Abstract
The γ-LiFeO2 micro-cubes were synthesized using a simple solid-state method. Their electrochemical performance as anode material for lithium ion batteries was firstly investigated. Pure γ-LiFeO2 without nanosizing or carbon coating can deliver the first discharge capacity of 1055.3 mAh/g, and 611.5 mAh/g can be maintained after 50 cycles, around 80 % of the second discharge capacity. γ-LiFeO2 also demonstrates nice rate capabilities. These results indicate that γ-LiFeO2 is a promising anode material candidate for lithium ion batteries. The charge/discharge mechanism was also investigated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Energy storage and conversion is one of the hottest topics both in the academic and industrial societies [1–3]. Especially, lithium ion batteries (LIBs) are extensively studied because of their versatile advantages, like light weight, no pollution and high energy density. Their electrochemical properties depend largely on the electrochemical activities of the electrode materials. So, it is being continuously interesting to look for new electrode materials to meet the updating requirements for LIBs in modern society.
Lithium intercalation compounds with the formula LiMO2 (M: Fe, Co, Ni and Mn) are one type of attractive candidate cathodes for LIBs because of the relatively easy intercalation and de-intercalation of Li+ ions [4, 5]. Among them, LiCoO2 is one of the most successful cathode materials that has been largely commercialized. However, its intrinsic drawbacks, like less available Co source and toxicity, restrict its further applications. Accordingly, Fe-based electrode materials should be promising because of the more available and non-toxic Fe source [6–10].
Until now, there are ten polymorphs of LiFeO2 have been discovered as listed in Table 1 and Fig. 1. They are: (1) α-LiFeO2 (Fm-3m Footnote 1, Pearson code cF8) with the cubic NaCl-type structure;8 (2) β-LiFeO2 (I4/mmm, Pearson code tI4) with the tetragonal CoO-type structure;9 (3) β’-LiFeO2 (C2/c, Pearson code mS32) with the monoclinic NaErO2-type structure;9 (4) γ-LiFeO2 (I41/amd, Pearson code tI16) with novel tetragonal structure [12]; (5) layered LiFeO2 (R-3m, hR12) with the rhombohedral α-NaFeO2-type structure [13, 14]; (6) goethite-type LiFeO2 (Pbnm, oP12) with the α-FeOOH-type structure [15]; (7) hollandite-type LiFeO2 (I4/m, tI24) with the β-FeOOH-type structure [15]; (8) corrugated layer LiFeO2 (Pmmn, oP8) with the orthorhombic β-NaMnO2-type structure [16]; (9) ordered rock-salt LiFeO2 (Fd-3m, cF64) with the LiTiO2-type structure [11, 14] and (10) T-LiFeO2 (Pna21, oP16) with the orthorhombic β-NaFeO2-type structure [17]. Among them, only several of them have been reported being electrochemical-active. Especially for α-LiFeO2, its electrochemical behaviors have been investigated extensively. While for γ-LiFeO2, a superstructure polymorph of α-LiFeO2, its electrochemical performance was particularly depressed when it was employed as the cathode material for LIBs [12, 15].
It is well known that transition-metal oxides are one type of most promising anode materials for LIBs [18, 19], and iron oxides have attracted much interest among them because of the advantages of Fe element [20–22]. Comparing LiFeO2 with Fe2O3, it is possible to employ the former as anode material, too. In fact, there are several reports about the application of α-LiFeO2 as anode recently. [23–27]. However, so far, there is no such study on γ-LiFeO2, which may be caused by the facts that γ-LiFeO2 has very bad electrochemical performance and γ-phase can’t be experimentally easily available.
Recently, γ-LiFeO2 micro-cubes were occasionally obtained by us using a simple solid state method. Later on, the synthesis method was optimized and pure γ-LiFeO2 micro-cubes could be obtained reproducibly. Here, the synthesis, various characterizations and electrochemical performance of γ-LiFeO2 as the anode material for LIB are firstly reported and discussed.
Experimental
Synthesis and material characterization
Pure γ-LiFeO2 was synthesized using a facile solid state method. 158.2 mg Li2CO3 (99.0 %, aladdin) and 341.8 mg Fe2O3 (99.9 %, aladdin) with the molar ratio of 1:1 were mixed, ground to a fine powder and pressed to a pellet, then the pellet was placed into a corundum crucible, heated in a muffle furnace from room temperature to 600°C in 4 hours and maintained for 48 hours.
Powder X-ray diffraction (PXRD, Bruker D8 Advance) pattern was collected at 40 kV and 100 mA for CuKα radiation (λ = 1.5406 Å) with a scan speed of 5°/minutes. Field emission scanning electron microscopy (FE-SEM, Hitachi S-4800II) and transmission electron microscopy (TEM, Philips Tecnai12) were used to investigate the sample’s morphology. Raman spectra (Renishaw inVia) measurement was done assuming a linear baseline in the spectral range 200–2000 cm−1.
Electrochemical characterization
The electrode slurry was prepared using γ-LiFeO2, super C65 and polyvinylidene fluoride (PVDF) with the molar ratios of 7: 2: 1 dissolved in N,N-2-methyl pyrrolidone (NMP). The slurry was magnetically stirred for 1 day, then it was coated onto a Cu foil, dried at 120 °C for overnight using a vacuum drying oven and cut the dried foil into Φ1.6 cm disks. The weight of electrode material is around 3 mg. The CR-2032 type coin cell was assembled using a Li foil as the counter electrode and a Celgard 2325 film as the separator in a glovebox filled with argon. The electrolyte used was 1 M LiPF6 in a mixed solvent of DEC and EC with a volume ratio of 1:1. The galvanostatic charge/discharge tests were carried out at a constant current density of 0.1 C in the potential range of 0.01–3 V using NEWARE CT-3008 charge–discharge system. The cells were first discharged to 0.01 V, and then the cycle number was counted. Cyclic voltammetry (C–V) (electrochemical workstation, CHI660D) curves were measured in 0.01–3 V with the scan rate of 0.5 mV/s.
Results and discussion
It has to be mentioned that the synthesis of γ-LiFeO2 has to be handled very seriously. Even small adjustment of the heating profiles, the ratios of the raw materials and the insufficient grind would result in the failure to get γ-phase. The solid-state method employed here is really simple, and scalable γ-LiFeO2 micro-cubes could be obtained without any impurities.
The crystal structure of γ-LiFeO2 is shown in Fig. 2. It crystallizes in the tetragonal space group I41/amd representing a new structure type with Pearson symbol tI16. There is only one Fe, one Li and one O atom in its crystallographically independent unit. Both metal atoms coordinate with six O atoms to form distorted octahedra. Each FeO6 octahedron shares edge with four FeO6 octahedra and corner with the other four FeO6 octahedra. The whole structure can be described as FeO6 octahedra constructed 3-D network and Li+ ions occupy the octahedral interspaces.
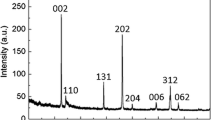
The PXRD pattern (Fig. 3) confirms that the as-prepared sample is pure γ-LiFeO2 as the measured one is well matched with the calculated one. The calculated PXRD pattern is simulated from the structure data of γ-LiFeO2 (ICSD No. 174086). The SEM image (Fig. 4a) indicate that the γ-LiFeO2 particles are cube-like with the diameter around 100–300 nm, while most of them are less than 200 nm. The high resolution TEM image (Fig. 4b) showing the uneven sizes of γ-LiFeO2 cubes are in good agreement with the SEM images. The lattice fringes of γ-LiFeO2 micro-cubes are obviously visible (Fig. 4c).
The charge–discharge curves of γ-LiFeO2 (Fig. 5) for the first three cycles indicate the work potential is around 1.1 V vs Li+/Li, close to the value of α-LiFeO2 [23–27]. The Coulombic efficiencies of γ-LiFeO2, Fe2O3 and Fe3O4 is 72.89, 68.30 and 51.66 % in the first cycle, indicating that it can effectively increase the first cycle’s Coulombic efficiency via compensating the lithium loss in the first cycle by adding some lithium into the anode, namely, from iron oxides to LiFeO2. Further, the Coulombic efficiency of γ-LiFeO2 is close to 100 % during the following cycles.
The cycling data at 0.1 C (Fig. 6) shows that the initial discharge capacity can be reached to 1055.3 mAh/g, higher than its theoretical capacity of 848 mAh/g. However, the first charge capacity is only 769.2 mAh/g, indicating that there is irreversible 286.1 mAh/g capacity loss. The discharge and charge capacities after the first cycle are reduced to 765.2 and 725.4 mAh/g, respectively. After the 50th cycle, there is 611.5 mAh/g capacity residual, which is 79.9 % of the 2nd cycle’s value. Except for the first cycle, the Coulombic efficiencies are very close to 100 % for all the 50 cycles, indicating its excellent reversible electrochemical reactions.
Compared to the electrochemical results of α-LiFeO2 as anode material, γ-LiFeO2′s capacity after 50 cycles is lower than those values of α-LiFeO2 and α-LiFeO2/C nanofibers with 3-D nano-architectures [23]; however, the capacity retention of γ-LiFeO2 is better. The lower capacity should be ascribed to the pristine γ-LiFeO2 without any nanosizing or carbon coating. We also tried to prepare the γ-LiFeO2/C composite, however, γ-phase was failed to be obtained once the carbon material introduced. The further optimization of the electrochemical performance of γ-LiFeO2 is still underway.
The rate performance of γ-LiFeO2 (Fig. 7) was studied at rates from 0.1 to 10 C with the interval of ten cycles, and finally return to 0.1 C. The highest discharge capacities are 1018, 660, 563, 428, 399, 378, 185, 68 and 705 mAh/g at 0.1, 0.2, 0.4, 0.8, 1, 2, 5, 10 and 0.1 C, respectively. When return to 0.1 C, the capacity can be recovered to 700 mAh/g, much close to the value attained without high-rate measurement.
The C-V (Fig. 8) measurements for the first three curves were recorded in 0.01–3 V. For the first cycle, the cathodic peak appears at around 0.55 V, lower than that of α-LiFeO2 and caused by the reduction of Fe3+ to Fe, the corresponding electrochemical reaction can be expressed as the reaction γ-LiFeO2 + 3e− + 3Li+ → 2Li2O + Fe [23–27]. The broad oxidation peak appeared at around 1.70 V, which might be consisted of two neighboring peaks according to its shape. It corresponds to the oxidation of Fe0 to Fe2+, and further to Fe3+ [23–27]. After the 1st cycle, γ-LiFeO2 demonstrates nice electrochemical reversibility as evidenced by the almost completely overlap of the C–V curves for the 2nd and 3rd cycles. The cathodic peak moves to 0.92 V for the latter two cycles, while the anodic peak keeps almost voltage unchanged, just the shape altered. Comparing the first three C–V curves of γ- and α-LiFeO2, it can be concluded that their shapes are similar, just the cathodic peaks’ locations are a little different. The subtle difference between the electrochemical mechanisms for γ-LiFeO2 and α-LiFeO2 might not be explained from their crystal structures, as the former has much worse electrochemical behavior than that of the latter when employed as cathode materials.
To better understand the first charge and discharge mechanism, two cells only undergone the first discharge and the first cycle, respectively, were opened and washed to get the anode materials, scraped the active materials from the copper foil, which were then subsequently characterized by powder XRD measurements. As shown in Fig. 9a, both the XRD peaks after first discharge and first charge become amorphous. Considering the C–V curves’ similarity of α- and γ-LiFeO2 after the first cycle, it could be presumed that γ-LiFeO2 should be amorphous after the first cycle, too. These results are also verified by the Raman spectra (Fig. 9b). It shows the existence of γ-LiFeO2 and carbon before electrochemical measurement, and the Raman peaks of γ-LiFeO2 disappeared after the first cycle.
The subtle difference between the electrochemical mechanisms for γ-LiFeO2 and α-LiFeO2 might not be explained from their crystal structures, as the former has much worse electrochemical behavior than that of the latter when employed as cathode materials. Here, the electrochemical data of γ-LiFeO2 as anode material was firstly studied with focusing on its facile synthesis method and promising electrochemical behavior.
Conclusion
In summary, a simple solid-state method was employed to synthesize γ-LiFeO2 micro-cubes. γ-LiFeO2 firstly studied here as the anode material demonstrates promising electrochemical behaviors. Without nanosizing or carbon coating efforts, it has the first discharge capacity of 1055.3 mAh/g, and 611.5 mAh/g can be maintained after 50 cycles, around 80 % of the second discharge capacity. It can be expected that its electrochemical performance will be largely enhanced through nano- or doping techniques. The electrochemical study of γ-LiFeO2 is still in its infancy. We hope this work can stimulate more studies on various polymorphs of “cathode material” LiFeO2 used as anode material for LIBs.
Notes
Almost all the literature described the space group of α-LiFeO2 as Fm3m, however, Fm-3m should be the right assignment after careful check of the data from inorganic crystal structure data (ICSD), Pearson’s crystal data (PCD) and related literature
References
Trogadas P, Ramani V, Strasser P, Fuller TF, Coppens MO (2015) Hierarchically structured nanomaterials for electrochemical energy conversion. Angew Chem Int Ed 54:122–148
Vlad A, Singh N, Galande C, Ajayan PM (2015) Design considerations for unconventional electrochemical energy storage architectures. Adv Energy Mater 5:201402115
Hu CG, Song L, Zhang ZP, Chen N, Feng ZH, Qu LT (2015) Tailored graphene systems for unconventional applications in energy conversion and storage devices. Energy Environ Sci 8:31–54
Wu NT, Zhang Y, Guo Y, Liu SJ, Liu H, Wu H (2016) Flakelike LiCoO2 with exposed 010 facets as a stable cathode material for highly reversible lithium storage. ACS Appl Mater Interfaces 8:2723–2731
He P, Yu HJ, Li D, Zhou HS (2012) Layered lithium transition metal oxide cathodes towards high energy lithium-ion batteries. J Mater Chem 22:3680–3695
Kang B, Ceder G (2009) Battery materials for ultrafast charging and discharging. Nature 458:190–193
Barpanda P, Nishimura S, Yamada A (2012) High-voltage pyrophosphate cathodes. Adv Energy Mater 2:201100772
Yan MY, Zhang GB, Mai LQ (2016) In operando observation of temperature dependent phase evolution in lithium-incorporation olivine cathode. Nano Energy 22:406–413
Zhang L, Xiang HF, Wang HH (2012) Synthesis of LiFePO4/C composite as a cathode material for lithium-ion battery by a novel two-step method. J Mater Sci 47:3076–3081
Muruganantham R, Sivakumar M, Subadevi R, Wu NL (2015) A facile synthesis and characterization of LiFePO4/C using simple binary reactants with oxalic acid by polyol technique and other high temperature methods. J Mater Sci Mater Electron 26:2095–2106
Vucinic-Vasic M, Antic B, Blanusa J, Rakic S, Kremenović A, Nikolic AS, Kapor A (2006) Formation of nanosize Li-ferrites from acetylacetonato complexes and their crystal structure, microstructure and order-disorder phase transition. Appl Phys A 82:49–54
Barré M, Catti M (2009) Neutron diffraction study of the β’ and γ Phases of LiFeO2. J Solid State Chem 182:2549–2554
Chappel E, Holzapfel M, Chouteau G, Ott A (2000) Effect of cobalt on the magnetic properties of the LiFe1−xCoxO2 layered system (0 ≤ x ≤ 1). J Solid State Chem 154:451–459
Hirayama M, Tomita H, Kubota K, Kanno R (2011) Structure and electrode reactions of layered rocksalt LiFeO2 nanoparticles for lithium battery cathode. J Power Sources 196:6809–6814
Li JG, Li JJ, Luo J, Wang L, He XM (2011) Recent advances in the LiFeO2-based materials for Li-ion batteries. Int J Electrochem Sci 6:1550–1561
Catti M, Montero-Campillo M (2011) Lithium diffusion pathways and vacancy formation in the Pmmn Li1−xFeO2 electrode material. Phys Chem Chem Phys 13:11156–11164
Armstrong AR, Tee DW, Mantia FL, Novák P, Bruce PG (2008) Synthesis of tetrahedral LiFeO2 and its behavior as a cathode in rechargeable lithium batteries. J Am Chem Soc 130:3554–3559
Jiang J, Li YY, Liu JP, Huang XT, Yuan CZ, Lou XW (2012) Recent advances in metal oxide-based electrode architecture design for electrochemical energy storage. Adv Mater 24:5166–5180
Yu XG, Marks TJ, Facchetti A (2016) Metal oxides for optoelectronic applications. Nature Mater 15:383–396
Zhou H, Ruther RE, Adcock J, Zhou W, Dai S, Nanda J (2015) Controlled formation of mixed nanoscale domains of high capacity Fe2O3–FeF3 conversion compounds by direct fluorination. ACS Nano 9:2530–2539
An QY, Lv F, Liu QQ, Han CH, Zhao KN, Sheng JZ, Wei QL, Yan MY, Mai LQ (2014) Amorphous vanadium oxide matrixes supporting hierarchical porous Fe3O4/graphene nanowires as a high-rate lithium storage anode. Nano Lett 14:6250–6256
Du DJ, Yue WB, Ren Y, Yang XJ (2014) Fabrication of graphene-encapsulated CoO/CoFe2O4 composites derived from layered double hydroxides and their application as anode materials for lithium-ion batteries. J Mater Sci 49:8031–8039
Büyükyazi M, Mathur S (2015) 3D nanoarchitectures of α-LiFeO2 and α-LiFeO2/C nanofibers for high power lithium-ion batteries. Nano Energy 13:28–35
Obrovac MN, Dunlap RA, Sanderson RJ, Dahn JR (2001) The electrochemical displacement reaction of lithium with metal oxides. J Electrochem Soc 148:A576–A588
Krummacher J, Passerini S, Balducci A (2015) Ionic liquid assisted solid-state synthesis of lithium iron oxide nanoparticles for rechargeable lithium ion batteries. Solid State Ionics 280:37–43
Rahman MM, Wang JZ, Hassan MF, Chen ZX, Liu HK (2011) Synthesis of carbon coated nano crystalline porous α-LiFeO2 composite and its application as anode for the lithium ion battery. J Alloys Compd 509:5408–5413
Li KY, Chen H, Shua FF, Xue DF, Guo XW (2014) Facile synthesis of iron-based compounds as high performance anode materials for Li-ion batteries. RSC Adv 4:36507–36512
Acknowledgements
We gratefully acknowledge the financial support by National Natural Science Foundation of China (Grant No. 21173183), the Higher Education Science Foundation of Jiangsu Province (No. 15KJB150031), State Key Laboratory of Structural Chemistry Fund (No. 20150009), the Priority Academic Program Development of Jiangsu Higher Education Institutions and the Qing Lan project. We would also like to acknowledge the technical support received from the Testing Center of Yangzhou University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guo, SP., Ma, Z., Li, JC. et al. First investigation of the electrochemical performance of γ-LiFeO2 micro-cubes as promising anode material for lithium-ion batteries. J Mater Sci 52, 1469–1476 (2017). https://doi.org/10.1007/s10853-016-0441-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-016-0441-3