Abstract
Characterization, Fe/Mo ordering and magnetic properties of Ca2FexMo2−xO6 (x = 0.9, 1.0, 1.1 and 1.2) (CFMO) double perovskite have been studied. Sonochemical methodology is adopted to synthesize Ca2FexMo2−xO6 double perovskite at low temperature (950 ∘ C) under Ar/H2 atmosphere. Single-phase monoclinic structure by space group P21/n, lattice parameters (a, b and c) and unit cell volume (V ) have been confirmed from X-ray diffraction (XRD) patterns. Average grain sizes of 145 nm with a variation of porosity were observed from scanning electron microscopy (SEM) pictures. Elemental analysis using energy-dispersive X-ray spectroscopy (EDS) confirmed the presence of elements Ca, Fe, Mo and O with no trace of foreign elements, and the compound is found to be pure. Fourier transform infrared spectroscopy (FTIR) data confirmed perovskite formation by identifying three characteristic absorption bands in the wave number range 400–1000 cm− 1 for Mo-O and Fe-O vibrations. Magnetic properties like Ms, Mr and Hc were studied for CFMO series using vibrating sample magnetometer (VSM). Saturation magnetization is found to vary with Fe composition (x).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Manganese perovskites based on LaMnO3 materials exhibit intrinsic colossal magnetoresistance (CMR) [1, 2], which undergo a huge change in electrical resistance for applied magnetic field. This effect plays a crucial role in fundamental and technological applications such as read/write heads, magnetic sensors, magnetic random access memory devices and spintronic devices [3,4,5]. As a substitute to perovskite manganites, a family of half-metallic ferromagnets, A\(_{\mathrm {2}}\textit {BB}\prime \textit {O}_{\mathrm {6}}\) (A = alkali earth and B, B\(\prime \quad =\) transition metals) has been identified [6,7,8,9,10]. A low-field CMR at room temperature was exhibited by Sr2FeMoO6 double perovskite oxides; in SFMO, each Fe and Mo atom is occupied alternately, in such a way that each FeO6 octahedron is corner-linked to six MoO6 octahedra, and vice versa [6]. When Fe replaces Mo, or vice versa, a B site disorder or antisite (AS) disorder appears, depending on Fe/Mo ordering structural transition that took place for SFMO [11,12,13,14,15,16]. Studies have been done on structural, magnetic and magnetoresistance properties of A2FeMoO6, where A = Sr, Ba and Ca [17,18,19]. Sr2FeMoO6, Ba2FeMoO6 and Ca2FeMoO6 compounds were found to be promising with great technological applications, but among them, Ca2FeMoO6 has exhibited an increase of magnetoresistance from 16.7 to 44.2% between 4 and 300 K at 7 T, which is far better than SFMO [20]. This makes CFMO alternative and attractive for CMR applications [21,22,23]. CFMO compound has been characterized by a solid-state method and a sol-gel method. A number of studies have been done on perovskites of type ABO3 using sonochemical methodology [24,25,26,27,28,29]. The sonochemical methodology has not been adopted for CFMO till date. Similarly, Fe/Mo ordering studies of CFMO have not been reported.
In view of this, in the present work, we have investigated Fe/Mo ordering of CFMO compound using the sonochemical method. All prepared samples were tested for confirmation on structure, grain size, purity, perovskite formation and magnetic properties using characterization techniques XRD, scanning electron microscopy (SEM), energy-dispersive X-ray spectroscopy (EDS), Fourier transform infrared spectroscopy (FTIR) and VSM, respectively.
2 Experimental
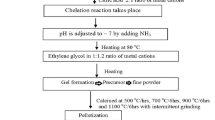
Polycrystalline samples of Ca2FexMo2−xO6 (x = 0.9, 1.0, 1.1 and 1.2) were synthesized by the sonochemical method. Exact proportional amounts of analytical grade chemicals such as Ca(NO3)3, Fe(NO3)2⋅9H2O and H2MoO4 were added in a beaker of 200 ml double-distilled water and stirred with a magnetic stirrer at room temperature (RT) until a clear solution is formed, and to this citric acid added in 2:1 mole ratio (citric acid to metal ion), the solution is again stirred for 10 min to obtain a clear solution. A highly intense sonication of frequency 20 kHz was carried out with a horn made up of titanium at a temperature of 353 K (Sonics Vibra-Cell, USA) for about 20 min. After sonication, ammonia is added to the solution to adjust pH to \(\sim \)?7. The solution is kept on a hot plate of a magnetic stirrer at 80 ∘ C, and ethylene glycol is added in 1:1.2 ratio (metal ion to ethylene glycol). The resulting solution is kept in a hot air oven at 230 ∘ C for 24 h, and the residue formed was collected and dried on a Bunsen burner at 250 ∘ C for about 5 min to obtain a required composition and was further ground to get homogenous powder.
The resulting powders were taken into crucibles and made to undergo heat treatment at 400, 600 and 800 ∘ C/6 h with intermittent grounding. The obtained powders were pressed into pellets of 1 cm diameter and 2 mm thickness using a die by applying 2 tons/m2. The resulting pellets were sintered at 950 ∘ C for 6 h. For reducing the Mo6+ to Mo5+ or losing of oxygen, the pellets were heated at 950 ∘ C in a stream mixture of 10% H2/Ar gas for about 6 h.
The final materials are subjected to X-ray diffraction (Philips PW 1830) studies to confirm the structure. SEM (Zeiss INCA) studies are done to confirm the grain size, and EDS (Oxford INCA EDX) is carried out on all samples to check purity and elements present. FTIR (PerkinElmer spectrum 2) spectra are used to analyse all samples to confirm perovskite formation. Magnetic measurements were done on the CFMO compound using VSM (EZ9; Microsense, Inc., USA) at applied magnetic field from − 20 to + 20 kOe.
3 Results and Discussion
3.1 Crystal Structures
The purity of phases of all CFMO samples is shown in Fig. 1. Compound was found to crystallize in monoclinic structure with space group P21/n as per X-ray diffraction patterns which are in excellent accord with the reported literature [30,31,32,33,34,35]. Lattice parameters a, b and c with unit cell volume (V ) were evaluated from indexed (hkl) values (110), (112), (220), (222), (204), (224) and (332) and are tabulated in Table 1. It is observed from Fig. 2 with an increase in Fe composition (x), lattice parameters and a decrease in unit cell volume, reasons for deviation of lattice parameter are valence disproportion and difference in radii of Fe3+ and Mo5+ [36, 37] of CFMO compound, which is in accordance with Vegard’s law [38].
3.2 SEM
Sintered pellets Ca2FexMo2−xO6 (x = 0.9, 1.0, 1.1 and 1.2) were characterized to find grain size parameter using SEM, and resulting micrographs are shown in Fig. 3a–d. It is found that grain boundaries are clearly seen, and average grain size was around 145 nm and varying with Fe composition (x).
3.3 EDS
Elemental data analyses for all samples of CFMO compound were carried out to check the elements present and the purity of the compound. Figure 4a–d shows the EDS spectra of Ca2FexMo2−xO6 (x = 0.9, 1.0, 1.1 and 1.2). From EDS spectra, it is confirmed that all required or raw preparation composition elements (Ca, Fe, Mo and O) were present and no other elements (foreign/impurities) are existing.
3.4 FTIR
Figure 5 shows FTIR spectra of the CFMO samples in the spectral wave number range 1000–400 cm− 1 at room temperature. To confirm the perovskite phase formation, the FTIR spectra should have three characteristic absorption bands between 850 and 400 cm− 1 with respect to composition [39]. From Fig. 5, FTIR spectra of the CFMO samples under investigation detected bands for Fe and Mo, i.e. one strong band in the high wavenumber range (\(\sim \)873.76 cm− 1) associated to the Mo-O symmetric stretching mode of MoO6-octahedra and another band at \(\sim \) 679.40 cm− 1 assigned to the antisymmetric stretching mode of the MoO6 octahedra, due to the higher charge of this cation [40]. One weak absorption band at about 479.31 cm− 1 is ascribed to Fe-O vibration absorption of FeO6 octahedra.
3.5 Atomic Ordering
In a unit cell, a degree of ordering is a study of favourable occupation of different atoms in respective sites [14]. For a solid solution Mo2−xFex, the order of phase of is MoFe. The degree of ordering (η) can be calculated as follows: η = \(P_{\text {Mo}}^{\mathrm {(1)}}\) − \(P_{\text {Mo}}^{\mathrm {(2)}}\) = \(P_{\text {Fe}}^{\mathrm {(2)}}\) − \(P_{\text {Fe}}^{\mathrm {(1)}}\), where \(P_{\text {Mo}}^{\mathrm {(1)}}\), \(P_{\text {Mo}}^{\mathrm {(2)}}\), \(P_{\text {Fe}}^{\mathrm {(1)}}\) and \(P_{\text {Fe}}^{\mathrm {(2)}}\) are relative occupancies of Mo and Fe atoms on the Mo site and Fe site, respectively; i.e. \(P_{\text {Mo}}^{\mathrm {(1)}}\) + \(P_{\text {Fe}}^{\mathrm {(1)}}\) = 1 and \(P_{\text {Mo}}^{\mathrm {(2)}}\) + \(P_{\text {Fe}}^{\mathrm {(2)}}\) = 1. Therefore, when complete disorder occurs, \(P_{\text {Mo}}^{\mathrm {(1)}}\) = \(P_{\text {Mo}}^{\mathrm {(2)}}\) = 1 − x / 2, \(P_{\text {Fe}}^{\mathrm {(1)}}\) = \(P_{\text {Fe}}^{\mathrm {(2)}}\) = x / 2 and η = 0. When the maximum ordering is achieved, \(P_{\text {Mo}}^{\mathrm {(1)}}\) = 1, \(P_{\text {Mo}}^{\mathrm {(2)}}\) = 1 − x, \(P_{\text {Fe}}^{\mathrm {(1)}}\) = 0, \(P_{\text {Fe}}^{\mathrm {(2)}}\) = x and \(\eta _{\max }\) = x for Mo-rich compounds (x ≤ 1), and \(P_{\text {Fe}}^{\mathrm {(2)}}\) = 1, \(P_{\text {Fe}}^{\mathrm {(1)}}\) = x − 1, \(P_{\text {Mo}}^{\mathrm {(2)}}\) = 0, \(P_{\text {Mo}}^{\mathrm {(1)}}\) = 2 − x and \(\eta _{\max }\) = 2 − x for Fe-rich compounds (x ≥ 1).
In the present case, A is Ca, B is Fe and B\(\prime \) is Mo in double perovskite A\(_{\mathrm {2}}\textit {B}_{x}\textit {B}\prime _{2-x}\textit {O}_{\mathrm {6}}\), and Fe and Mo alternatively occupy the B and B\(\prime \) sites, respectively. For B-rich compounds (x ≥ 1.0), the maximum degree (\(\eta _{\max })\) is 2 – x, and for B\(\prime \)-rich compounds (x ≤ 1.0), the maximum degree is x in Ca2FexMo2−xO6 (i.e. A\(_{\mathrm {2}}\textit {B}_{x}\textit {B}\prime _{2-x}\textit {O}_{\mathrm {6}})\) compounds. The values are tabulated in Table 2. A plot showing the maximum degree (\(\eta _{\max })\) as a function of composition is shown in Fig. 6. It is found from the figure that the degree of the B/B\(\prime \) site ordering is maximum at x = 1.0 and deviates (decreases at x = 0.9, 1.1 and 1.2) from there on in the Ca2FexMo2−xO6 (x = 0.9, 1.0, 1.1 and 1.2) compound.
3.6 Magnetization Measurements
Figure 7 shows a magnetization versus applied magnetic field graph of Ca2FexMo2−xO6 samples (x = 0.9, 1.0, 1.1 and 1.2) recorded at room temperature. Magnetic properties like saturation magnetization (Ms), remanent magnetization (Mr) and coercivity (Hc) for CFMO series have been obtained from Fig. 7 and are tabulated in Table 3. The variations of saturation magnetization (Ms) with Fe composition (x) of CFMO compound are plotted in Fig. 8.
It is evident from Fig. 8 that saturation magnetization was found to be maximum at x = 1.0 compared to other compositions. The values of saturation magnetization were found to be 18.25, 29.20, 29.02 and 24.03 emu/g for x = 0.9, 1.0, 1.1 and 1.2, respectively. This type of trend for saturation magnetization with Fe composition (x) was correlated with a degree of Fe/Mo ordering for Sr2FexMo2−xO6 and had been reported [41, 42]. Mis-site (Fe-Mo) imperfection, oxygen deficiency and valence disproportion were quoted as the reasons [43]. From Fig. 9, it is confirmed that a correlation exists between the degree of ordering and the magnetic properties of CFMO compound.
4 Conclusions
It is reported here that the Ca2FexMo2−xO6 (x = 0.9, 1.0, 1.1 and 1.2) compound prepared by the sonochemical method is in a single phase with a monoclinic structure and lattice parameters were found to vary with Fe composition (x). The average grain size (145 nm) of the compound was confirmed from SEM pictures. Purity and elements present in the final compound were investigated using the EDS technique. Perovskite structure formation is confirmed for FTIR spectra. Magnetic properties of all samples in the compound were investigated using the VSM technique, and from M–H loop, Ms, Mr and Hc were evaluated. Saturation magnetization was clearly varying with Fe composition (x), and which is in correlation with the degree of Fe/Mo ordering.
References
Ramirez, A.P.: J. Phys.: Condens. Matter 9, 8171 (1997)
Rao, C.N.R., Raveau, B.: Colossal Magnetoresistance and Other Related Properties In 3d Oxides. World Scientific, Singapore (1998)
Retuerto, M., Martinex-Lope, M.J., Garcia-Hernandez, M., Alonso, J.A.: J. Phys.: Condens. Matter 21, 186003 (2009)
Moritomo, Y., Xu, S., Machida, A., Akimoto, T., Nishibori, E., Takata, M., Sakata, M.: Phys. Rev. B 61, R7827 (2000)
Yang, C.W., Fang, T.T.: J. Electrochem. Soc. 159, 35 (2012)
Kobayashi, K.I., Kimura, T., Sawada, H., Terakura, K., Tokura, Y.: Nature 395, 677 (1998)
Garcia-Landa, B., Ritter, C., Ibarra, M.R., Blasco, J., Algarabel, P.A., Mahendiran, R., Garcia, J.: Solid State Commun. 110, 435 (1999)
Maignan, A., Raveau, B., Martin, C., Hervieu, M.J.: Solid State Chem. 144, 224 (1999)
Kobayashi, K.I., Kimura, T., Tommioka, Y., Sawada, H., Terakura, K., Tokura, Y.: Phys. Rev. B 59, 1159 (1999)
Kim, T.H., Uehara, M., Cheong, S.W., Lee, S.: Appl. Phys. Lett. 74, 1737 (1999)
Aldica, G., Plapcianu, C., Badica, P., Valsangiacom, C., Stoica, L.: J. Magn. Magn. Mat. 311, 665 (2007)
Valsangiacom, C., Plapcianu, C., Stoica, L., Aldica, G., Kumcser, V.: J. Opto. Avd. Mat. 10, 845 (2008)
Alonso, J.L., Fernandez, L.A., Guinea, F., Lesmes, F., Martin-Mayor, V.: Phys. Rev. B 67, 214423 (2003)
Liu, G.Y., Rao, G.H., Feng, X.M., Yang, H.F., Ouyang, Z.W., Liu, W.F., Liang, J.K.: J. Alloy Compd. 353, 42–47 (2003)
Liu, G.Y., Rao, G.H., Feng, X.M., Ouyang, Z.W., Liu, W.F., Liang, J.K.: Phys. B 334, 229–233 (2003)
Markandeya, Y., Suresh, K., Bhikshamaiah, G.: J. Alloys Compd. 509, 9598–9603 (2011)
Liang, P., Jiang, J.-J., Ma, X.-G., Tian, B.: Trans. Nonferrous Met. Soc. China 17, 109 (2007)
Liu, X.-J., Huang, Q.-J., Niu, D.-L., Sheng, X. U., Zhang, S.-Y.: Chin. Phys. Lett. 21, 2281 (2004)
Goko, T., Endo, Y., Morimoto, E., Arai, J., Matsumoto, T.: Physica B 329–333, 837–839 (2003)
Sarma, D.D., Sampath Kumaran, E.V., Ray, S., Nagarajan, R., Majumbar, S., Kumar, A., Nalini, G., Guru Row, T.N.: S S Com. 114, 465 (2000)
Borges, R.P., Thomas, R.M., Cullinan, C., Coey, J.M.D., Suryanarayanan, R., BenDor, L., Pinsard-Gaudart, L., Revcoleschi, A.: J Phys. Conden. Mat. 11, L445 (1999)
Perry, A.S., Mcintyre, N.S.: J. Phys. Chem. 97, 11020
Ritter, C., Ibarra, M.R., Morellon, L., Blaso, J., Garcia, J., de Teresa, J.M.: J Phys. Conden. Mat. 12, 8295 (2000)
Moghtada, R.A.: https://www.sciencedirect.com/science/journal/13504177. Ultrason. Sonochem. 41, 127 (2018)
Charoonsuk, T., Vittayakorn, W., Vittayakorn, N., Seeharaj, P., Maensiri, S.: https://www.sciencedirect.com/science/journal/02728842. Ceram. Int. 41, 87 (2015)
Moghtada, A., Ashiri, R.: https://www.sciencedirect.com/science/journal/13504177. Ultrason. Sonochem. 33, 141 (2016)
Dutta, D.P., Tyagi, A.K.: https://www.sciencedirect.com/science/journal/0167577X. Mater. Lett. 164, 368 (2016)
Moghtada, A., Shahrouzianfar, A., Ashiri, R.: https://www.sciencedirect.com/science/journal/09218831. Adv. Powder Technol. 28, 1109 (2017)
Utara, S., Hunpratub, S.: https://www.sciencedirect.com/science/journal/13504177. Ultrason. Sonochem. 41, 441 (2018)
Maignan, A., Raveau, B., Martin, C., Hervieu, M.: J. Solid State Chem. 144, 224 (1999)
Moreno, N.O., Barbosa, L.B., Reyes Ardila, D., Andreeta, J.P.: J Supercond. Nov. Magn. 12, 1723 (2012)
Nogues, D.J., Munoz, J.S., Fontcuberta, J.: Mater. Sci. Eng. B 126, 279–282 (2006)
Rubi, D., Frontera, C., Roig, A., Nogues, J., Munoz, J.S., Fontcuberta, J.: J Phys. Conden. Mat. 17, 8037 (2005)
Dai, J.M., Song, W.H., Wang, S.G., Ye, S.L., Wang, K.Y., Du, J.J., Sun, Y.P., Fang, J., Chen, J.L., Gao, B.J.: J Math. Sci. Eng. B 83, 217 (2001)
Xi, L., Qiao, W., Yan, S., Peng, C., Shi, X., Ge, S., Du, J., Ge, J.: J Math. Sci. Eng. B 170, 71 (2010)
Kim, J.H., Ahn, G.Y., Park, S.-I., Kim, C.S.: J. Magn. Magn. Mater. 282, 295–298 (2004)
Feng, X.M., Rao, G.H., Liu, G.Y., Yang, H.F., Liu, W.F., Ouyang, Z.W., Liang, J.K.: Phys. B 344, 21–26 (2004)
Vegard, L.: Die Konstitution der Mischkristalle und die raumfüllung der Atome. Zeitschrift für Physik 5, 17–26 (1921)
Mostafa, M.F., Ata-Allah, S.S., Youssef, A.A.A., Refai, H.S.: J. Magn. Magn. Mater. 320, 344 (2008)
Lavat, A.E., Baran, E.J.: Vib Spectrosc. 32(2), 167 (2003)
Zhang, Q., Rao, G.H., Dong, H.Z., Xiao, Y.G., Feng, X.M., Liu, G.Y., Zhang, Y., Liang, J.K.: Physica B 370, 228–235 (2005)
Markandeya, Y., Saritha, D., Vithal, M., Singh, A.K., Bhikshamaiah, G.: J. Alloys Compd. 509, 5195–5199 (2011)
Ogale, A.S., Ogale, S.B., Ramesh, R., Venkatesan, T.: Appl. Phys. Lett. 75, 537 (1999)
Acknowledgments
One of the authors (SVP) wishes to thank the Principal and HOD of the Department of Physics of JNTUACEP for providing the lab facility for synthesis; expresses his gratitude to Prof. G. Bhikshamaiah, Department of Physics, Osmania University, for providing the gas treatment facility; and thank Shri. M. Shiva Kumar (IITK) and Dr. K. Nageswara Rao (Director P&R, DRDL) for their technical inputs and unconditional support as well as Ln. K. Krishna Reddy (Chairman, KGRCET) for the encouragement.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Varaprasad, S., Thyagarajan, K., Markandeya, Y. et al. Investigation of Fe/Mo Ordering and Magnetic Properties of Ca2FexMo2−xO6 (x = 0.9, 1.0, 1.1 and 1.2) Double Perovskites Prepared by Sonochemical Method. J Supercond Nov Magn 32, 583–589 (2019). https://doi.org/10.1007/s10948-018-4738-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-018-4738-0