min period of sunlight irradiation.
3.7 Radical Trapping Experiment
To determine the involvement of in-situ generated ROS by 0.15SnS2/BiVO4 heterojunction composite towards CIP degradation, specific ROS scavenging studies were performed. Various ROS scavengers like, chloroform (1mM) [41], ammonium oxalate (AO, 1mM) [42], and isopropyl alcohol (IPA 1mM) [43], were used as the quenchers for O2¯•, h+, and •OH radicals respectively (Fig. 6d). The photocatalytic performance of 0.15SnS2/BiVO4 significantly decrease with the addition of chloroform, whereas similar observation was obtained after the addition of ammonium oxalate as a quencher. However, in case of IPA as a quencher slight considerable decrease in the degradation rate was observed, which indicate that superoxide anion radical (O2¯•) and hole (h+) play more pivotal role than hydroxyl (•OH) radicals in degradation process.
Apart from the excellent photocatalytic degradation performance, the re-usability, and leaching of the different metal species during the photocatalytic degradation were also playing a major role in the application. Figure 7a shows the re-usability of 0.15SnS2/BiVO4 photocatalyst for the photocatalytic degradation of CIP up to five cycles, which shows a similar type degradation pattern in each cycle, reflecting the photocatalyst’s high stability. The excellent structural stability of the 0.15SnS2/BiVO4 photocatalyst was further confirmed by the unchanged XRD pattern (Table S3) and morphology of pristine and used photocatalyst (Fig. 7b-c). Further, the toxicity level of the used 0.15SnS2/BiVO4 photocatalyst was accessed by the amount of leached metal species, i.e., Bi, V, and Sn, using the ICP-OES technique. The respective calibration plots of Bi, V, Sn, and S were used to determine the amount of leached concentration (Fig. S2 of Supporting Information). It is observed that the leaching concentrations correspond to ~ 0.93% Bi (306 µg/L), 1.5% V (295.12 µg/L), and 0.8% Sn (285.32 µg/L) of the total weight of the catalyst, which is well within the permissible limits (Table S4).
3.8 Photocatalytic Mechanism
The photoluminescence spectra of BiVO4, 0.10SnS2/BiVO4, 0.15SnS2/BiVO4, and 0.20SnS2/BiVO4 nanocomposite exhibit broad spectra with a peak at a wavelength of ~ 514 nm. From Fig. 8a, it is clearly visible that BiVO4 owns a strong PL emission peak at ~ 514 nm, while the emission peaks of the SnS2/BiVO4 nanocomposites show a decrease in PL intensity as compared to that of BiVO4. These results indicated that the recombination of charge carriers is strongly suppressed in SnS2/BiVO4, and it favoured the interfacial charge transfer in SnS2/BiVO4 because of heterojunction formation. These separated charge carriers move to the surface and react with the molecular oxygen and water to form reactive oxygen species (ROS) [44]. To verify the efficient charge transfer of SnS2, BiVO4 and 0.15SnS2/BiVO4 nanocomposites, the photocurrent, and EIS characterizations of SnS2, BiVO4, and 0.15SnS2/BiVO4 nanocomposite were performed and results are shown in Fig. 8b-c respectively. The SnS2 and BiVO4 exhibits a low photocurrent attributing to heavily charge recombination. However, the photocurrent performance of 0.15SnS2/BiVO4 NCs was higher than individual components SnS2 and BiVO4, which demonstrates that the 0.15SnS2/BiVO4 can separate electron-hole pairs more effectively. The charge carrier mobility of BiVO4, SnS2, and SnS2/BiVO4 nanocomposite was further evaluated by EIS Nyquist plot (Fig. 8c). The radius of the impedance arc for 0.15SnS2/BiVO4 was minimum as compared to that of BiVO4 and SnS2 (Fig. 8c). Smaller impedance arc of 0.15SnS2/BiVO4, attributed to high electron-hole separation or high interface charge transfer [45]. Therefore, the rapid charge separation and the transport of charge carriers in 0.15SnS2/BiVO4 result in enhanced photocatalytic performance [46, 47].
In this work, a Mott–Schottky experiment was performed to determine the positions of the ECB and EVB of SnS2 and BiVO4, and to analyze the charge transfer route. The Mott-Schottky curve can be obtained by using the equation (Section S3) and plotting 1/Cs2-E with potential corresponding to 1/Cs2 [48]. As shown in Fig. 8d-e, for SnS2 and BiVO4, the intercepts of the linear region and X-axis is − 1.11 V and + 0.48 V (vs. Ag/AgCl), respectively [49]. Therefore, the ECB of SnS2 and BiVO4 will be − 0.89 V and + 0.70 V vs. NHE, respectively. Meanwhile, the EVB of SnS2 and BiVO4 is + 1.31 V and + 3.11 V vs. NHE respectively.
As the ECB of SnS2 shows more negative potential than BiVO4, the electron from the ECB of SnS2 migrates to the EVB of BiVO4, according to the type-II heterojunction system. In this type of charge transfer mode, the electron present in the ECB of BiVO4 could not reduce O2 into a superoxide radical anion (O2¯•) because the ECB of BiVO4 has more positive potential than that of the superoxide radical anion (O2¯•, – 0.33 eV vs. N.H.E) [50]. Thus, the electron migration in type-II heterojunction is not favorable for producing the superoxide radical anion, which is the main reactive oxygen species responsible for the degradation of CIP, according to the radical trapping experiment (Scheme 1b). Based on the above characterization results, a possible Z-scheme mechanism for the degradation of antibiotics using SnS2/BiVO4 heterojunction photocatalyst was proposed, as shown in scheme-1a-b. Under sunlight irradiation, both SnS2 and BiVO4 absorb visible light photons to produce photogenerated e‾ and h+. In SnS2/BiVO4 heterojunction, the photogenerated e‾ in the ECB of BiVO4 (\({e}_{CB}^{-}\)) would migrate to EVB of SnS2 to recombine immediately with the photogenerated holes (\({h}_{VB}^{+})\), while the EVB holes of BiVO4 remain on it to oxidize CIP directly. In addition, the e‾ in the ECB of SnS2 could easily migrate to the O2 molecules adsorbed on the surface SnS2/BiVO4 heterojunctions to produce the strong oxidant i.e., superoxide radicals that oxidize CIP. The photogenerated charge carrier transfer process can be described as:
4 Conclusion
A Z-scheme SnS2/BiVO4 heterogeneous photocatalyst with excellent photocatalytic efficiency towards CIP degradation under natural sunlight was synthesized by in-situ hydrothermal method. The apparent rate constant for CIP photocatalytic degradation by 0.15SnS2/BiVO4 photocatalyst was about 4.83 times and 6.41 times higher than that of the BiVO4 photocatalyst. The enhanced photocatalytic performance is attributed to the improved visible light response and efficient charge separation due to the formation of a Z-scheme heterojunction. The excellent re-usability and chemical stability of SnS2/BiVO4 nanocomposite make it an effective photocatalyst for the removal of antibiotics from wastewater.
Data Availability
All the datasets used and/or analyzed in this study are available in the manuscript and supplementary information can be asked from the corresponding author upon request.
References
R. Singh, A.P. Singh, S. Kumar, B.S. Giri, K.H. Kim, J. Clean. Prod. 234, 1484 (2019)
J. Rivera-Utrilla, M. Sánchez-Polo, M.Ã. Ferro-García, G. Prados-Joya, R. Ocampo-Pérez, Chemosphere 93, 1268 (2013)
J. Wang, R. Zhuan, Sci. Total Environ. 701, 135023 (2020)
S. Sharma, A. Bhattacharya, Appl. Water Sci. 7, 1043 (2017)
S. Manzetti, R. Ghisi, Mar. Pollut Bull. 79, 7 (2014)
R. Zhang, J. Tang, J. Li, Z. Cheng, C. Chaemfa, D. Liu, Q. Zheng, M. Song, C. Luo, G. Zhang, Sci. Total Environ. 197, 450–451 (2013)
J. Fick, H. Söderström, R.H. Lindberg, C. Phan, M. Tysklind, D.G.J. Larsson, Environ. Toxicol. Chem. 28, 2522 (2009)
M.Z. Akbari, Y. Xu, Z. Lu, L. Peng, Environ. Adv. 5, 100111 (2021)
N.A. Khan, S. Ahmed, I.H. Farooqi, I. Ali, V. Vambol, F. Changani, M. Yousefi, S. Vambol, S.U. Khan, A.H. Khan, TrAC- Trends Anal. Chem (2020). https://doi.org/10.1016/j.trac.2020.115921
C. Adams, Y. Wang, K. Loftin, M. Meyer, J. Environ. Eng. 128, 253 (2002)
I. Michael, L. Rizzo, C.S. McArdell, C.M. Manaia, C. Merlin, T. Schwartz, C. Dagot, D. Fatta-Kassinos, Water Res. 47, 957 (2013)
Q.T. Dinh, E. Moreau-Guigon, P. Labadie, F. Alliot, M.J. Teil, M. Blanchard, M. Chevreuil, Chemosphere. 168, 483 (2017)
M.J.F. Calvete, G. Piccirillo, C.S. Vinagreiro, M.M. Pereira, Coord. Chem. Rev. 395, 63 (2019)
J.C. Durán-Álvarez, E. Avella, R.M. Ramírez-Zamora, R. Zanella, Catal. Today. 266, 175 (2016)
Y. Wang, K. Ding, R. Xu, D. Yu, W. Wang, P. Gao, B. Liu, J. Clean. Prod. (2020). https://doi.org/10.1016/j.jclepro.2019.119108
R. Yang, Z. Zhu, C. Hu, S. Zhong, L. Zhang, B. Liu, W. Wang, Chem. Eng. J. 390, 124522 (2020)
Y. Wang, D. Yu, W. Wang, P. Gao, S. Zhong, L. Zhang, Q. Zhao, B. Liu, Sep. Purif. Technol. 239, 116562 (2020)
N. Wetchakun, S. Chaiwichain, B. Inceesungvorn, K. Pingmuang, S. Phanichphant, A.I. Minett, J. Chen (2012). https://doi.org/10.1021/am300812n
C. Lai, M. Zhang, B. Li, D. Huang, G. Zeng, Chem. Eng. J. 358, 891 (2019)
M. Yan, Y. Wu, Y. Yan, X. Yan, F. Zhu, Y. Hua, W. Shi, ACS Sustain Chem. Eng 4(3), 757–766 (2016)
T. Soltani, A. Tayyebi, B. Lee, J. Environ. Manage. 232, 713 (2019)
Y. Deng, L. Tang, C. Feng, G. Zeng, J. Wang, Y. Zhou, Y. Liu, B. Peng, H. Feng, J. Hazard. Mater. 344, 758 (2018)
M. Ge, L. Liu, Z. Zhou, CrystEngComm 14(3), 1038–1044 (2012)
A. Raja, P. Rajasekaran, K. Selvakumar, M. Arunpandian, K. Kaviyarasu, S.A. Bahadur, M. Swaminathan, Sep. Purif. Technol. 233, 115996 (2020). https://doi.org/10.1016/j.seppur.2019.115996
W. Yin, W. Wang, L. Zhou, S. Sun, L. Zhang, J. Hazardous Mater. 173(1–3), 194–199 (2010)
Q. Tang, X. Luo, S. Yang, Y. Xu, Sep. Purif. Technol. 248, 117039 (2020)
H. Li, K. Yu, X. Lei, B. Guo, H. Fu, Z. Zhu, J. Phys. Chem. 119(39), 22681–22689 (2015)
X. Chen, J. Zhou, Y. Chen, Y. Zhou, L. Ding, H. Liang, X. Li, Process Safety Environ. Protect 145, 364–377 (2021)
H. Jiang, H. Endo, H. Natori, M. Nagai, K. Kobayashi, Mater. Res. Bulletin 44(3), 700–706 (2009)
M. Long, W. Cai, J. Cai, B. Zhou, X. Chai, Y. Wu, J. Phys. Chem. 110(41), 20211–20216 (2006)
Z. Qiang, X. Liu, F. Li, T. Li, M. Zhang, H. Singh, M. Huttula, W. Cao, Chem. Eng. J. 403, 126327 (2021)
J. Sun, C.H. Shen, J. Guo, H. Guo, Y.F. Yin, X.J. Xu, Z.H. Fei, Z.T. Liu, X.J. Wen, J. Colloid Interface Sci. 588, 19 (2021)
J. Mao, B. Hong, J. Wei, J. Xu, Y. Han, H. Jin, D. Jin, X. Peng, J. Li, Y. Yang, J. Gong, H. Ge, X. Wang, ChemistrySelect 4, 13716 (2019)
X. Luo, Z. Chen, S. Yang, Y. Xu, J. Colloid Interface Sci. 532, 456 (2018)
S. Chen, D. Huang, G. Zeng, W. Xue, L. Lei, P. Xu, Chem. Eng. J. 382, 122840 (2020)
S. Singla, S. Basu, P. Devi, J. Ind. Eng. Chem. 118, 119 (2023)
Y. Li, X. Wang, L. Gao, J. Mater. Sci. Mater. Electron. 30, 16015 (2019)
X. Gao, G. Huang, H. Gao, C. Pan, H. Wang, J. Yan, Y. Liu, H. Qiu, N. Ma, J. Gao, J. Alloys Compd. 674, 98 (2016)
G. Kumar, R.K. Dutta, J. Phys. Chem. Solids. 164, 110639 (2022)
J. Tauc, R. Grigorovici, A. Vancu, Phys. Status Solidi. 15, 627 (1966)
G.K.I. Mukherjee, M.D.B.P. Vellenki, Int. J. Environ. Sci. Technol. 20, 2903 (2022)
G. Kumar, V. Cilamkoti, R.K. Dutta, Colloids Surf. Physicochem Eng Asp. 639, 128368 (2022)
G. Kumar, J. Kumar, M. Bag, R. Kumar, Dutta, Sep. Purif. Technol. 292, 121040 (2022)
G. Kumar, R. Kumar, Environ. Sci. Pollut Res. 29, 57758 (2022)
K. Bisht, G. Kumar, R.K. Dutta, Ind. Eng. Chem. Res. 61(46), 16946–16961 (2022)
Z. Li, X. Meng, Z. Zhang, Catal. Today. 315, 67 (2018)
J. Yi, H. Mo, B. Zhang, J. Song, D. Liu, G. Zhuo, Sep. Purif. Technol. 211, 474 (2019)
F. Deng, Y. Luo, H. Li, B. Xia, X. Luo, S. Luo, D.D. Dionysiou, J. Hazard. Mater. 383, 121127 (2020)
Q. Zhou, W. Huang, C. Xu, X. Liu, K. Yang, D. Li, Y. Hou, D.D. Dionysiou, Chem. Eng. J. 420, 129582 (2021)
G. Kumar, R.K. Dutta, Process. Saf. Environ. Prot. 159, 862 (2022)
Acknowledgements
GK are thankful to the University Sophisticated Instrument Facility (USIF) AMU Aligarh for providing XRD, FESEM and HRTEM facility. The authors also thank Department of Interdisciplinary Nanotechnology Centre of Aligarh Muslim University (INDIA) for the instrumental facilities.
Funding
“The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.“
Author information
Authors and Affiliations
Contributions
GK contributed to conception and design experiments, data analysis and preparation of manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Ethical Approval
Not applicable.
consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumar, G. Sunlight Responsive 2D/2D SnS2/BiVO4 Nanocomposite for Photocatalytic Removal of Ciprofloxacin Antibiotic from Aqueous Medium. J Inorg Organomet Polym 33, 2710–2720 (2023). https://doi.org/10.1007/s10904-023-02711-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-023-02711-y