Abstract
Effect of Ga addition on structure, resistivity and magnetoresistance of Ba2Fe1−xGaxMoO6 (x = 0.0, 0.1, 0.25 and 0.3) double perovskite. The sol–gel procedure was used to synthesize Ba2Fe1−xGaxMoO6 double perovskite and consolidated at 1150 °C under Ar/H2 atmosphere. Single phase with cubic crystal structure confirmed for all the samples through X-ray diffraction studies. Variation of grain size behavior on the samples surface has been studied by scanning electron microscopy (SEM). The spectra from energy dispersive X-ray spectroscopy (EDS) showed that all the elements Ba, Fe, Ga, Mo and O are present in the samples and there are no impurities in the materials. Fourier transform infrared spectroscopy (FTIR) spectral analysis of samples produced three characteristic absorption bands of Mo–O and Fe–O vibrations in the wave number range 400–1000 cm−1 which confirmed the present materials are double perovskite phase. Magneto-resistance (MR %) of these samples reduce with the addition of Ga content at room temperature (RT). Whereas, MR% at 5 K exhibits opposite trend to the MR% at RT with the Ga-content. It is found that all samples show semiconductor behavior at 5 K and changes into metallic nature as temperature increases showing a semiconductor-metallic transition. This value is larger for composition x = 0.3 and smaller for parent compound.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Room temperature negative colossal magneto-resistance at the low magnetic field observed in polycrystalline ordered double type perovskite Sr2FeMoO6 oxides in 1998 [1]. Since then, these classes of materials greatly attracted researcher for the fundamental and technological importance [1,2,3,4,5]. Typically, the double perovskite oxides have the formula unit A2BB′O6, where A (Sr, Ba, Ca), B (Fe) and B′ (Mo, Re, W) are the metal ions of + 2, + 3 and + 5, respectively. These double perovskites possess the tetragonal or the cubic crystal structure with a space group symmetry of I4/mmm, or \(Fm\bar {3}m\) depends upon chemistry or doping, respectively [6,7,8]. It is reported that huge low field magnetoresistance (LFMR) at room temperature in double perovskite compound owing to the spin dependent scattering of electrons at the magnetic domain boundaries or the grain boundaries extend to inter grain tunneling effect [1, 9]. This inter grain tunneling arises due to insulating grain boundaries where the charge carrier electrons are spin-polarized [10, 11]. Double perovskite A2FeMoO6 have an ordered structure, which consists of alternating MoO6 and FeO6 octahedra along the axis of the crystal, while the voids in between the octahedral are occupied by ‘A’ cation [1, 12]. Double perovskite A2FeMoO6 exhibits metallic and ferrimagnetic properties below Curie temperature (Tc). The phenomena had been explained using the antiferromagnetic coupling between the itinerant down spin Mo+5(4d1) and the localized up spin Fe3+(3d5), due to this magnetic structure of double perovskite, saturation magnetization (Ms) of 4 µB per formula achieved theoretically in the ferrimagnetic state [13]. However, the observed value of Ms is lower than the reported value [1, 14]. The less value of Ms is due to ascribed to the mixed site (or lattice site) disorder between Mo and Fe ions [1, 15].
Replacement of Ba atom to Sr in (Sr1−xBax)2FeMoO6 compound results in enhanced saturation magnetization whereas the Curie temperature is reduced. The saturation magnetization and Curie temperature of the Sr2FeMoO6 and Ba2FeMoO6 compound are 3.45 and 3.70 µB/f.u., 420–450 and 320–340 K, respectively [2, 10,11,12,13, 16,17,18,19,20,21,22,23,24,25]. The MR% at 7 T and 330 K for the Ba2FeMoO6 compound is 15% [2] which is double than that of Sr2FeMoO6 compound [1]. Therefore, the Ba contained double perovskite exhibits improved compare magnetic properties to Sr2FeMoO6. Further doping of the + 3 ions at B or B′ site of the A2BB′O6 double perovskite may produce further enhanced magnetic and magnetoresistance properties of Ba2FeMoO6 compound. Lu et al. [26] reported that improved magnetoresistance of Sr2Fe1−xGaxMoO6 compound with Ga addition. It is interesting to investigate the effect of Ga ions replacement for the Fe in Ba2FeMoO6 compound on the transport and magnetoresistance properties. Therefore, we have investigated transport and magnetoresistance properties with the Ga concentration in Ba2Fe1−xGaxMoO6 (x = 0.0, 0.1, 0.25, and 0.3) compounds, which are synthesized by sol–gel method. Further these materials were aslo characterized by XRD, SEM, EDS and FTIR.
2 Experimental
Double perovskite of Ba2Fe1−xGaxMoO6 (x = 0.0, 0.1, 0.25 and 0.3) (BFGMO) samples were prepared by sol–gel procedure by talking stoichiometric ratio of Ba(NO3)2, Fe(NO3)39H2O, Ga(NO3)3 and H2MoO4 (Analytical Reagent grade from Aldrich). Details of sample (sol–gel) preparation were reported [27,28,29,30,31,32,33,34,35,36]. The powders of BFGMO samples were pelletized into dimensions 1 cm dia and about 2 mm thick using harden steel dies and a hydraulic press at 2 ton m−2. These pellets were sintered at 1200 °C for 6 h. To reduce Mo6+ to Mo5+, samples were subjected to heat-treated at 1150 °C for 3 h under partial hydrogen (10% H2 + 90% Ar). Phase purity, lattice parameter and crystal structure were evaluated using the X-ray diffraction (XRD) technique. XRD profiles of the samples were collected using Philips PW-1830 (40kV × 25 mA) with Cu-Kα radiation in step mode with a step width (2θ) of 0.02° and time of 0.5 s in the range between of 20° and 80°. The surface morphology and micro structural studies of these double perovskites were analyzed using the SEM with (Model No. Joel JSM 5600) combined micro analyzer. The elemental analysis of the BFGMO was determined by EDS (Model: OXFORD). These materials were analyzed using FTIR by Bruker Tensor 27 DTGS TEC detector spectrophotometer in the wave number range 400–1000 cm−1 by the KBr pellet method.
Electrical resistivity measurements were carried out by the standard four-probe method in the temperature range 5–300 K at the constant magnetic field of 0 and 5 T using laboratory-made resistivity meter that inserts along with OXFORD superconducting magnet system. Electrical resistivity data were also collected at constant temperature 5 and 300 K by varying the magnetic field from 0 to 8 T. MR (%) of the samples was calculated using this resistivity data as a function of magnetic field as well as the temperature.
3 Results and discussion
3.1 Characterization
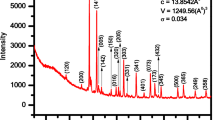
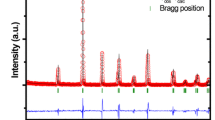
Figure 1 shows the XRD profiles of BFGMO samples at temperature 300 K. The X-ray pattern of all the samples reveals the single phase double perovskite structure of Ba2FeMoO6 with cubic crystal structure having space group of \(Fm\bar {3}m\) [7, 9, 13, 22, 33, 34, 37, 38]. The lattice parameter ‘a’ and volume of unit cell ‘V’ of individual samples in BFGMO series were evaluated using Bragg’s angle with the corresponding (hkl) which are in Table 1. Lattice parameter decreases with Ga concertation, inset to Fig. 1. It is evident that the considerable reduction of lattice parameter and unit cell volume arises due to the replacement of the smaller Ga3+ (0.62 Å) ions in the Fe3+ (0.645 Å) ionic sites [39, 40], which obeys the Vegard’s law [41]. JCPDS card no. 01-083-3584 was used to index XRD profile of the Ba2Fe1−xGaxMoO6 double perovskite. Estimated crystallite (diffracted domain) size from the XRD profiles for all the samples using peak broadening (Williamson-Hall) method are above 150–280 nm due to very small value of full width half maxima (FWHM) of 0.112°–0.0876°, which are very close to instrumental broadening of 0.058° for the 2θ of 31.34°.
The tolerance factor (t) is a semi-quantitative estimation of double perovskite to know the closeness of cubic structure and its stability. The tolerance factor (t) adopted to A2B(1−x)B″xB′O6 double perovskite [26, 31, 34, 38, 42] given by
where rA, rB, rB″, rB′ and rO are ionic radii of the respective ions [43]. They calculated tolerance factor of Sr2Fe1−xGaxMoO6 using Eq. (1) and symmetry of lattice was described. In this investigation, the value tolerance factor (t) for Ba2Fe1−xGaxMoO6 samples were evaluated by using Eq. (1) and the values are given in Table 1. It is observed that the tolerance factor (t) rises towards the unity with increase of Ga content in BFGMO samples owing to lowering of distortion from double perovskite structure [26, 44]. Thus symmetric nature of the present double perovskite compounds increases due to the substitution of Ga at Fe-site in Ba2FeMoO6.
The FE-SEM secondary electron images of BFGMO samples observed from freshly cleaved surfaces are shown in Fig. 2. Cleaved surfaces of all these samples exhibits well grown faceted grains with size of above 1 μm. Energy dispersive spectra’s (EDS) of BFGMO samples are shown in Fig. 3. The results from EDS analysis showed that the elements Ba, Fe, Ga, Mo and O are present and no other impurities exists in the present samples. Measured chemical compositions of BFGMO samples are given Table 2.
Figure 4 shows FTIR spectra of the BFGMO samples in the spectral wave number range 1000–400 cm−1 at room temperature. The FTIR spectra of the perovskite structure have three characteristic absorption bands between 850 and 400 cm−1, respective to composition and these are usually used to identify the perovskite phase formation [32, 35, 36, 45]. From the FTIR spectra of the BFGMO samples investigated in the present study, three bands for Fe and Mo are detected. These bands are, one strong band in the high wave number range (~ 824 cm−1) associated to the Mo–O symmetric stretching mode of MoO6-octahedra, another band at ~ 654 cm−1 assigned to the anti-symmetric stretching mode of the MoO6-octahedra, due to the higher charge of this cation [32, 35, 36, 46]. In BFGMO double perovskite, the highly charge Mo5+ cation octahedra, the MoO6, act as independent groups, the vibration spectrum therefore arises from such MoO6-octahedra. Mo–O symmetric stretching mode of MoO6-octahedra at about 824 cm−1 is usually an infrared inactive vibration, but in double perovskite, both Fe and Mo ions exist in Fe and Mo sites, it becomes partially allowed due to lowering site symmetry [32, 35, 36, 46]. One more weak absorption band at about 498 cm−1 is ascribed to Fe–O vibration absorption of FeO6-octahedra. For all the samples, these bands confirm the formation of perovskite phase.
3.2 Electrical resistivity
Temperature dependent electrical resistivity (ρ) at constant magnetic fields of 0 and 5 T are shown in Fig. 5a–d. The resistivity of all compositions at low temperature decreases with the increase of temperature, and a further increase of temperature at a certain point, called transition temperature, resistivity starts increasing, which infers that all samples at low temperature behave like semiconductor and after transition temperature behaves like metal. Since all the BFGMO samples behaved like as semiconductor and metallic at lower and higher temperature regimes, respectively [26, 27, 30, 38, 39, 47,48,49,50] a semiconductor to metallic transition (TSM) occurs in this BFGMO series. The TSM’s are obtained from Fig. 5a–d which are given in Table 3. It is known from Table 3 that TSM is high for composition x = 0.3 and low for composition x = 0.0 since resistivity is greater and smaller, respectively in BFGMO samples. The TSM decreases with the increase of magnetic field in BFGMO samples, which might be due to a decrease in resistivity with a magnetic field. It is shown from Fig. 5a–d that with change in Ga-content in BFGMO, there is no systematic variation of resistivity which may be due to disorder in the grain boundaries which vary randomly in the samples [33, 49, 51]. Higher resistivity values of Ga-containing samples than parent compound Ba2FeMoO6 may be due to larger grain boundaries [9]. It may also be expected that Ga3+ acts as a barrier to electron transport, reducing the number of pathways for electron percolation [30, 47, 49, 52].
The magnetic field dependence of electrical resistivity (ρ) of BFGMO samples in the magnetic field range 0–8 T keeping temperatures constant at 5 and 300 K are shown in Figs. 6a–d and 7a–d respectively. It is seen from Figures that the resistivity of BFGMO samples decreases with increase of magnetic field at temperatures 5 and 300 K. The decrease in resistivity may be due to increase of magnetic ordering with magnetic field which in turn reduces electron-magnon scattering [30, 53].
3.3 Magnetoresistance
Magnetoresistance (MR%) of the material is given by
where ρ(0,T) and ρ(H,T) are the resistivities at zero magnetic field and H magnetic field at temperature T respectively.
Figure 8 shows variation of MR (%) with temperature at 5 T (Tesla) for all BFGMO samples. It is observed that the variation of MR (%) increases with the decrease of temperature at the constant field of 5 T for BFGMO samples. It is well known from the literature that the grain boundary effect dominates the MR (%) as temperature decreases below Curie temperature [1, 38, 52, 54, 55]. Since grain boundary effects the MR (%) of BFGMO sample, goes to higher value as spin polarization increases with the decrease of temperature [1, 30, 56]. The magnetic field dependence MR (%) at constant temperatures of 5 and 300 K are shown in Fig. 9a, b for BFGMO samples. It is understood from Fig. 9a, b that the magnitude of MR (%) raises with an increase of magnetic field at constant temperatures of 5 and 300 K, is owing to the lowering of spin scattering at grain boundaries in the presence of magnetic field. This enhancement of MR (%) with magnetic field occurs below Curie temperature of the samples, where the polarization of carriers is large [16, 38, 52, 53, 55,56,57].
The values of MR (%) at 1 T, and 5 and 300 K, are obtained from Fig. 9a, b and given in Table 3. It is noticed from Table 3 that the values of MR (%) enhanced by varying the Ga in BFGMO at 5 K. The increase in MR (%) with Ga in BFGMO sample is due to the weakening of the double exchange barrier after Ga doping in Ba2FeMoO6 samples [26, 33, 38, 55, 58]. In such a case, for electron transport along the chain in the ferromagnetic segregation Ga ions may act as a barrier and weaken the ferromagnetic exchange.
It is found from Fig. 9a that low-field magneto-resistance has occurred for BFGMO samples. The value of LFMR (%) for composition x = 0.3 is largest, i.e., 10.62% at 1 T and 5 K compared to other samples in BFGMO series. In the double perovskite systems, magneto-resistance response originates from tunneling of spin-polarized charge carriers through insulating barriers. These barriers may be Fe–Mo disorder defects, grain boundaries (giving inter-granular magneto-resistance), and some domain boundaries (giving intra-granular magneto-resistance) [4, 16, 38, 55]. Spin polarization of charge carriers plays an important role in both of these tunneling magneto-resistance. The high magnetic softness of the material is also important as it increases the magnetic field response of magneto-resistance. The similar LFMR results had been reported by Liu at al [26] at 5 K and magnetic field range 0–2 T in Sr2Fe1−xGaxMoO6 samples. The spin polarization in the double perovskite systems is very sensitive to temperature and to anti-site defects (ASDs). It is observed from Table 3 that the MR (%) decreases with the addition of Ga in Ba2FeMoO6 at temperature 300 K. It is ascribed to an increase in anti-site defects or a decrease of spin polarization of charge carriers [16, 30, 33, 38, 47, 49, 55, 56]. The MR (%) of the samples depends on various parameters like as temperature, magnetic field and grain boundary effect (grain size). (MR%) of the material is defined as \({\text{MR}}(\% )=\left\{ {{{\left[ {\rho \left( {{\text{H}},{\text{T}}} \right) - \rho \left( {0,{\text{T}}} \right)} \right]} \mathord{\left/ {\vphantom {{\left[ {\rho \left( {{\text{H}},{\text{T}}} \right) - \rho \left( {0,{\text{T}}} \right)} \right]} {\rho \left( {0,{\text{T}}} \right)}}} \right. \kern-0pt} {\rho \left( {0,{\text{T}}} \right)}}} \right\} \times 100\), where ρ(0,T) and ρ(H,T) are the resistivities at zero magnetic field and H magnetic field at temperature T respectively. The value change of resistivity with magnetic field and without magnetic field reduces at room temperature may be attributed to increase of grain boundaries (decrease of grain size) by addition of Ga content. Since MR (%) decreases with Ga content at room temperature due to increase of grain boundaries are one of the reason.
4 Conclusions
The authors studied the structure, charcterization, resistivity and magnetoresistance of the Ba2Fe1−xGaxMoO6 (0 ≤ x ≤ 0.3) double perovskite materials. The value of lattice parameter decreases with increase in Ga content in BFGMO. FTIR spectra of BFGMO samples showed three characteristic absorption bands between 860–400 cm−1 indicating formations of double perovskite structure. It was found from results at room temperature that the value of MR (%) decreased by doping of Ga in the BFGMO samples. The enhancement of MR (%) in the magnetic field range 0–8 T occurred by addition of Ga-content at temperature of 5 K in the BFGMO series. The largest value of LFMR (%), i.e. 10.62%, obtained at 1 T and 5 K for composition x = 0.3 in BFGMO series. The value of MR (%) of BFGMO samples increases with increases of composition at low temperature (5 K) while these values decreases with composition at room temperature (300 K). Novelty of the present work is that the opposite trend is followed in variation of MR (%) with composition at temperature 5 and 300 K in the BFGMO samples.
References
K.I. Kobayashi, T. Kimura, H. Sawada, K. Terakura, Y. Tokura, Nature 395, 677 (1999)
A. Maignan, B. Raveau, C. Martin, M. Hervieu, J. Solid State Chem. 144, 224 (1999)
H.Y. Hwang, S.W. Cheong, N.P. Ong, B. Batlog, Phys. Rev. Lett. 77, 2041 (1996)
D.D. Sarma, S. Ray, K. Tanaka, M. Kobayashi, A. Fujimori, P. Sanyal, H.R. Krishnamurthy, C. Dasgupta, Phys. Rev. Lett. 98, 157205 (2007)
H.F. Zhao, L.P. Cao, Y.J. Song, S.M. Feng, X. Shen, X.D. Ni, Y. Yao, Y.G. Wang, Solid State Commun. 204, 1–4 (2015)
D.D. Sarma, Curr. Opin. Solid State Mater. Sci. 5, 261 (2001)
S.B. Kim, B.W. Lee, S.R. Yoon, C.S. Kim, J. Magn. Magn. Mater. 245–255, 580–582 (2003)
Q. Zhang, Z.F. Xu, J. Liang, J. Pei, H.B. Sun, J. Magn. Magn. Mater. 354, 231–234 (2014)
V. Pandey, V. Verma, R.P. Aloysius, G.L. Bhalla, V.P.S. Awana, H. Kishan, R.K. Kotnala, J. Magn. Magn. Mater. 321, 2239–2244 (2009)
H. Han, B.J. Han, J.S. Park, B.W. Lee, S.J. Kim, C.S. Kim, J. Appl. Phys. 89, 7687 (2001)
A.S. Ogale, S.B. Ogale, R. Ramesh, T. Venkatesan, Appl. Phys.Lett. 75, 537 (1999)
T. Goko, Y. Endo, E. Morimoto, J. Arai, T. Matsumoto, Phys. B 333–329, 837–839 (2003)
H. Han, C.S. Kim, B.W. Lee, J. Magn. Magn. Mater. 254–255, 574–576 (2003)
W.Y. Lee, H. Han, S.B. Kim, C.S. Kim, B.W. Lee, J. Magn. Magn. Mater. 254–255, 577–579 (2003)
A.G. Floresa, M. Zazo, J. Íῆiguez, V. Raposo, C. de Francisco, J.M. Muῆoz, W.J. Padilla, J. Magn. Magn. Mater. 254–255, 583–585 (2003)
M. García-Hernández, J.L. Martínez, M.J. Martínez-Lope, M.T. Casais, J.A. Alonso, Phys, Rev.Lett 86, 2443 (2001)
F.K. Patterson, C.W. Moeller, R. Ward, Inorg, Chem 29, 196 (1963)
M. Itoh, I. Ohta, Y. Inaguma, Mater. Sci. Eng. B 41, 55 (1996)
E.K. Hemery, G.V.M. Williams, H.J. Trodahl, Curr. Appl. Phys. 6, 312–315 (2006)
H.M. Yang, H. Han, B.W. Lee, J. Magn. Magn. Mater. 272–276, 1831–1833 (2004)
Q. Zhang, G.H. Rao, H.Z. Dong, Y.G. Xiao, X.M. Feng, G.Y. Liu, Y. Zhang, J.K. Liang, Phys. B 370, 228–235 (2005)
V. Pandey, V. Verma, R.P. Aloysius, G.L. Bhalla, R.K. Kotnala, Solid State Commun. 149, 869–873 (2009)
X.M. Feng, G.H. Rao, G.Y. Liu, W.F. Liu, Z.W. Ouyang, J.K. Liang, J. Phys. Condens. Matter 16, 1813–1821 (2004)
J. Kim, J.G. Sung, H.M. Yang, B.W. Lee, J. Magn. Magn. Mater. 290–291, 1009–1011 (2005)
K. Ramesh, A.S. Prakash, Mater. Sci. Eng., B 164, 124–130 (2009)
M.F. Lu, J.P. Wang, J.F. Liu, W. Song, X.F. Hao, De F. Zhou, X.J. Liu, Z.J. Wu, J. Meng. J. Alloys Compd. 428, 214–219 (2007)
Y. Markandeya, D. Saritha, M. Vithal, A.K. Singh, G. Bhikshamaiah, J. Alloys Compd. 509, 5195–5199 (2011)
Y. Markandeya, K. Suresh, G. Bhikshamaiah, J. Alloys Compd. 509, 9598–9603 (2011)
S. Ferau, P. Samoila, A.I. Borhan, M. Ignat, A.R. Iordan, M.N. Palamaru, Mater. Charact. 84, 112–119 (2013)
Y. Markandeya, Y. Suresh Reddy, S. Bale, G. Bhikshamaiah, J. Mater. Sci.: Mater. Electron. 29, 6711–6719 (2018)
Y. Suresh Reddy, Y. Markandeya, B. Appa Rao, G. Bhikshamaiah, J. Mater. Sci.: Mater. Electron. 29, 2966–2973 (2018)
Y. Markandeya, Y. Suresh Reddy, S. Bale, C. Vishnuvardhan Reddy, G. Bhikshamaiah, J. Bull. Mater Sci 38, 1603–1608 (2015)
Y. Markandeya, Y. Suresh Reddy, A. Manjula Devi, K. Suresh, G. Bhikshamaiah, IOP Conf. Ser.: J. Mater. Sci. Eng. 73, 012097 (2015)
G. Rajender, Y. Markandeya, A.K. Singh, G. Bhikshamaiah, IOP Conf. Ser.: J. Mater. Sci. Eng. 330, 012007 (2018)
S. Varaprasad. K. Thyagarajan. Y. Markandeya, K. Suresh, G. Bhikshamaiah J. Mater. Sci.: Mater. Electron. (2018). https://doi.org/10.1007/s10854-018-9488-z
S. Varaprasad, K. Thyagarajan, Y. Markandeya, K. Suresh, G. Bhikshamaiah J. Supercond. Nov. Magn. (2018). https://doi.org/10.1007/s10948-018-4738-0
H.-E.M.M. Saad, N. Rammeh, Phys. B 481, 217–223 (2016)
S. Vasala, M. Karppinen, J. Prog. Solid State Chem. 43, 1–36 (2015)
J.H. Kim, G.Y. Ahn, S.-I. Park, C.S. Kim. J. Magn. Magn. Mater. 282, 295–298 (2004)
X.M. Feng, G.H. Rao, G.Y. Liu, H.F. Yang, W.F. Liu, Z.W. Ouyang, J.K. Liang, Phy. B 344, 21–26 (2004)
L. Vegard, Die Konstitution der Mischkristalle und die Raumfüllung der Atome. Zeitschrift für Physik 5, 17–26 (1921)
T. Tiittannen, M. Karppinen, J. Solid State Chem. 246, 245–251 (2017)
R.D. Shannon, C.T. Prwitt, Acta Cryst. B 25, 925–946 (1969)
D.A.L. Tellez, D.P. Llamosa, C.E.D. Toro, A.V.G. Rebaza, J. Roa-Rojas, J. Mol. Struct. 1034, 233–237 (2013)
M.F. Mostafa, S.S. Ata-Allah, A.A.A. Youssef, H.S. Refai, J. Magn. Magn. Mater 320, 344 (2008)
A.E. Lavat, E.J. Baran, Vib. Spectrosc. 32(2), 167 (2003)
L. Harnagea, P. Berthet, J. Solid State Chem. 222, 112–115 (2015)
B. Ufacial, C. Adriano, R. Lora-Serrano, J.G.S. Duque, L. Mendovca-Ferreira, C. Rojas-Ayala, E. Baggio-Saitovitch, E.M. Bittar, P.G. Pagliuso, J. Solid State Chem. 212, 23–29 (2014)
Y. Lan, X. Feng, X. Zhang, Y. Shen, D. Wang, Phys. Lett. A 380, 2962–2967 (2016)
M. Khlifi, M. Wali, E. Dhahri, J. Phys. B 449, 36–41 (2014)
E.K. Hemery, G.V.M. Williams, H.J. Throdahl, Phys. B 394, 74–80 (2007)
F. Sher, A. Venimadhav, M.G. Blamire, B. Dabrowski, S. Kolesnik, J.P. Attfield, Solid State Sci. 7, 912–919 (2005)
A. Dhahri, M. Jemmali, E. Dhahri, E.K. Hlil, J. Dalton Trans. 44, 620 (2015)
Y.-Q. Zhai, J. Qiao, Z. Zhang, E-J. Chem. 8(S1), S189–S194 (2011)
D. Serrate, J.M. De Teresa, P.A. Algarabel, J. Galibert, C. Ritter, J. Blasco, M.R. Ibarra, Phys. Rev. B 75, 165109 (2007)
A. Gaur, G.D. Varma, H.K. Singh, J. Alloy. Compd. 460, 581–584 (2008)
F. Sher, A. Venimadhav, M.G. Blamire, K. Kamenev, J.P. Attifield, Chem. Mater. 17, 176 (2005)
Y. Sui, X.J. Wang, Z.N. Qian, J.G. Cheng, Z.G. Liu, J.P. Miao, Y. Li, W.H. Su, C.K. Ong, Appl. Phys. Lett. 85, 269 (2004)
Acknowledgements
The authors thank Dr. Rajeev Rawat of UGC-DAE, Consortium for Scientific Research, Indore for providing magnetoresistance facilities. The authors acknowledge Department of Science and Technology, New Delhi, India for providing financial assistance to carry out this work under the research project (File No.SB/S2/CMP-004/2013). The authors thank to Dr. N. Venkateshwarlu, Asst. Professor, University College of Law, Prof. S. Ramchandram, Vice Chancellor, Osmania University, Head, Department of Physics, University College of Science, Principal, Nizam College, Osmania University, Hyderabad, India for his encouragement and providing necessary facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Markandeya, Y., Suresh Reddy, Y., Varaprasad, S. et al. Synthesis, characterization, resistivity and magnetoresistance of Ba2Fe1−xGaxMoO6 double perovskite. J Mater Sci: Mater Electron 29, 16639–16646 (2018). https://doi.org/10.1007/s10854-018-9756-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-018-9756-y