Abstract
The dielectric and EMI shielding behaviors of styrene acrylonitrile (SAN)/polyaniline (PANI) polymer blends are investigated using impedance analyzer. The incorporation of PANI forms the phase separated morphology in SAN matrix and by increasing the concentration of PANI, the interconnected network of PANI phase develops within the SAN matrix. Near the percolation threshold concentration, the dielectric constant (\(\varepsilon^{\prime }\)) and dielectric loss (\(\varepsilon^{\prime\prime}\)) of SAN/PANI blends are enhanced up to five and eight orders of magnitude at 1 kHz, respectively. Moreover, total shielding efficiency (SET) was determined in the radio frequency range. SAN/PANI polymer blends show the total shielding efficiency at very less thickness of about 150 µm. Overall, shielding efficiency in SAN/PANI blends is improved owing to the increased number of charge dipoles accumulation at the insulator-conductor interfaces.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In recent times, the developments in the field of telecommunication and electronic devices have greatly enhanced the problem of electromagnetic interference (EMI) [1]. Blending the combinations of insulative and conductive polymer matrices for designing the functional materials with improved dielectric properties are becoming the potential candidates for the electromagnetic interference (EMI) shielding and miniaturization of the electronics. Although conductive polymers show particular characteristics which make them novel dielectric material but their practical applications are limited owing to their poor processability [2]. Therefore, to utilize the inherent specific characteristics, conductive polymers are blended with insulative polymers to improve the processability [3–10]. The formation of polymer blends in which conductive polymer acts as a guest in the insulative polymer matrix imparts attractive synergistic properties to fill the gap among insulating polymers and highly conductive metals.
Polyaniline (PANI) is commonly used as conductive matrix owing to the high conductivity, ease of preparation and good environment stability [11, 12]. Various polymer blends of PANI have been prepared to improve the mechanical properties as well as the electrical conductivity of the overall system [13–19]. The conductivity value of 0.1 Scm−1 for 20 wt% of PANI was observed for PS/PANI blends prepared by in situ polymerization [20]. Vicentini et al. [21] reported in situ polymerization and solution method for the preparation of TPU/PANI blends and found that the values of electrical conductivity at 30 wt% of PANI reached to 1.2 × 10−1 Scm−1 for in situ polymerization, which was 10 times higher than the value of TPU/PANI blends prepared by solution method. Moreover, the electrical conductivity of polyvinyl formal (PVF)/PANI blends was enhanced to 2.5 × 10−4 S cm−1 with the increase of PANI concentration up to 65 wt% [22]. Besides the electrical behaviors, dielectric properties of PANI and its blends have been of interest as well. For PVA/PANI polymer blends, high k-value ranging from 200 to 1000 was observed [23]. For PU/PANI systems [18], dielectric constant was around 1120 at 1 kHz but no data about dielectric loss was reported for these systems. In case of PVDF/PANI blends at 23 vol%, the dielectric constant reached to 2000 while dielectric loss tangent was around 1.75 at 100 Hz [16]. Epoxy/PANI blends [19] at 25 wt% prepared by in situ polymerization reached the dielectric constant close to 3000 and dielectric loss tangent around 0.5 at 10 kHz. The increase in the dielectric properties as well as electrical behavior of these polymer blends can be utilized for shielding EMI radiation signals which can influence the system performance due to the electrostatic discharge.
In this research work, styrene acrylonitril (SAN) copolymer is used as an insulating matrix in which concentrations of conductive PANI are varied from 5 to 50 wt% using solution method to investigate the dielectric and EMI shielding properties of SAN/PANI polymer blends. Studies on SAN/PANI blends are rarely reported although few reports are available on the dielectric properties of SAN with graphite and carbon nanotubes [24–27]. Phase separated heterogeneous morphology is formed in SAN/PANI polymer blends as characterized by SEM. The influence of PANI on the dielectric behavior of SAN is pronounced in the lower frequency region while at higher frequency region, dielectric properties are close to each other for all the concentrations. Moreover, EMI shielding behavior of SAN/PANI polymer blends were investigated providing considerable shielding efficiency for samples of 150 µm thickness.
2 Experimental section
2.1 Materials
Styrene-acrylonitrile (SAN) of commercial grade was provided by the courtesy of ERKOL, Turkey. Aniline monomers were purchased from Uni CHEM chemical reagents and purified through distillation. Ammonium peroxydisulfate (APS) (99 % pure) was purchased from Daejung chemicals, Korea and Dodecylbenzenesulphonic acid (DBSA) from Sigma Aldrich. For the preparation SAN/PANI polymer blends, 1, 2-dichloroethane of analytical grade was used as solvent which was purchased from LAB-SCAN Analytical Sciences.
2.2 Sample preparation
Polyaniline (PANI) was prepared using chemical oxidative polymerization technique. To start the reaction, 6 g of aniline monomer (ANI) was added to 100 ml of Deionized water in flask containing a stirrer (located in ice bath). To make it conductive, 1 M solution of HCL acid was added and after continuous stirring aniline hydrochloride was prepared. 1 M solution of Sodium dodecyl sulphate (SDS) was added as surfactant. To initiate polymerization, an initiator APS (ammonium peroxide sulphate) (1.2 g) was dissolved in 20 g deionized water and then slowly added to the aniline solution with stirring for 1 h at a controlled temperature of 0 °C using an ice bath, this was followed by stirring for 1.5 h at room temperature. The polymer was precipitated with methanol, washed with distilled water several times and then dried in vacuum oven at 50 °C. The final product was obtained in the form of green colored powder of PANI.
For preparation of SAN/PANI polymer blends, solution method was used in which SAN was dissolved in 1, 2 dichloroethane and PANI was added in the SAN solution at various concentrations. The solution was kept for stirring for 7 h and sonicated for 1 h. Then, the blend was poured in a petri dish for overnight drying in a fume hood at ambient temperature, followed by drying in vacuum oven at 60 °C for 5 h. The solution casted films were about 150 µm thick. The concentration of PANI in SAN was varied from 5 to 50 wt%.
2.3 Characterization techniques
2.3.1 Scanning electron microscope
The morphology of the samples was determined using a scanning electron microscopy (SEM) (JEOL-instrument JSM-6490A). The samples were frozen in liquid nitrogen where it became fragile and broken to generate the fresh surface. The samples were mounted on aluminum stubs and coated the surfaces with gold.
2.3.2 Differential scanning calorimetry
The thermal behavior was investigated using Diamond DSC (Perkin Elmer Pyris) equipped with Liquid N2. Dry N2 gas was purged through DSC furnace at flow rate of 10 mL/min. The mass of the samples was kept ~5 mg and heating rate of 10 °C/min was used. The temperature and heat flow rates were calibrated with indium and zinc standards.
2.3.3 Impedance analyzer
Dielectric behavior was determined using precision impedance analyzer (Wayne Kerr 6500B) at room temperature. The capacitance C and dissipation D were measured through impedance analyzer in the frequency range of 100 Hz to 5 MHz. The circular samples with thickness of 0.15 mm and diameter of 13 mm were cut from casted films.
2.4 Analysis of dielectric data
The dielectric data is first expressed in terms of real (\( \varepsilon^{\prime } \)) and imaginary part of dielectric permittivity (\( \varepsilon^{{\prime \prime }} \)) which is obtained from the complex permittivity as follows [28];
Dielectric constant is the ability of a substance to store electrical energy in an electric field, which can be calculated by using the following expression,
where \( C \) is the observed capacitance of the sample, \( d \) is the thickness (meters), \( A \) is the area (meters) and \( \varepsilon_{o} \) is the permittivity of free space. Dielectric loss can be calculated from dielectric tangent loss (tanδ) which is observed directly from the instrument having the expression;
Tangent loss is the dissipation factor D which is a measure of the energy dissipated by the dielectric material under an oscillating field. The AC conductivity for the dielectric polymer nanocomposites is calculated using the expression;
All the above mentioned parameters are obtained as a function of frequency at room temperature.
3 Results and discussion
3.1 Morphology of SAN/PANI polymer blends
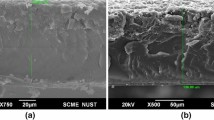
Figure 1 shows the SEM images of neat SAN and SAN/PANI polymer blends at different concentrations. For neat SAN (Fig. 1a), co-continuous morphology is discernable without showing any prominent features reflecting its completely amorphous nature. In case of SAN/PANI polymer blends, bright lines indicate the dispersion of PANI phase in the SAN matrix. The heterogeneous morphology of SAN/PANI polymers blends is clearly observed owing to the immiscibility of both phases with each other. PANI phase is uniformly distributed in the SAN phase at concentration of 10 wt% (Fig. 1b) and by increasing the concentration up to 40 wt% (Fig. 1c), the PANI phase is densified forming the interconnected network in the SAN matrix.
Figure 2 shows the differential scanning calorimetry (DSC) results of SAN/PANI polymer blends at various concentrations. Neat SAN shows the appearance of the step at 105 °C indicating its glass transition temperature (Tg). After the incorporation of PANI in SAN up to 10 wt%, the Tg is slightly reduced to 103 °C. However, beyond 10 wt% of PANI concentration, the step for Tg is broadened owing to the heterogeneous phase morphologies of the SAN/PANI blends, consistent with the SEM results.
SEM and DSC results have confirmed the formation of heterogeneous phase morphologies for SAN/PANI polymer blends. PANI is forming the interconnected phase at higher concentrations which would presumably influence the dielectric properties of the SAN. Thereby, the dielectric and shielding properties of the SAN/PANI polymer blends are investigated and discussed further.
3.2 Dielectric properties of SAN/PANI polymer blends
Figure 3 shows the dielectric constant (\(\varepsilon^{\prime }\)) behavior of SAN/PANI polymer blends for various concentrations at room temperature as a function of frequency. SAN is an insulating polymer having weak dipole moment resulting in low \(\varepsilon^{\prime }\) as well as \(\varepsilon^{\prime\prime}\) and behaves almost independent of frequency. The influence of PANI on the \(\varepsilon^{\prime}\) behavior of SAN is pronounced in the lower frequency region while at higher frequency region, \(\varepsilon^{\prime }\) value is close to each other for all the concentrations. In the lower frequency region (inset of Fig. 3a), \(\varepsilon^{\prime }\) value is in the similar range for SAN/PANI blends <30 wt% while ≥30 wt%, \(\varepsilon^{\prime }\) of SAN is remarkably enhanced. The maximum value of ε’ is increased by about five orders of magnitude from 1.9 to 8.9 × 105 at 1 kHz by the addition of 40 wt% of PANI in SAN and beyond that concentration (50/50 wt% of SAN/PANI blends) ε’ value is decreased again. The significant enhancement in \(\varepsilon^{\prime }\) value is pertaining to the Maxwell–Wagner polarization originating from the insulator-conductor interfaces. The evolvement of such interfacial polarization is due to the accumulation of the dipoles or space charges at the interfaces. The space charges have sufficient time to respond to the applied electric field in the low frequency region, while in the higher frequency range, the electric field changes too fast for the space charges to respond and thereby, the polarization effect does not appear.
Dielectric constant versus frequency of a SAN and SAN/PANI polymer blends at various concentrations, inset shows the zoom portion of lower frequency region, b values of dielectric constant at 1 kHz for SAN and SAN/PANI blends, inset shows the power law behavior of SAN/PANI polymer blends near percolation threshold concentration
The dependence of \(\varepsilon^{\prime }\) value for SAN with increasing concentration of PANI at 1 kHz is shown in Fig. 3b. The \(\varepsilon^{\prime}\) value suddenly jumps to the highest value at the concentration of 40 wt% of PANI in SAN and then reduced again above 40 wt% (vol fraction = 0.35) of concentration. Similarly, Lu et al. [19] reported 300 fold increase of \(\varepsilon^{\prime }\) value by the addition of 25 wt% of PANI in epoxy matrix owing to the formation of fine PANI network surrounded by epoxy matrix. The variation of \(\varepsilon^{\prime }\) near \( f_{c} \) is also given by power law as per the expression,
where s is the dielectric critical exponent. The inset of Fig. 3b shows the experimental values of \(\varepsilon^{\prime }\) which is in good agreement with Eq. 5 for f c = 0.35 (40 wt%) and s = −1.26. Such significant increase of permittivity constant value at 40 wt% is related to the percolation threshold concentration for the SAN/PANI polymer blends. Above percolation concentration, the electron conduction is probably occurring as near the percolation threshold concentration, the PANI phase connects with each other directly, bringing the leakage loss in the blends.
Figure 4 shows the dielectric loss (\(\varepsilon^{\prime\prime }\)) trend of pure SAN and SAN/PANI blends as a function of frequency at room temperature. \(\varepsilon^{\prime\prime }\) is independent of increasing frequency for pure SAN and SAN/PANI blends for concentrations less than 30 wt%. The difference appears in the lower frequency region when PANI concentration reached ≥30 wt% (inset of Fig. 4). The highest \(\varepsilon^{\prime\prime }\) value is achieved for 40 wt% of PANI concentration (eight orders of magnitude from 0.014 to 2.3 × 108) and beyond that the value is decreased again as shown in Fig. 4b. Recently, Gamal et al. [29] reported the significant increase in \(\varepsilon^{\prime }\) and \(\varepsilon^{\prime\prime }\) for PVA/PANI polymer blends in the low frequency region and the maximum values of \(\varepsilon^{\prime}\) and \(\varepsilon^{\prime\prime }\) are obtained for the addition of 70 wt% of PANI in PVA. Moreover, dissipation factor (tanδ) of SAN/PANI blends (Fig. 5) shows similar trend as observed for \(\varepsilon^{\prime\prime }\).
The AC conductivity (σAC) of pure SAN and SAN/PANI blends with increasing frequency at room temperature is shown in Fig. 6. The σAC is increased with increasing frequency as well as with the concentration of PANI. In the lower frequency region, the increase in σAC is pronounced particularly near the percolation threshold concentration of 40 wt% and then decreased above 40 wt% possibly due to the losses from the formation of conductive pathways above the percolation concentration. Notably, SAN/PANI blends ≥30 wt% show reduction in σAC near 1 kHz indicating the contribution of interfacial polarization to induce the conductivity started to disappear with the increase in frequency. Moreover, above 1 MHz, the conductivity of all the samples show upward trend as in this region hopping mechanism play the role to enhance the conductivity within the system. For comparison, all the values for \(\varepsilon^{\prime}, \varepsilon^{\prime\prime }\), tanδ and σAC for SAN and SAN/PANI blends at 1 kHz are listed in Table 1.
Overall, the dielectric properties of SAN/PANI blends are significantly increased particularly when the concentration is reached to the percolation threshold value (40 wt% of PANI). Although, this is the higher value of percolation concentration found for the insulative-conductive polymer blends. However, some particular methods are opted to reduce the percolation threshold concentration either by selective localization of the conductive network in one phase or by adding large aspect ratio fillers into the matrix [30–32]. We also utilized the selective localization approach to reduce the percolation concentration by adding nanofiller into the lower concentrations of SAN/PANI blends which will be published elsewhere.
3.3 EMI Shielding of SAN/PANI polymer blends
EMI shielding is a material property that can attenuate electromagnetic waves. The total shielding efficiency (SET) of a sample can be expressed by the following equation [33, 34],
where EIN and EOUT are the incident and transmitted EM waves passing through a shielding material, respectively. SEA and SER are the absorption and reflection shielding efficiency, respectively. The last term SEI is a positive or negative correction term related to multiple reflections of the EM wave inside the sample, and is typically neglected when SER > 10 dB. The total shielding efficiency is expressed in terms of decibels (dB). Shielding on the basis of SER occurs owing to the interaction of EM waves with the mobile charge carriers (electrons or holes) resulting in the reflection loss of the EM waves. SEA, on the other hand, is due to the electric or magnetic dipoles in the shielding material which would interact with the incident EM waves, thereby enhancing the shielding due to absorption. For better EMI shielding, the material should maximally attenuate the transmitted EM wave either by reflection or absorption losses. To calculate the EMI shielding behavior of SAN/PANI polymer blends, following equations are used [35];
where l is the thickness of the sample and α, n, and γ are defined by the following equations;
In the above equations, λ is wave length of EM waves, \(\varepsilon^{\prime}\) is the dielectric constant and tanδ is the dissipation factor of the material, ±sign is added to the equation for the positive and negative values of \(\varepsilon^{\prime }\).
Using the Eqs. 6, 7, 8, 10, and 11, SEA, SER, and SET values are calculated for SAN/PANI polymer blends and plotted against frequency as shown in Fig. 7. SEA values of SAN/PANI polymer blends (Fig. 7a) are minimal in the lower frequency region but indicate increasing trend as the frequency is increased. Notably, the trend of SEA value is highest by adding 40 wt% of PANI in SAN in the whole range of frequency. SER values (Fig. 7b) are negligible in the higher frequency region for all the concentrations of SAN/PANI blends. However, for the PANI concentrations above 20 wt%, SER values are increased in the lower frequency region. The highest SER value of 164 dB at 1 kHz is found for 40 wt% of PANI in SAN owing to the percolation concentration having highest \(\varepsilon^{\prime}\) and tanδ values. The SET values (Fig. 7c) follow the same trend as found for SER values indicating that maximum shielding occurred due to the reflection loss instead of absorption loss in SAN/PANI blends. The trend of SET validates the effect of interconnected network of PANI in SAN at percolation threshold concentration (40 wt%). As a result, better interaction between materials and EM waves occur for attenuating the EM interference. Similar observation is reported by Panwar et al. [35] for SAN/graphite sheets (GS) composites in the frequency range of 0.001–3 GHz in which maximum SET value of 201 dB is obtained at 1.57 GHz by the addition of 20 wt% of GS in SAN. Moreover, thickness of the sample plays important role for the attenuation of the EM waves. Recently, Joshi et al. [36] reported the shielding behavior of epoxy/PANI/graphene nanoribbons based hybrid composites in which by changing the thickness of the samples from 1.7 to 3.4 mm, the shielding effectiveness was increased from −44 to −68 dB for 5 wt% of graphene nanoribbons in epoxy/PANI system. It is worth noting that SAN/PANI blends are showing promising total shielding efficiency even though the thickness of SAN/PANI blend samples is ~0.15 mm which is much less than the reported thickness of the samples.
4 Conclusions
The dielectric and EMI shielding behaviors of styrene acrylonitrile (SAN) and polyaniline (PANI) based polymer blends are investigated impedance analyzer. PANI as a conductive polymer is dispersed into insulative SAN matrix at various concentrations ranging from 5 to 50 wt%. PANI forms phase separated morphology in the SAN matrix and near the percolation threshold concentration ~40 wt%, the interconnected network of PANI develops in the SAN matrix as characterized by SEM. The dielectric properties of SAN/PANI polymer blends are enhanced significantly near the percolation concentration; \(\varepsilon^{\prime}, \varepsilon^{\prime\prime }\) and σAC of SAN are increased five, eight and ten orders of magnitude, respectively. Moreover, shielding behavior of SAN/PANI blends is determined in the radio frequency range. Thin films of 150 µm (0.15 mm) of SAN/PANI blends are used to determine the total shielding efficiency. Total shielding efficiency (SET) was highest for SAN/PANI blends at 40 wt% of concentration owing to the interference of EM waves with the charge dipoles accumulation at the conductor–insulator interfaces. This study could further open up new avenues to design light weight and flexible thin films for EMI shielding.
References
J.L.N. Violette, D.R.J. White, F. Violette, Electromagnetic Compatibility Handbook (Van Nostrand Reinhold company, New York, 1987)
M.A. De Paoli, Conductive Polymer Blends and Composites. Handbook of Organic Conductive Molecules and Polymers, vol. 2 (Wiley, New York, 1997)
D. Zhu, Q. Guo, Z. Zheng, M. Matsuo, Synth. Met. 187, 165 (2014)
K. Lakshmi, H. John, K.T. Mathew, R. Joseph, K.E. George, Acta Mater. 57, 371 (2009)
A.D. Price, V.C. Kao, J.X. Zhang, H.E. Naguib, Synth. Met. 160, 1832 (2010)
R. Faiz, I.M. Martin, M.A. De Paoli, M.C. Rezende, J. Appl. Polym. Sci. 83, 1568 (2002)
S.M. Ebrahim, A.B. Kashyout, M.M. Soliman, J. Polym. Res. 14, 423 (2007)
C. Tian, Y. Du, P. Xu, R. Qiang, Y. Wang, D. Ding, J. Xue, J. Ma, H. Zhao, X. Han, A.C.S. Appl, Mater. Interfaces 7, 20090 (2015)
K. Yang, X. Huang, M. Zhu, L. Xie, T. Tanaka, P. Jiang, A.C.S. Appl, Mater. Interfaces 6, 1812 (2014)
L. Zhang, S. Yuan, S. Chen, D. Wang, B.Z. Han, Z.M. Dang, Compos. Sci. Technol. 110, 126 (2015)
Y. Wang, Int. J. Mater. Res. 105, 3 (2014)
P. Zhang, X. Han, L. Kang, R. Qiang, W. Liu, Y. Du, RSC Adv. 3, 12694 (2013)
S.W. Byun, S.S. Im, Polymer 39, 485 (1998)
H. Morgan, P.J.S. Foot, N.W. Brooks, J. Mater. Sci. 36, 5369 (2001)
P.S. Rao, D.N. Sathyanarayana, J. Appl. Polym. Sci. 86, 1163 (2002)
C. Huang, Q.M. Zhang, J. Su, Appl. Phys. Lett. 82, 3502 (2003)
V.N. Bliznyuk, A. Baig, S. Singamaneni, A.A. Pud, K.Y. Fatyeyeva, G.S. Shapoval, Polymer 46, 11728 (2005)
C.P. Chwang, C.D. Liu, S.W. Huang, D.Y. Chao, S.N. Lee, Synth. Met. 142, 275 (2004)
J. Lu, K.S. Moon, B.K. Kim, C.P. Wong, Polymer 48, 1510 (2007)
W.J. Bae, W.H. Jo, Y.H. Park, Synth. Met. 132, 239 (2003)
D.S. Vicentini, G.M.O. Barra, J.R. Bertolino, A.T.N. Pires, Eur. Polym. J. 43, 4565 (2007)
S. Ebrahim, A.H. Kashyout, M. Soliman, Curr. Appl. Phys. 9, 448 (2009)
P. Dutta, S. Biswas, S.K. De, Mater. Res. Bull. 37, 193 (2002)
V. Panwar, R.M. Mehra, Eur. Polym. J. 44, 2367 (2008)
N.K. Shrivastava, S. Suin, S. Maiti, B.B. Khatua, Ind. Eng. Chem. Res. 52, 2858 (2013)
A. Goldel, G.R. Kasaliwal, P. Potschke, G. Heinrich, Polymer 53, 411 (2012)
K.T. Kim, W.H. Jo, J. Polym. Sci. Part A Polym. Chem. 48, 4184 (2010)
S. Havriliak Jr., S.J. Havriliak, Dielectric and Mechanical Relaxation in Materials: Analysis, Interpretation and Application to Polymers (Hanser Publishers, Munich, 1997)
S.E. Gamal, A.M. Ismail, R.E. Mallawany, J. Mater. Sci. Mater. Electron. 26, 7544 (2015)
M.H. Al-Saleh, U. Sundararaj, Compos. Part A 39, 284 (2008)
M.H. Al-Saleh, U. Sundararaj, Eur. Polym. J. 44, 1931 (2008)
C. Mao, Y. Zhu, W. Jiang, A.C.S. Appl, Mater. Interfaces 4, 5281 (2012)
Z. Liu, G. Bai, Y. Huang, Y. Ma, F. Du, F. Li, T. Guo, Y. Che, Carbon 45, 821 (2007)
J. Joo, A.J. Epstein, Appl. Phys. Lett. 65, 2278 (1994)
V. Panwar, B. Kang, J.O. Park, S. Park, R.M. Mehra, Eur. Polym. J. 45, 1777 (2009)
A. Joshi, A. Bajaj, R. Singh, A. Anand, P.S. Alegaonkar, S. Datar, Compos. Part B 69, 472 (2015)
Acknowledgment
ANK acknowledges the financial support obtained from Higher Education Commission (HEC) Pakistan under the NRPU R&D Project-20-3052.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saboor, A., Khan, A.N., Cheema, H.M. et al. Effect of polyaniline on the dielectric and EMI shielding behaviors of styrene acrylonitrile. J Mater Sci: Mater Electron 27, 9634–9641 (2016). https://doi.org/10.1007/s10854-016-5021-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-016-5021-4