Abstract
In the present work, the effect of reflow time on wetting behaviour, microstructure and shear strength of the eutectic Sn–0.7Cu lead-free solder on Cu substrate were studied. The reflow time was varied from 10 to 10,000 s. The contact angle decreased with the increase in reflow time. The growth of intermetallic compounds (IMCs) increased with the reflow time. The thickness of the Cu6Sn5 IMC layer formed during a reflow time of 10 s was about 3.76 μm and its thickness increased to 3.89, 6.2, 6.68, 7.6 and 19.83 μm during 100, 300, 500, 1,000 and 10,000 s reflow time respectively. The joint shear test was performed to assess the integrity of Sn–0.7Cu solder solidified on copper substrate surfaces. The shear strength decreased with increase in reflow time after an optimum value.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The awareness of the adverse effects of toxic Pb on both health and the environment, coupled with the implementation of legislation to ban the use of lead based solders, has stimulated search for green electronic packaging using Pb-free solders. The eutectic Sn–0.7 wt% Cu solder alloy is one of the most favoured lead free alloys used for soldering in electronic applications. It is inexpensive and is principally used in wave soldering. In addition, this alloy is considered as a better alloy for flip chip applications compared to eutectic Sn–Ag and Sn–Ag–Cu [1–3]. Cu is the most common conductor metal, which is utilized as substrate in contact with solders owing to its good solderability characteristic and excellent thermal conductivity performance. Cu gets oxidized quickly when exposed to environmental condition, leading to reduction in wettability and solderability. Surface finishes are used to prevent the copper metallization on the printed circuit boards (PCB) from oxidizing, also to prevent contamination and mishandling prior to assembly. Typical pad metallizations include electroless nickel immersion gold (ENIG), organic solderability preservative (OSP), Ni–Au, Ni–Pd, immersion Sn (ImSn), immersion Ag (ImAg) etc. OSP finish can be used for short-term reliability applications since it is economical compared with ImSn, ENIG, and ImAg. OSP being an organic finish is always prone to contamination, which can lead to reliability problems. Applications prone to tin whisker formation should avoid ImSn finish. ImAg finish is highly sensitive to plating chemistry and hence is prone to champagne voids, and should be avoided as a primary choice for PCB finish [4, 5]. ENIG is preferable for long term reliability applications to ImSn, ImAg, and OSP finishes but during applications at higher frequencies skin effect comes into picture due to high magnetic permeability of nickel [6]. Hence pure copper was considered as the substrate in our present work.
An electronic package experiences various mechanical and thermal loads during manufacturing, storage, transport, and operation. The performance and quality of the solder are very essential to the integrity of a solder joint, which in turn is vital to the overall functioning of the assembly [1, 7]. During soldering, the solder alloy melts and then reacts with the substrate to form IMCs at the joint interface. The reliability of solder joints in electronic packaging is governed by the type and morphology of the interfacial IMCs formed between the solder and substrates during soldering. A good metallurgical bond can be achieved with the formation of a thin IMC layer, whereas excessive IMC growth may have a deleterious effect on the quality of the joint. This is due to inherent brittle nature of the IMC which has a greater tendency to generate structural defects. A large increase in the thickness of the IMC layer at the solder/substrate interface degrades the reliability of the solder joint. This implies that there exists a critical thickness of the IMC layer at which the mechanical properties of the solder joint are optimum [8–10]. With the longer reflow time, the solder joint results in the growth of thicker IMCs, leading to weakening of the solder joint strength. The failure of solder joints often occurs at the interface between the IMC and solder or between the IMC and metallic substrate, and consequently leads to the loss of function in interconnects and results in product failure [11]. It is therefore necessary to understand and control the factors that govern the kinetics of interfacial reaction.
Yoon et al. [2] studied the effect of reflow time on interfacial reaction and shear strength of Sn–0.7Cu solder/Cu joint for various reflow time between 1 s and 30 min at a fixed reflow temperature of 225 °C. Only Cu6Sn5 IMC layer was found to be formed at the interface and the morphology of the IMC changed from round or scallop to elongated scallop or rod shaped with the increase of reflow time. The thickness of the IMCs also increased with the increase of reflow time. In all the samples, the fracture mainly occurred in the bulk solder. Shear strength was found to be insensitive to the amount of IMCs formed at the interface even for an extended reflow time of about 30 min at 255 °C. Dariavach et al. [12] investigated the IMC growth kinetics Sn–0.7Cu on Cu substrates. A thick scallop layer of η-Cu6Sn5 and a thin layer of ε-Cu3Sn were observed at the interface. The total thickness of the IMC and the grain size of the η phase increased with increase in soldering time and IMC layer exhibited a decreasing growth rate with increasing time. Shin et al. [10] studied the effect of micro structural evolution and IMC layer growth on shear strength of Sn–1.5 wt% Cu/Cu and Sn–2.5 wt% Cu/Cu solder joints at a reflow temperature of 270 °C. The maximum shear strength was attained at the IMC layer thickness of about 1.2 μm corresponding to the reflow time of 15 s. At the critical IMC layer thickness, the shearing fracture occurred in the bulk of solder, indicating that the maximum shear strength of joints represents the bulk solder strength. The shear fracture of the joints occurred near the solder/IMC interface as the IMC layer grew over the critical thickness. Islam et al. [13] studied the interfacial reactions of Sn–0.7Cu solder with Ni/Au surface finished Cu pad for various reflow time of 5, 10, 30, 120, and 180 min at a fixed reflow temperature of 250 °C. At the time of ball shear test mostly ductile fracture was observed within the solder and solder—IMC interface for the reflowed samples. The solder gave relatively stable ball shear strength from 1.98 to 1.86 kgf over the whole duration of reflow. The shear strength value did not change much as a function of reflow time. Satyanarayan et al. [14] studied the solder joint reliability of Sn–0.7Cu on smooth and rough Cu substrate reflowed at 270 °C for 40 min. Long needle shaped Cu6Sn5 IMCs with a thickness of 8 μm were formed at the interface of solder and smooth Cu substrate where as short IMCs were found at the interface on rough Cu substrate. Solder on smooth Cu substrate gave a mean shear force of 341 N under shear test and suffered a ductile failure while solder on rough Cu substrate gave a mean shear force of 287 N and suffered a interfacial failure. Smooth surfaced copper substrates yielded higher solder joint strength compared to rough substrates.
The effect of reflow time on the microstructures and mechanical properties of the Sn–0.7Cu/Cu solder joint has not yet been sufficiently studied in detail. The present study is focused on the interfacial reaction between eutectic Sn–0.7Cu solder and Cu substrate and related joint reliability. The shear strengths of solder joints were determined as a function of the reflow time. The dependence of the shear strength on the corresponding interfacial reaction at the solder joints was investigated.
2 Experimental procedures
The commercial Sn–0.7 wt% Cu solder alloy (Multicore Manufacturers, UK) was used in the present study. The procured solder rod (Ø 6 mm) was drawn into solder wires having a diameter of about 1.4 mm. Spherical solder balls of weight 0.080 gm were obtained by melting the solder wire using a solder station (ERSA DIGITAL 2000 A). These solder balls were then used for wettability study by placing on Cu substrates (Ø 12 mm × 8 mm). Cu substrates were polished using SiC papers of different grit sizes (1/0–4/0 grade) and velvet cloth disk polish using diamond-lapping compound to obtain smooth surface finish. The surface profiles of substrates were measured using Form Talysurf 50 surface profiler. The arithmetic mean roughness (Ra) values were maintained in the range of 0.01 ± 0.006 μm. Contact angle measurements were carried out using FTA 200 dynamic contact angle analyzer. The equipment has a flexible video system for measuring contact angle, surface and interfacial tensions. Spherical balls of solder alloy were placed on the substrate and the solder/substrate system was kept inside the environmental chamber after coating the substrate surface with flux (Inorganic acid, Alfa Aesar, USA). Temperature of the environmental chamber was maintained at 270 °C throughout for all the experiment. The spreading process was recorded and the captured images were analyzed using FTA software (FTA 32 Video 2.0) to determine the wetting behavior of solder. Zeiss stereomicroscope (Stemi 2000-C) was used for obtaining macro images of the solder sessile drop after spreading. The shear test was performed on solder droplet samples by using the bond tester (Nordson DAGE 4000 Plus). The height of shear tool and the shear speed chosen were 1 μm and 200 μm/s. The force–displacement curves were recorded during the shear test. Shear test was performed at least three times to ensure consistency in results. After the ball shear test, the sheared surfaces were examined using stereo microscope and scanning electron microscope (SEM, JEOL JSM 6380LA) with energy dispersive spectroscopy (EDS) in back scattered electron mode for assessing the failure mode. The solder drop bonded to the substrate was sectioned along the axis and polished using SiC papers of different grit sizes. The final polishing was carried out on velvet cloth disc polisher using diamond lapping compound and then etched with 5 % nital (a mixture of C2H5OH and Conc. HNO3 in the ratio of 95:5) for about 3–5 s. The solder/substrate interfacial region was also examined using scanning electron microscope (SEM, JEOL JSM 6380LA). X-ray diffraction (XRD) study was carried out to identify and characterise the IMC at the solder/substrate interface using JEOL JDX-8P-XRD system.
3 Results and discussion
3.1 Spreading behaviour
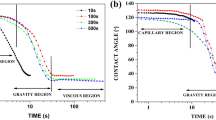
Dynamic contact analyser is depicted in Fig. 1 and typical relaxation curves for the spreading of Sn–0.7Cu solder on Cu substrate as a function of reflow time are presented in Fig. 2. Each experiment was repeated at least three times. The wetting or spreading behaviour of solder involves continuos change in the contact and drop base diameter with time. The contact angle decreased sharply up to 100 s indicating the start of solder dissolution and precipitation of IMCs during that period. The contact angle stabilized with further increase in time. As the environmental chamber attains the reflow temperature, the lattice distance between atoms increases due to thermal activation. The alloy transforms from solid to liquid state when the activation energy of solder atoms exceeds the forces between them in the solid state. Thus, the contact angle of solder suddenly drops in few seconds and the molten solder completely contacts the surface of Cu substrate followed by reactive wetting of liquid solder with copper. As the dissolution of molten solder completes, the excess Cu will be precipitated as intermetallic compounds (IMCs). The spreading of liquid solder stops as equilibrium state is approached. The contact angle and base radius (drop base area) of the solder alloy exhibited significant variation with time. Figure 3 presents the variation of the base radius of the solder with time. Spreading of the molten solder on Cu substrate occurred uniformly in the radial direction. The values of equilibrium contact angle obtained at the end of spreading of Sn–0.7Cu solder on Cu substrates at 270 °C are given in Table 1. The equilibrium contact angle decreased with increase in reflow time showing a better wetting of solder on the substrate. The macroscopic images (top view) of the stabilized droplets of Sn–0.7Cu solder on Cu substrate are shown in Fig. 4. Spreading of the solder alloy showed three regimes, namely, capillary, gravity and viscous regime with a sharp change in the spreading rate. Regimes of solders during spreading are identified by the change of slope in plot of ln D versus ln t, where D is the drop base diameter (mm) during spreading and t is the time (ms). The average spreading rate in each regime were calculated in terms of base diameter and found to be 1.025, 1.45 and 1 mm/ms in capillary, gravity and viscous regime respectively.
The wetting of a molten solder on metallic surfaces is a complex phenomenon. In addition to physical spreading due to surface tension reduction, diffusion and chemical reactions take place in the solder/substrate interfacial region. Wetting of the liquid solder on a substrate is accompanied by the formation of intermetallic compounds at the interface. Figure 4 shows a halo zone that appeared at the solder front Cu substrate and its area increased with the increase in reflow time. It is believed that the formation of the halo region is related to an interfacial reaction and to the formation of intermetallic layer [3, 15]. The instant of time when the halo starts to appear at the droplet edge corresponds to the transition from a fast spreading to a relatively slow spreading stage. This indicates that a resistance for the wetting kinetics has begun at the interface. This phenomenon may be related to the formation of an intermetallic layer [16]. The reliability of solder joints in electronic packaging is controlled by the type and morphology of interfacial IMCs formed between Sn–0.7Cu solder and substrates.
3.2 Interfacial microstructure of Sn–0.7Cu/Cu joint
Figure 5 shows the SEM micrographs of the interface between Sn–0.7Cu solder and Cu substrate reflowed at 270 °C for different reaction times. Back-scattered electron mode image of SEM was captured to show boundaries of the interfacial layers more distinct. At the initial stages of solder alloy spreading on the substrate, the metallic atoms from the metallic substrate (such as Cu) contacting to the molten solder, rapidly dissolve into the molten solder and quickly become supersaturated in the interface regions. Subsequently, the IMC phases start to nucleate and grow at the interface due to the local metastable equilibrium solubility of metallic atoms in the liquid solder alloy [12]. These IMCs grew into the solder field from the interface. The chemical composition of IMCs revealed that the IMCs were composed of Cu and Sn atoms. The atomic percentage of Cu decreases from the root to the tip of the IMCs. In all the reaction couples, Cu6Sn5 IMC was detected between the solder and Cu layer. Cu3Sn IMC with a layer-type morphology was observed between Cu6Sn5 layer and Cu substrate when the reflow time was 10,000 s. The Cu6Sn5 IMC formed on the Cu substrate at a reflow time of 10 s had a discontinuous but a well-known morphology of round scallop shape. At a reflow time of 100 and 300 s a continuos layer of IMC with round scallop morphology was formed. With an increase in the reflow time to 500 and 1,000 s, the round morphology of the IMC changed to elongated scallop morphology. At a reflow time of 10,000 s, faceted scallop morphology was formed. The increase in reaction times lead to the increase in the thickness of the Cu6Sn5 IMC layer. This is due to the greater dissolution of Cu atoms into the molten solder during longer reflow time intervals. The thickness of the Cu6Sn5 IMC layer formed during a reflow time of 10 s was 3.76 μm and its thickness increased to 3.89, 6.2, 6.68, 7.6 and 19.83 μm during 100, 300, 500, 1,000 and 10,000 s reflow time respectively. Thickness of the Cu3Sn layer formed during 10,000 s of reflow time was about 2 μm. The activation energy for the growth of Cu3Sn was found to be more compared to Cu6Sn5, thus explaining the slower growth rate of Cu3Sn [17]. According to Park et al. [18] Cu3Sn grows at the expense of Cu6Sn5 phase reacting with copper, only in the absence of sufficient supply of Sn. Figures 6 and 7 show the EDAX pattern of location represented by P1 and P2 in Fig. 5f. XRD analysis was carried out to confirm the composition of intermetallics formed at the solder/substrate interface. Figure 8 shows the XRD pattern of Sn–0.7Cu solder on Cu surface reflowed at 10, 100, 300, 500, 1,000 and 10,000 s. The XRD pattern confirmed the formation of Cu6Sn5 IMCs at the interface during all the reflow times and also confirmed the formation of Cu3Sn IMC exclusively during reflow for 10,000 s.
EDAX pattern of the position represented by P1 in Fig. 5f
EDAX pattern of the position represented by P2 in Fig. 5f
The thickening or growth kinetics of the IMC layer was studied. Figure 9 graphically represents the growth of intermetallic layer thickness as a function of square root of reflow time. The layer thickness is given by the equation
where w is the intermetallic layer thickness, t is the reflow time, k is the IMC growth rate constant, and w0 is the growth intercept of the IMC layer. The correlation coefficient value for this equation is 0.9947. The linear fit of the curve reveals that the growth of intermetallic layer follows the diffusion-controlled mechanism [15, 19].
3.3 Shear test results
Ball shear tests were performed to evaluate the effect of the interfacial reactions on the reliability of solder joints as a function of reflow time. During the shear test, the solder joint fractures along the weak point which indicates the failure mode of the solder joint. It was reported that, if the applied stress by the shear tool on the interface is higher than the solder strength, although the net interfacial stress during the shear test is lower than the interfacial adhesion strength, the solder ball will fail inside the solder. On the other hand, if the adhesion strength between the IMC layer and the substrate metallisation layer (or any other layer) is lower than the net interfacial stress during the shear test, the solder ball will fail through the interface [20]. Figure 10 shows the force–distance curves obtained during the shear test on Sn–0.7Cu/Cu surfaces and the error bars indicate the standard deviation in the shear strength values while carrying out three sets of tests. The maximum shear force obtained from the joint shear testing experiment is given in Table 1. Force versus distance curve exhibited a gradual decrease after reaching a peak value. Shear force was maximum for solder/substrate reflowed for 100 s. A drop in shear force was observed for the samples reflowed for higher time intervals in the later stages showing the existence of a optimum reflow time between 100 and 300 s. In addition, a reduction in the shear force for the solder joints was recorded with increasing thickness of interfacial Cu–Sn IMC layer. This is mainly due to the continuous depletion of Sn from the bulk Sn–Cu solder during the growth of interfacial Cu–Sn IMC at the solder joint. This will lead to a lower tin content and hence lower the joint strength as long as the fracture crack is confined in the bulk solder [21]. The shear energy was calculated by measuring the area under the force–displacement curve. The shear energy values obtained from the joint shear testing experiment are given in Table 1. All the samples exhibited ductile failure. A gradual decrease observed in the force versus distance curve, instead of a sudden failure signifies ductile failure. Shear marks on the fractured surfaces indicated the occurrence of failure predominantly in the bulk of the solder regardless of the reflow time. Fractured surface analysis of surface using EDAX confirmed that fracture has taken place in the bulk solder. Each test was performed three times. Figure 11 shows the SEM images of fractured surfaces. Shear marks indicate the direction of the shear action. For the samples reflowed for 10,000 s, hardly one or two dimples were found on the fractured surface (Fig. 11h). The needle shaped IMCs that were intact with shear loading were found inside the dimple. These needle shape intermetallics were confirmed to be Cu6Sn5 by EDS analysis. It clearly indicates that, the IMCs that are formed at the interface are not completely exposed to the shear. The results of the fracture analysis showed that the shear strength could not be explicitly related to the thickness of the IMC layer.
4 Conclusion
In this study, interfacial reactions and shear strength between eutectic Sn–0.7 wt% Cu solder ball and Cu substrate were studied as a function of reflow time. The reflow temperature was 270 °C and reflow times were 10, 100, 300, 500, 1,000 and 10,000 s. Contact angle decreased with the increase in the reflow time. Shear force showed a maximum value for the samples reflowed at 100 s. Cu6Sn5 was found to be the only intermetallic compound that formed at the interface in samples reflowed for 10, 100, 300, 500 and 1,000 s. Cu3Sn layer was formed between Cu6Sn5 and Cu substrate for samples reflowed at 10,000 s. The IMC thickness increased with the increase in reflow time and the morphology of IMC changed from round scallop to elongated and then to faceted scallop. The examination of sheared surfaces indicated ductile mode of failure in the bulk solder. A reflow time of 100 s significantly improved the integrity of solder joints. The investigation clearly suggests that good wettability does not necessarily imply a higher shear strength. An optimum reflow time is essential for the development of the peak shear strength.
References
J. Yoon, D. Kim, S. Kim, S. Jung, J. Koo, J. Electron. Mater. 33, 1190 (2004)
J. Yoon, S. Kim, S. Jung, J. Alloys Compd. 385, 192 (2004)
Satyanarayan, K.N. Prabhu, Adv. Colloid Interface Sci. 166, 87 (2011)
A. Choubey, H. Yu, M. Osterman, M. Pecht, F. Yun, L. Yonghong, X. Ming, J. Electron Mater. 37, 1130 (2008)
M. Sona, K.N. Prabhu, J. Mater. Sci. Mater. Electron. 24, 3149 (2013)
A. Aggarwal, P.M. Raj, B. Lee, M. J. Yim, A. Tambawala, M. Iyer, M. Swaminathan, C. P. Wong, T. Rao, in Proceedings of the Electronic Components and Technology Conference, 2007, pp. 905–913
M. Abtew, G. Selvaduray, J. Mater. Sci. Eng. R. 27, 95 (2000)
J. Shen, Y.C. Liu, H.X. Gao, J. Mater. Sci. 42, 5375 (2007)
H. Tsukamoto, T. Nishimura, S. Suenaga, K. Nogita, J. Mater. Sci. Eng. B. 171, 162 (2010)
C.K. Shin, Y.J. Baik, J.Y. Huh, J. Electron Mater. 30, 1323 (2001)
D. Kim, J. Kim, S. Ha, B. Noh, J. Koo, D. Park, M. Ko, S. Jung, J. Alloys Compd. 458, 253 (2008)
Z. Dariavach, P. Callahan, J. Liang, R. Fournelle, J. Electron Mater. 35, 1581 (2006)
M.N. Islam, Y.C. Chan, J. Mater. Sci. Eng. B. 117, 246 (2005)
K.N. Prabhu, Satyanarayan, Mater. Sci. Technol. (2013). doi:10.1179/1743284713Y.0000000287
J. Liang, N. Dariavach, P. Callahan, D. Shangguan, Mater. Trans. 47, 317 (2006)
H. Zhao, D.R. Nalagatla, D.P. Sekulic, J. Electron Mater. 38, 284 (2009)
X. Ma, F. Wang, Y. Qian, F. Yoshida, Mater. Lett. 57, 3361 (2003)
Y.W. Park, T.S.N. Sankara Narayanan, K.Y. Lee, Wear 262, 320 (2007)
M.J. Rizvi, C. Bailey, Y.C. Chan, M.N. Islam, H. Lu, J. Alloys Compd. 438, 122 (2007)
J.W. Yoon, S.B. Jung, Mater. Sci. Technol. 25, 1478 (2009)
Y.C. Chan, C.K. Alex So, J.K.L. Lai, J. Mater. Sci. Eng. 55, 5 (1998)
Acknowledgments
Authors thank the Defence Research Development Organization (DRDO), Govt. of India, New Delhi for the financial support under a Research Grant: ERIP/ER/1006009M/01/1356.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sona, M., Prabhu, K.N. The effect of reflow time on reactive wetting, evolution of interfacial IMCs and shear strength of eutectic Sn–Cu solder alloy. J Mater Sci: Mater Electron 25, 1446–1455 (2014). https://doi.org/10.1007/s10854-014-1749-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-014-1749-x