Abstract
Solder interconnect reliability is exceedingly influenced by the solder material properties, the intermetallics formed during soldering, and the environmentally imposed loads. The current study involves the study of the wetting behaviour, interfacial reactions and growth of intermetallic compounds (IMCs) in Sn–2.5Ag–0.5Cu lead free solder solidified on Cu substrate as function of reflow time. The reflow temperature was maintained at 270 °C and reflow time was varied from 10 to 10,000 s. The solder alloy showed better wettability on the substrate with the increase in reflow time. A growth in Cu6Sn5 IMC thickness was observed up to a reflow time of 500 s. IMC layer formed during a reflow time of 10 s was 3.09 μm and its thickness increased to 4.40, 8.21, 10.96 μm during 100, 300 and 500 s reflow time respectively. A decrease in the thickness of IMC was observed for samples reflowed for 1000 s. The IMC thickness increased with further increase in reflow time. The joint reliability of Sn–2.5Ag–0.5Cu solder solidified on copper substrate surfaces was assessed by performing bond shear test. The average shear strength decreased with an increase in reflow time after an optimum value.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The Pb–Sn alloys have been the mainstay of the soldering technology for past several decades due to their distinct blend of material properties and low cost [1]. Recently, there has been much activity in the development of Pb-free solder alloys for microelectronic packaging applications, due to concern over the toxicity of Pb in Pb–Sn solders [2]. Nevertheless, Pb-free solder reveals superior mechanical properties in terms of maximum strength and ductility when compared to Sn-Pb solder alloy [3]. Among the currently considered lead-free solder alloy compositions, Sn–Ag–Cu alloys are the most established alternatives for Pb-bearing solders [4]. Silver (Ag) plays a significant role in decreasing the melting temperature of the Sn–Ag–Cu alloy where as Cu increases the mechanical properties [5, 6]. Advances in packaging technologies are driven by the aspiration for miniaturization and increased circuit speed. The severe operating conditions under which components have to perform raise reliability concerns in solder joint [7]. Cu is the most common conductor metal, which is utilized as substrate in contact with solders owing to its good solderability characteristic and high thermal conductivity [8]. To form a proper metallurgical bond between two metals, wetting must take place. This implies a specific interaction between the liquid solder and the solid surface of the parts to be soldered. Intermetallic compound (IMC) formation at the solder/substrate interface is the result of reactive reaction between solder and the substrate [9, 10]. The nucleation and growth of intermetallic compounds (IMC) are vital steps in the formation of reliable solder joints. Therefore existence of IMC is a sign of an adequate metallurgical bond in solder joints and is critical in determining the reliability of the soldered joint during its service period [11].
The present investigation is aimed at the study of the interfacial reaction between Sn–2.5Ag–0.5Cu solder and Cu substrate and the related joint reliability as a function of the reflow time. Shear test was performed to assess the joint reliability. Shear strength values signify a measure of the joint strength under shear stress.
2 Experimental Procedure
Sn–2.5Ag–0.5Cu solder alloy was procured in wire form (Ø 0.8 mm) from Antex Electronics Manufacturers, UK. The solder wire was melted using a solder station (ERSA DIGITAL 2000 A) to prepare spherical solder balls of weight 0.080 g. These solder balls were used for wettability study by placing on Cu substrates. Copper substrates (Ø12 mm × 8 mm) were prepared from rolled round bar of EC grade copper (99.9 % purity) procured from Hi Tech Sales Corporation, Mangalore. Copper substrates were polished using SiC papers of various grit sizes (1/0–4/0 grade) and velvet cloth disk polish using diamond-lapping compound to achieve smooth surface finish. Form Talysurf 50 surface profiler was used to measure the surface profiles of substrates. The arithmetic mean roughness (Ra) values were maintained in the range of 0.01 ± 0.006 µm. Contact angle measurements were carried out using FTA 200 dynamic contact angle analyzer. The equipment has a flexible video system for measuring contact angle. Spherical balls of solder alloy were positioned on the substrate and the solder/substrate system was kept inside the environmental chamber after coating the substrate surface with an inorganic acid flux (Alfa Aesar, USA). The temperature of the environmental chamber was maintained at 270 °C throughout the experiment. The spreading process was recorded and the captured images were analyzed using FTA software (FTA 32 Video 2.0) to determine the wetting behavior of solder. Figure 1 shows the schematic sketch of the experimental set-up for measurement of contact angle and Fig. 2 shows the photograph of the dynamic contact angle analyzer (FTA 200 Å). The shear test was performed on solder droplet samples by using the Nordson DAGE 4000Plus bond tester (Fig. 3). The height of shear tool and the shear speed chosen were 1 µm and 200 µm/s respectively. The force–displacement curves were recorded during the shear test. Shear test was performed at least on three samples to ensure consistency in results. After the ball shear test, the sheared surfaces were examined using scanning electron microscope (SEM, JEOL JSM 6380LA) with energy dispersive spectroscopy (EDS) in back scattered electron mode for assessing the failure mode. The solder drop bonded to the substrate was sectioned along the axis and mirror polished and then etched with 5 % nital (a mixture of C2H5OH and Conc. HNO3 in the ratio of 95:5) for about 3–5 s. The solder/substrate interfacial region was also examined using scanning electron microscope (SEM, JEOL JSM 6380LA).
3 Results and Discussion
3.1 Spreading Behavior and Interfacial Microstructure of Sn–2.5Ag–0.5Cu/Cu Joint
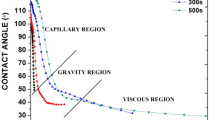
The relaxation behavior and various regimes involved during the spreading of Sn–2.5Ag–0.5Cu solder on copper substrate at 270 °C for various reflow times are shown in Fig. 4. The various regions involved during spreading of Sn–2.5Ag–0.5Cu on Cu substrate are capillary, diffusion/reaction and viscous zones. Average relaxation rates in capillary, reaction and viscous zones are 2.94 ± 0.05, 0.32 ± 0.1 and 0.01°/s respectively. As the environmental chamber attains the reflow temperature, solder starts melting. Capillary zone exists for a period of about 8 s of reflow time. This zone is followed by the diffusion/reaction zone which ranges between 8 and ≈100 s of reflow time and involves reactive wetting. As the dissolution of molten solder completes, the excess Cu gets precipitated as intermetallic compounds (IMCs). Once Cu6Sn5 grains nucleate, they are more inclined to grow laterally covering the complete Cu substrate within few seconds [12]. Figure 5a shows the formation of a continuous layer of Cu6Sn5 on Cu substrate during a reaction time of 10 s. The third region of solder spreading is viscous zone. The spreading process approaches equilibrium in this region and solder spreading completely ceases. The final contact angle (θf) attained at the completion of the spreading is given in Table 1. The final contact angle decreases with the increase in reflow time.
Figure 5a–f show SEM images of the Sn–2.5Ag–0.5Cu/Cu interface reflowed at 270 °C for various reflow times. Cu atoms from the substrate quickly diffuse into the molten solder and get dissolved during initial stages of soldering. Dissolution is a nonequilibrium process [13]. After a small interval of time, the layer of molten solder, adjacent to the Cu metal becomes supersaturated with the dissolved Cu throughout the interface. Thermodynamically, at the local equilibrium solubility, the solid IMCs begin to form in this part of the solder. Metal solutes were taken out of the saturated liquid solder for the formation of IMCs resulting in further dissolution of the contacted metal, particularly if the intermetallic layer is not uniform on top of the substrate [12]. Hence a change in morphology of IMCs was observed with the reflow time. The primary IMC found at the interface was Cu6Sn5. EDS analysis confirmed the formation of Cu6Sn5 at the interface of all Sn–2.5Ag–0.5Cu/Cu system in the present study. Sn–2.5Ag–0.5Cu/Cu exhibited both Cu6Sn5 and Ag3Sn IMCs in the bulk of the solder alloy and at the interface, Cu6Sn5 IMCs protruded into the solder matrix with few precipitates of Ag3Sn. A thin layer of Cu3Sn IMC was found underneath the Cu6Sn5 IMCs at the interface during reflow for 10,000 s. Table 2 gives the EDS analysis results of marked regions in Fig. 5f for Sn–2.5Ag–0.5Cu solder on Cu substrate. The presence of Ag3Sn IMCs is due to the presence of Ag in solder alloy. Figure 6 shows the XRD pattern of Sn–2.5Ag–0.5Cu solder on Cu surface reflowed at 10, 100, 300, 500, 1000 and 10,000 s. The XRD pattern confirmed the formation of Cu6Sn5 IMCs at the interface during all the reflow times and the formation of Cu3Sn IMC only during reflow for 10,000 s. IMC exhibited scallop shape morphology at the interface during a reflow for 10 s. With an increase in reflow time to 100 s, the morphology of the IMC changed from continuous scallop to long needle morphology and then to coarse needle morphology for the samples reflowed for 300 and 500 s. The interfacial IMCs had highly coarse needle morphology for samples reflowed for 1000 and 10,000 s. The increase in reaction times lead to the increase in the thickness of the Cu6Sn5 IMC layer. The thickness of the Cu6Sn5 IMC layer formed during a reflow time of 10 s was 3.09 μm and its thickness increased to 4.40, 8.21, 10.96, 6.62 and 14.66 μm during 100, 300, 500, 1000 and 10,000 s reflow time respectively. The thickness of Cu3Sn IMC was measured and it was found to be 2.53 μm. The thickness of Cu6Sn5 decreases at the beginning of formation of Cu3Sn at the interface. This is because at the beginning of formation process, Cu3Sn grows at the expense of Cu6Sn5. On formation of the Cu3Sn layer, Cu6Sn5 layer starts growing again, due to the reaction between Cu atoms diffusing through the intermetallic layers and Sn atoms from the solder [13]. In our present work, we have noticed a drop in Cu6Sn5 IMC thickness for the samples reflowed for 1000 s and later a growth in thickness of the same layer was observed for samples reflowed for 10,000 s. The observed drop in thickness is attributed to the initiation of Cu3Sn formation.
3.2 Shear Test Results
The effect of reflow time on joint reliability was assessed by conducting a joint shear test. Force Vs distance curves obtained during the joint shear test of Sn–2.5Ag–0.5Cu solidified on Cu substrate are shown in Fig. 7. The error bars represent the standard deviation in shear strength values obtained whilst carrying out three sets of experiments for consistency. Table 1 gives the average shear force values obtained during the joint shear test and shear energy was calculated by measuring the area under the force–displacement curve. Average shear force shows a maximum value for samples reflowed for 500 s. The shear force showed a maximum value for Sn–2.5Ag–0.5Cu/Cu system when compared to Sn–0.7Cu/Cu [8]. The increase in shear force and shear energy for the Ag bearing solder is mainly due to the presence of minor Ag content. A decrease in average shear force value is observed for samples reflowed for 1000 and 10,000 s in the subsequent stages indicating the existence of an optimum reflow time between 300 and 500 s. A steady decline in the force Vs distance curve instead of a sudden breakdown indicates a ductile failure in the sample. Figure 8 shows the SEM images of fractured surfaces. EDS analysis on the fractured surface shows maximum Sn content indicating that fracture has occurred in the bulk solder. Figure 9 shows the EDS analysis results of the fractured surface. A definite relationship between the IMC thickness and joint shear strength was not found in solder/substrate systems reflowed for various reflow times. All the samples showed fracture in bulk solder rather than in the interface even in the presence of IMCs of varying thickness.
EDS area analysis result of fractured surface shown in Fig. 8g
4 Conclusion
The interfacial reaction and joint shear strength of Sn–2.5Ag–0.5Cu solder solidified on Cu substrate were studied as a function of reflow time ranging from 10 to 10,000 s at 270 °C reflow temperature. An increase in the Cu6Sn5 IMC thickness was observed up to a reflow time of 500 s and the samples reflowed for 500 s showed maximum value of average shear strength. Samples reflowed for 1000 s showed a decrease in Cu6Sn5 IMC thickness where as the sample reflowed for 10,000 s showed an increment in Cu6Sn5 IMC thickness. A Cu3Sn layer was also found at substrate in addition to the Cu6Sn5 layer. A drop in shear strength was observed for samples reflowed beyond 500 s. Reflow for 500 s showed considerable improvement in the solder joint reliability. The fracture occurred in the bulk solder for all samples.
References
Hammad A E, Mater Des 50 (2013) 108.
Chen T, and Dutta I, J Electron Mater 37 (2008) 347.
Keller J, Baither D, Wilke U, and Schmitz G, Acta Mater 59 (2011) 2731.
Watanabe H, Hidaka N, Shoji I, and Ito M, in Proceedings of Materials Science and Technology (MS&T) (2006) 135.
Lee J H, Yu A M, Kim J H, Kim M S, and Kang N, Met Mater Int 14 (2008) 649.
Sona M, and Prabhu K N, J Mater Sci Mater Electron 24 (2013) 3149.
Lee J G, and Subramanian K N, J Electron Mater 32 (2003) 523.
Sona M, and Prabhu K N, J Mater Sci Mater Electron 25 (2014) 1446.
Mookam N, and Kanlayasiri K, J Mater Sci Technol 28 (2012) 53.
Abtew M, and Selvaduray G, Mater Sci Eng 27 (2000) 95.
Chung C K, Duh J G, and Kao C R, Scr Mater 63 (2010) 258.
Laurila T, Vuorinen V, and Kivilahti J K, Mater Sci Eng R 49 (2005) 1.
Lee L M, and Mohamad A A, Adv Mater Sci Eng. doi:10.1155/2013/123697.
Acknowledgments
Authors thank the Defence Research Development Organization (DRDO), Govt. of India, New Delhi for the financial support under a research Grant: ERIP/ER/1006009M/01/1356.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sona, M., Prabhu, K.N. Assessment of Joint Reliability of Sn–2.5Ag–0.5Cu Solder/Cu as a Function of Reflow Time. Trans Indian Inst Met 69, 941–947 (2016). https://doi.org/10.1007/s12666-015-0590-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12666-015-0590-0