Abstract
Double perovskite oxides Sr2 Fe x Mo2−x O6 (x = 0.8, 1.0, 1.2, 1.3 and 1.4) (SFMO) of different compositions were prepared by sol–gel growth followed by annealing under reducing atmosphere conditions of H2/Ar flow. X-ray powder diffraction studies revealed that the crystal structure of the samples changes from tetragonal to cubic at around x = 1.2. Lattice parameters and unit cell volume of these samples found to decrease with the increase in Fe content. The characteristics absorption bands observed in the range 400–1000 cm−1 of Fourier transform infrared spectra indicate the presence of FeO6 and MoO6 octahedra and confirm the formation of double perovskite phase. The value of g ∼ 2.00 obtained from electron spin resonance studies indicates that Fe is in 3+ ionic state in the SFMO samples. Dilatometric studies of these samples reveal that the average value of coefficient of thermal expansion (\( {\overline {\alpha }}\)) increases with the increase in temperature or Fe content in SFMO samples. The low value of coefficient of thermal expansion 1.31 × 10−6 ∘C−1 obtained for Sr2Fe0.8Mo1.2O6 in the present study in the temperature range of 40–100∘C makes it useful as anode material in fuel cells. The coefficient of thermal expansion (\( {\overline {\alpha }}\)) and the unit cell volume (V) of SFMO samples vary inversely with composition in agreement with Grüneisen relation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Double perovskite materials have gained lot of research interest in recent years owing to their significant applications such as anode materials in fuel cells, magnetic storage devices. Especially double perovskite oxide Sr2FeMoO6 doped with several elements were extensively studied for magnetoresistance and magnetization.[1–17] This compound crystallizes in tetragonal lattice with space group I4/mmm.[1] The structure of Sr2FeMoO6 can be viewed as a regular arrangement of corner-sharing FeO6 and MoO6 octahedra, alternating along the three directions of the crystal with the Sr cations occupying the voids in between the octahedra.[1,3]Thermal expansion studies give information regarding the expansion coefficient of a material and its applicability as sealants. However, there is no work reported on thermal expansion studies on these materials except on stoichiometric A2FeMoO6 (A = Sr, Ba, Ca).[11] In addition, Fe and Mo are d-block elements and their ions are of almost same size. In view of this, the authors have taken up synthesis of Sr2Fe x Mo2−x O6 by interchanging Fe and Mo content in their sites with an objective to characterize them using X-ray diffraction (XRD), scanning electron microscopy (SEM), Fourier transform infrared (FTIR) spectroscopy, electron spin resonance (ESR) and study thermal expansion at elevated temperatures.
2 Experimental
Different composition of Sr2Fe x Mo2−x O6 (x = 0.8, 1.0, 1.2, 1.3 and 1.4) (SFMO) powders were synthesized by the sol–gel method using AR grade Sr(NO3)2, Fe(NO3)3⋅9H2O and H2MoO4. The details of the synthesis of the SFMO powders are given in earlier publications[7,8]from this laboratory. The powders of SFMO were pressed into pellets of dimensions 25 mm × 6 mm × 6 mm using a dies under a pressure of 5 t m−2. These pellets were sintered at 1200∘C for 6 h and then heated at 1000∘C in a stream mixture of gas (10% H2 + 90% Ar) for 3 h for reducing Mo6+ into Mo5+. These pellets were subjected to XRD studies using Philips PW 1830 diffractometer with Cu Kα radiation (40 kV × 25 mA) to confirm the crystal structure. Surface morphology of the samples was found using the SEM (Model no. Joel JSM-5600) with combined microanalyzer. FTIR spectra of the samples were recorded on Bruker Tensor 27 DTGS TEC detector spectrophotometer in the wavenumber range of 400–1000 cm−1 by the KBr pellet method. ESR spectra were recorded at room temperature using a JOEL-1X-ESR spectrometer that works in X-band frequency range (8–12.5 GHz) with 100 kHz field modulation. Thermal expansion measurements were performed using Netzsch 402 PC dilatometer in the temperature range of 40–400∘C during heating cycle.
3 Result and discussion
3.1 Characterization
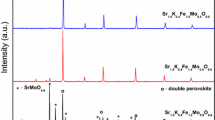
XRD patterns of various compositions of SFMO samples are shown in figure 1. These patterns reveal that the observed diffraction profiles belong to double perovskite structure of Sr2FeMoO6.[4–6]All the profiles of SFMO are indexed and found to be in single phase. The lattice parameters of SFMO were evaluated by Cohen’s least square method using (hkl) values and Bragg’s angles with a suitable computer program. It has been found that SFMO crystallizes in tetragonal lattice (space group I4/mmm) for 0.8 ≤ x ≤ 1.2 and cubic lattice (space group \(\text {Fm}\bar {3}\mathrm {m})\) for x > 1.2. The values of lattice parameters ‘a’ and ‘c’ of SFMO are given in table 1. The unit cell volume V = a 3 for cubic and 2 a 2 c for tetragonal structure were calculated using lattice parameters and are included in table 1. It is found that the value of ‘a’, ‘c’ and V decreases with the increase of Fe content in both tetragonal and cubic phase. Since the ionic radius of Fe 3+ (0.645 Å) is larger than that of Mo 5+(0.61 Å), the decrease in ‘a’, ‘c’ and V might be attributed to cation or oxygen vacancies or valence disproportion as observed in double perovskites.[18–20]Further, it can be noted from table 1 that the structural transition from tetragonal to cubic lattice occurs at around x = 1.2 in SFMO.[21] The tolerance factor of SFMO double perovskite is given by
where r Sr, r Fe, r Mo and r O are the ionic radii of Sr, Fe, Mo and O ions, respectively. The tolerance factor (t) of SFMO compounds was calculated by employing equation (1) and the values are included in table 1. It is found from table 1 that the deviation of tolerance factor from unity in SFMO samples increases with the increase of Fe content, indicating the increase of distortion from ideal double perovskite structure with Fe content.
The SEM photograph of SFMO sample is shown in figure 2a–e. It is observed from these figures that the porosity is present in the samples. The pore size changes from sample to sample. The pore size is very small and material is closely packed with fewer voids for composition x = 1.3. It is slightly larger for rest of the compositions in SFMO samples.
Figure 3 shows FTIR spectra of the Sr2Fe x Mo2−x O6 (x = 0.8, 1.0, 1.2, 1.3 and 1.4) samples in the spectral wavenumber range of 400 and 1000 cm−1. Three characteristic absorption bands between 400 and 860 cm −1 are usually used to identify the perovskite phase formation.[12] In the present study, FTIR spectra of the SFMO samples show three absorption bands corresponding to Fe–O and Mo–O vibrations, namely, strong bands in the high-wavenumber range ∼809 and 858 cm−1 with associated with the Mo–O symmetric stretching mode of MoO6octahedra; broad band at ∼630 cm−1 and weak band at ∼702 cm−1 assigned to the antisymmetric stretching mode of the MoO6 octahedra due to the higher charge of this cation.[13] The weak absorption band obtained at about 422 cm −1 is ascribed to Fe–O vibration absorption of FeO6 octahedra. In SFMO double perovskite samples, the highly charged Mo cation octahedra (MoO6) acts as independent group, the vibration spectrum therefore arises from such MoO6 octahedra. Mo–O symmetric stretching mode of MoO6 octahedra at about 809 and 858 cm−1 is usually an infrared inactive vibration, but in a double perovskite, both Fe and Mo ions exist in Fe and Mo sites and it becomes partially allowed due to lowering of site symmetry.[13] The bands obtained in the present study confirm the formation of perovskite phase.
ESR spectra of Sr2Fe x Mo2−x O6 (x = 1.0, 1.2, 1.3 and 1.4) samples taken at room temperature are shown in figure 4a–d. All the figures show a similar spectrum. It is observed from spectra that a peak is resolved at around H = 310 mT for all samples. The value of g-factor of SFMO samples were evaluated using g = h ν/β H, where h is Planck’s constant (6.625 × 10 −34 J s), ν the frequency of magnetic field, β the Bohr magneton (9.274 × 10−24 J Tesla−1), H the applied magnetic field. The value of g-factor thus obtained is included in table 1. It is found from table 1 that the value of g-factor is close to 2.00, indicating Fe is in 3+ state in SFMO samples. Therefore, the ESR spectra of SFMO samples obtained in this study correspond to the localized 3d5 Fe cores in the band picture as reported by Kobayashi et al.[1] This rules out the possibility of assigning the observed spin resonance to localized Fe2+ in SFMO samples. Because its ground state in octahedral environment is a triplet with an expected g-factor about 3.4. On the other hand, if Fe 2+ ions were also present, their short relaxation time would render their ESR line undetectable as in the case of FeO. If this was the case, the Fe 2+–Fe 3+ coupling would induce a g-shift to the Fe 3+ ESR line.[14] Hence, in the present study, it can be concluded that SFMO samples contain Fe 3+ ions only since the observed g-value remains constant in the whole composition range. Further, the small distortion observed in the shape of spectrum for the samples x > 1.2 in figure 4c and d might be attributed to structural phase change from tetragonal to cubic at x = 1.2 in conformity with results obtained from XRD studies.
3.2 Thermal expansion
The thermal expansion characteristics (ΔL/L o) of Sr2Fe x Mo2−x O6 (x = 0.8, 1.0, 1.2, 1.3 and 1.4) in the temperature range from 40 to 400 ∘C obtained using dilatometer are shown in figure 5. The thermal expansion depends on the electrostatic forces within the lattice, which depend on the concentration of positive and negative charges and their distances within the lattice.[22] The thermal expansion of a solid is characterized by the coefficient of thermal expansion (α) caused by the thermal lattice vibrations given by
where L o and L 1 are the lengths of sample at temperature T o and T 1, respectively.
The experimental data of thermal expansion (ΔL/L o) as a function of temperature (T) of SFMO samples in the present study can be fitted to a polynomial of degree 2 as
where A, B and C are the constants and given in table 2 for all the samples. The value of α then can be determined using the first derivative of equation (3) with respect to temperature as
Equation (4) shows that the value of α at any temperature can be obtained using constants B and C.
The average value of coefficient of thermal expansion (\(\bar {\alpha })\) in the temperature range from T o to T 1 can be determined using the relation
Thus, the value of \(\bar {\alpha }\) was evaluated in different temperature ranges namely 40–100, 40–200, 40–300 and 40–400 ∘C for all SFMO materials and are given in table 2. The plot of \(\bar {\alpha }\) vs. Fe composition of SFMO in the temperature ranges 40–300 and 40–400 ∘C is shown in figure 6 and it is observed that the value of \(\bar {\alpha }\) increases with the Fe content and temperature. Falcon et al[23] investigated the oxidation profiles of Sr2FeMoO6−δ oxide, results showed that oxygen was relatively easily incorporated into the perovskite structure. Increase in the oxygen vacancy concentration leads to oxygen permeation and lattice expansion which becomes much more pronounced.[24] Therefore, in the present study increase in the value of \(\bar {\alpha }\) of SFMO samples with the increase in temperature may be attributed to increase in concentration of oxygen vacancy. Zhang et al[11] have reported that the value of \(\bar {\alpha }\) of Sr2FeMoO6−δ in the temperature range 30–1000 ∘C is 13.9 × 10 −6 ∘C −1. The value of \(\bar {\alpha }\) of Sr2FeMoO6 obtained in the present study in the temperature ranges 40–400 ∘C −1 is 13.8 × 10 −6 ∘C −1 is very much in agreement with that reported by Zhang et al.[11] It is also found from figure 6 that the value of \(\bar {\alpha }\) increases with the increase in Fe content. This increase in \(\bar {\alpha }\) may be attributed to substitution of larger ionic size of Fe 3+ (0.645 Å) at smaller ionic size of Mo 5+ site (0.61 Å). Further, it is to be noted that the average value of coefficient of thermal expansion of Sr2Fe0.8Mo1.2O6 is found to be 1.31 × 10−6 ∘C−1 in the temperature range 40–100 ∘C makes this material in fuel cell applications as anodes.
It is observed from table 1 and figure 6 that the value of unit cell parameters decrease and the value of \(\bar {\alpha }\) increases with the increase in Fe content, indicating an inverse relation between the value of \(\bar {\alpha }\) and unit cell parameters in accordance with the following Gruneisen’s relation:[22,25]
where α v is the coefficient of volume thermal expansion, γ G Gruneisen’s constant, C v the heat capacity at constant volume, χ the compressibility, and V the volume of the unit cell.
4 Conclusions
-
(1)
It has been found that the lattice parameters and unit cell volume decreases with the increase of Fe content in SFMO samples.
-
(2)
A structural transition from tetragonal to cubic at around x = 1.2 is observed in SFMO samples.
-
(3)
The FTIR spectra of SFMO showed three characteristic absorption bands in the range of 860–400 cm−1, indicating the formation of perovskite structure.
-
(4)
ESR results of the samples show that the iron is in Fe3+ state in SFMO samples.
-
(5)
The results of thermal expansion showed that the average value of coefficient of thermal expansion (\(\bar {\alpha })\) increases with the increase in temperature or Fe content in SFMO samples.
References
Kobayashi K I, Kimura T, Sawada H, Terakura K and Tokura Y 1998 Nature (Lond.) 395 677
Ray S, Middey S, Jana S, Banerjee A, Sanyal P, Rawat R, Gregoratti L and Sarma D D 2011 J. EPL 94 47007
Lu M F, Wang J P, Liu J F, Song W, Hao X F, Zhou D F, Liu X J, Wu Z J and Meng J 2007 J. Alloys Compd. 428 214
Gaur A, Varma G D and Singh H K 2008 J. Alloys Compd. 460 581
Bufaiçal L, Adriano C, Lora-Serrano R, Duque J G S, Mendonça-Ferreira L, Rojas-Ayala C, Baggio-Saitovitch E, Bittar E M and Pagliuso P G 2014 J. Solid State Chem. 212 23
Feng X M, Rao G H, Liu G Y, Yang H F, Liu W F, Ouyang Z W and Liang J K 2004 Physica B 344 21
Markandeya Y, Suresh K and Bhikshamaiah G 2011 J. Alloys Compd. 509 9598
Markandeya Y, Saritha D, Vithal M, Singh A K and Bhikshamaiah G 2011 J. Alloys Compd. 509 5195
Huo G, Ren X, Qian L, Zhang N, Liu S and Yan X 2013 , J. Magn. Magn. Mater. 343 119
Mohamed Musa Saad H -E 2012, Physica B 407 2512
Zhang L, Zhou Q, He Q and He T 2010 J. Power Sources 195 6356
Mostafa M F, Ata-Allah S S, Youssef A A A and Refai H S 2008 J. Magn. Magn. Mater. 320 344
Lavat A E and Baran E J 2003 Vib. Spectrosc. 32 167
Gulley J E and Jaccarino V 1972 Phys. Rev. B 6 58
Kapusta C., Riedi P C, Zajac D, Sikora M, De Teresa J M, Morellon L and Ibarra M R 2002 J. Magn. Magn. Mater. 242–245 701
Kim S B, Lee B W and Kim C S 2002 J. Magn. Magn. Mater. 242–245 747
Xue J, Shen Y and He T 2011 J. Power Sources 196 3729
Topfer J and Goodenough J B 1997 Chem. Mater. 9 1467
van Roosmalen J A M, van Vlaanderen P, Cordfunke E H P, Ijdo W L and Ijdo D J W 1995 J. Solid State Chem. 114 516
Takeda Y, Kanno K, Takada T, Yamamoto O, Takano M, Nakayama N and Bando Y 1986 J. Solid State Chem. 63 237
Yoshida K, Keuchi S I, Shimizu H, Okayasu S and Suzuki T 2011 J. Phys. Soc. Jpn. 80 044716
Prasanth Kumar V, Reddy Y S, Kistaiah P, Prasad G and Vishnuvardhan Reddy C 2008 Mater. Chem. Phys. 112 711
Falcon H, Barbero J A, Araujo G, Casais M T, Martinez-Lope M J, Alonso J A and Fierro J L G 2004 Appl. Catal. B—Environ. 53 37
Onuma S, Yashiro K, Miyoshi S, Kaimai A, Matsumoto H, Nigara Y, Kawada T, Mizusaki J, Kawamura K, Sakai N and Yokokawa H 2004 Solid State Ion. 174 287
Eastabrook J N 1957 Philos. Mag. 2 1421
Acknowledgements
One of the authors (GB) wish to thank DST for providing financial assistance to carry out this work through a project OU-DST-PURSE-Scheme No. A.60. We express our gratitude to the Head, Department of Physics, Osmania University and the Head, Department of Physics and Principal, Nizam College, Osmania University, Hyderabad, for their encouragement. We also thank Prof V Seshubai, University of Hyderabad, for providing ESR measurements.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
MARKANDEYA, Y., REDDY, Y.S., BALE, S. et al. Characterization and thermal expansion of Sr2Fe x Mo2−x O6 double perovskites. Bull Mater Sci 38, 1603–1608 (2015). https://doi.org/10.1007/s12034-015-0972-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12034-015-0972-2