Abstract
A new and simple colorimetric receptor 1, based on the combination of 2-amino-4-methylphenol moiety and julolidine moiety, has been designed and synthesized. 1 showed a selective colorimetric sensing ability toward F− ion by changing color from yellow to orange, and could be utilized to monitor F− without any inhibition by competitive anions such as CH3COO− and CN−. Based on Job plot, ESI-mass spectrometry and the 1H NMR titration, the binding mode of 1 for F− was proposed to be 1:1. Moreover, the sensing mechanism for F− was theoretically supported by DFT and TD-DFT calculations.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recognition and detection of various anions have been consistently paid much attention, due to their important roles in chemical sciences, biological, medical and environmental [1–3]. Among the various anions, especially, fluoride is most concerned, because it is very useful in a wide range of biological, medical, chemical processes, and etc. [4–7]. Also, intake of suitable amount of fluoride can prevent dental disease and osteoporosis [8]. Despite the advantages of fluoride, however, chronic intake of fluoride can cause accumulation in our body [9]. Such hyperingestion of fluoride can bring many problems due to its toxicity, such as gastric and kidney disorders, dental and skeletal fluorosis, urolithiasis, or even death [10–15]. These various effects of fluoride drove many scientists to develop chemosensors for fluoride recognition in the specific concentration range [16–18]. Nevertheless, many chemosensors are still unable to distinguish fluoride effectively from anions such as CH3COO− and CN− because they have similar basicity to F− and easily form hydrogen bonds [19]. Therefore, it is still an important challenge to develop a chemosensor for highly selective detection of fluoride.
Several methods, such as inductively coupled-plasma atomic emission spectrometry [20], atomic absorption spectroscopy [21], electrochemical methods [22] and so on, have been developed to detect anions. Among the methods, the most attractive one may be colorimetric sensors, which can detect targets via naked-eye of the color change. In addition, colorimetric materials have advantages such as low cost, rapid response rate, easy detection, no requirement of a specific equipment and high selectivity [23–29].
Schiff bases have been utilized to develop novel chemosensors with selectivity and sensitivity toward anions. In particular, Schiff bases containing phenolic groups are known as one of strategies of designing sensor for anions because of the ability of the phenolic-OH group to interact with anions through hydrogen bonding [30, 31]. In addition, the julolidine moiety is one of the well-known chromophores [32, 33]. Moreover, we expected that the combination of the methylphenol moiety having the electron-donor methyl group and the julolidine moiety may show interesting electronic and sensing properties toward anions.
Herein, we report a highly selective chemosensor 1 for F−, which was synthesized in one step by the condensation reaction of 2-amino-4-methylphenol with 8-hydroxyjulolidine-9-carboxaldehyde (Scheme 1). Chemosensor 1 can selectively detect F− by color change from yellow to orange via the ‘naked-eye’. Also, it was able to distinguish F− from competitive anions such as CH3COO− and CN−. Moreover, a deprotonation mechanism for sensing of F− was proposed, which was supported by the DFT/TD-DFT calculation method.
Results and discussion
The colorimetric chemosensor 1 for fluoride was synthesized by condensing 2-amino-4-methylphenol with 8-hydroxyjulolidine-9-carboxaldehyde (Scheme 1) and characterized by 1H NMR and 13C NMR, ESI-mass spectrometry, and elemental analysis. The binding abilities of 1 were investigated by UV–vis, 1H NMR spectroscopy and the DFT/TD-DFT studies.
UV–vis study of 1-F−
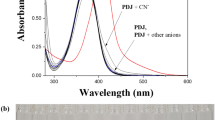
The sensing abilities of 1 were observed by “naked-eye” and confirmed by UV–vis spectroscopy. The absorption response of 1 toward the tetraethylammonium (TEA) salts (F−,CN−, Cl−, Br−, I−) or tetrabuthylammonium (TBA) salts (AcO− H2PO4 −, BzO−, N3 −, SCN−) was carried out in a mixture of bis–tris buffer/DMSO (3:97, v/v, 10 mM bis–tris, pH 7.0) (Fig. 1a). Upon the addition of each anion to 1, only F− induced a clear spectral change while other anions showed either no or a little change in the absorption spectra relative to the spectra of 1. In addition, only F− showed a color change from yellow to orange by the ‘naked-eye’ (Fig. 1b). By contrast, other anions did not cause any color change of solution. This observation suggested that the receptor 1 can serve as a potential candidate of a “naked-eye” chemosensor for F−.
a Absorption spectral change of 1 (10 μM) in the presence of 240 equiv of various anions in a mixture of DMSO/bis–tris buffer (97:3, v/v, 10 mM bis–tris, pH 7.0). b Color change of receptor 1 (10 μM) in the presence of 240 equiv of various anions in a mixture of DMSO/bis–tris buffer (97:3, v/v, 10 mM bis–tris, pH 7.0)
The binding properties of 1 with F− were studied by UV–vis titration experiments (Fig. 2). Upon the gradual addition of F− into a solution of 1, a new band at 500 nm appeared and the absorption band at 403 nm gradually decreased, resulting in a color change from yellow to orange. Simultaneously, two clear isosbestic points were observed at 358 and 442 nm, indicating the formation of the only one species between 1 and F−. This bathochromic shift indicates that the proton of phenol in 4-methylphenol might be deprotonated by F−, which leads to the formation of phenolate. The phenolate donates the electron density into the π-system, thus resulting in formation of extended π-conjugated system [34, 35]. On the other hand, the increase of an amount of the bis–tris buffer rendered 1 lose the sensing property toward F−. This might be due to the inhibition of deprotonation of 1 by water.
The binding mode between 1 and F− was identified to be a 1:1 stoichiometry through Job plot analysis (Fig. 3), which was also confirmed by ESI-mass spectrometry analysis (Fig. 4). The positive ion mass spectrum of ESI-mass showed that a peak at m/z = 581.20 was assignable to [1-H++2TEA]+ [calcd, m/z: 581.48], which supports the deprotonation mechanism of 1 by fluoride. From the UV–vis titration data, the association constant for 1 with F− was determined as 6.15 × 102 M−1 on the basis of Benesi-Hildebrand equation (Fig. S1) [36]. The detection limit (3σ/k) of receptor 1 for the analysis of F− ions was calculated to be 31.4 μM (Fig. S2) [37].
An important feature to develop a practical sensor is its high selectivity toward other competitive species. To confirm the properties of 1 as the practical sensor, the selectivity of 1 was studied in the presence of various competing anions. The receptor 1 was treated with 240 equiv of F− in the presence of 240 equiv of other anions (Fig. 5). There was no interference for the detection of F− toward other anions. This observation suggested that 1 could be an excellent sensor for detecting F− in the presence of all the competing anions.
1H NMR study of 1-F−
1H NMR titration experiments were performed to understand the interaction between 1 and fluoride at room temperature (Fig. 6). In absence of fluoride, the hydroxyl proton (H10) of the julolidine moiety was not observed maybe due to hydrogen bonding, while the hydroxyl proton (H11) of methylphenol moiety appeared as a singlet at 14.37 ppm, and the aromatic protons (H1, H2, H4, and H6) were resonated in the range of 7.09–6.79 ppm. Upon addition of F− (1 equiv), the H11 at 14.37 ppm disappeared immediately. The further addition of F− (1–10 equiv) rendered all aromatic protons to shift upfield. In contrast, H5 moved to down-field, suggesting that the electron density of the imine moiety decreased due to the hydrogen bonding between N atom and -OH group. Then, a new peak at 16 ppm appeared, indicating the formation of [FHF−] species [38]. These results indicates that the negative charge originated from deprotonation of 1 by F− might be delocalized through the receptor molecule [39]. Therefore, the 1H NMR titration would support the proposal that the binding mechanism of 1 for fluoride might occur by the deprotonation pathway, resulting in color change [40]. Based on Job plot, ESI-mass spectrometry and 1H NMR titration, we proposed the mechanism of 1-F− binding pathway as shown in Scheme 2.
Density function theory (DFT) studies
To understand the sensing mechanisms of 1 with F−, theoretical calculations were performed in parallel to the experimental studies. As it was proposed that the hydroxyl proton of 4-methylphenol group in 1 was deprotonated by F−, based on ESI-mass spectrometry analysis and 1H NMR titrations, 1 − species was optimized by DFT/B3LYP/6-31G**/DMSO level. The significant structural properties of the energy-minimized structures for 1 and 1 − were shown in Fig. 7. The energy-minimized structure of 1 indicated a skew structure between 4-methylphenol and 8-hydroxy-9-iminomethyljulolidine [dihedral angle (1C, 2C, 3 N, 4C) = 148.7o], and the hydrogen bond was observed between 3N and 5H (Fig. 7a). 1 − showed a planar structure [dihedral angle (1C, 2C, 3N, 4C) = −179.6o], and the hydrogen bond was also observed between 3N and 5H (Fig. 7b).
To further gain an insight into colorimetric sensing mechanism of 1 toward F−, time-dependent density functional theory (TD-DFT) calculations were carried out at the optimized geometries (Figs. S3 and S4). In order to clearly understand the absorption energies, the UV–vis spectra of 1 and 1 − were compared to their TD-DFT calculations (B3LYP/6-31G**/DMSO) [Figs. S(3a) and S(4a)]. In case of 1, the main molecular orbital (MO) contribution of the first lowest excited state was determined for HOMO → LUMO transition with oscillator strength of 1.1019 [390.34 nm, Fig. S(3b)]. The main MO contribution of the first excited state of 1 − was determined for HOMO → LUMO with oscillator strength of 0.754 [483.86 nm, Fig. S(4b)]. As shown in Fig. 8, the deprotonation of 4-methylphenol group in 1 showed mainly that HOMO-1 (−5.791 eV) of 1 destabilized to HOMO (−3.823 eV) of 1 − by +1.968 eV, whereas LUMO of 1 (−1.488 eV) destabilized to LUMO (−0.907 eV) of 1 − by +0.581 eV. The difference of the destabilization energy induced the red shifted spectrum, resulting in the color change from yellow to orange.
Conclusion
We have presented a simple imine-based naked-eye chemosensor 1 for the detection of F−. The receptor 1 obviously showed selectivity toward F− through color change (yellow to orange), which was explained by theoretical calculations. Based on the Job plot, 1H NMR titration and ESI-mass spectrometry analysis, we suggested that the receptor 1 interacted with F− in a 1:1 stoichiometric manner. Moreover, the receptor 1 could distinguish F− in the presence of other anions. Therefore, we believe that this type of easy-to-synthesized naked-eye chemosensor 1 could contribute to developing a new ‘fluoride sensor’.
Experiment
General information
Unless otherwise specified, all the solvents and reagents (analytical grade and spectroscopic grade) were obtained commercially and used without further purification. 1H NMR and 13C NMR measurements were performed on a Varian 400 and 100 MHz spectrometer and chemical shifts were recorded in ppm. Absorption spectra were recorded using a Perkin Elmer model Lambda 25 UV–Vis spectrometer at room temperature. Electrospray ionization mass spectra (ESI-mass) were collected on a Thermo Finnigan (San Jose, CA, USA) LCQ™ Advantage MAX quadrupole ion trap instrument. The spray voltage was set at 4.2 kV, and the capillary temperature was set at 80 °C. Elemental analysis for carbon, nitrogen, and hydrogen was carried out by using a Flash EA 1112 elemental analyzer (thermo) in Organic Chemistry Research Center of Sogang University, Korea.
Synthesis of receptor 1
To a solution of 2-amino-4-methylphenol (0.12 g, 1 mmol) in methanol (10 mL), 8-hydroxyjulolidine-9-carboxaldehyde (0.22 g, 1 mmol) was added, followed by addition of three drops of phosphoric acid into the reaction mixture. The reaction mixture was stirred for 3 h at room temperature, whereupon the red-brown powder was produced. The powder was collected by filtration, washed with ethyl acetate, and air-dried. The yield was 93 % and the melting point 150 °C. 1H NMR (400 MHz, DMSO-d 6) δ: 14.33 (s, 1H), 8.49 (s, 1H), 7.07 (s, 1H), 6.79 (m, 3H), 3.21 (m, 4H), 2.57 (m, 4H), 2.23 (s, 3H), 1.85(m, 4H); 13C NMR (100 MHz, DMSO-d 6, ppm): 162.01, 157.56, 147.70, 147.14, 133.83, 129.83, 128.45, 126.43, 118.69, 116.18, 112.80, 108.59, 105.73, 49.66, 49.30, 27.00, 21.82, 20.85, 20.52, 20.29 ppm. ESI–MS m/z [1-H+]−: calcd, 321.4; found, 321.4. Anal. Calcd for C20H22N2O2: C, 74.51; H, 6.88; N, 8.69 %. Found: C, 74.85; H, 6.43; N, 8.92 %.
UV–vis study of 1 with various anions
Receptor 1 (3.22 mg, 0.01 mmol) was dissolved in DMSO (1 mL) and 3 μL of the receptor 1 (10 mM) was diluted with 2.997 mL DMSO/bis–tris buffer (97:3, v/v) to make the final concentration of 10 μM. Stock solutions (100 mM) of tetraethylammonium salts (F−, Br−, Cl−, I−, and CN−) or tetrabutylammonium salts (SCN−, BzO−, N3 −, AcO− and H2PO4 −) were separately prepared in DMSO. 75 μL of the stock solution (100 mM) of each anion was transfered to 3 mL of receptor 1 solution (10 μM) prepared above. After mixing them for a few seconds, UV–vis spectra of 1 were taken at room temperature.
UV–vis titration of 1
The receptor 1 (3.22 mg, 0.01 mmol) was dissolved in DMSO (1 mL) and 3 μL of the receptor 1 (10 mM) was dilluted to 2.997 mL of DMSO-buffer solution (97:3, v/v, 10 mM bis–tris, pH 7.0) to make the concentration of 10 μM. Tetraethylammonium fluoride ((TEA)F, 26.1 mg, 0.1 mmol) was also dissolved in DMSO (1 mL) and 1.5–75 μL of the F− solution (100 mM) was transferred to the solution of 1 (10 μM, 3 mL) prepared above. After mixing them for a few seconds, UV–vis spectra were taken at room temperature.
Job plot measurement
The receptor 1 (3.22 mg, 0.01 mmol) was dissolved in DMSO (1 mL) and 0.09 mL of the solution was diluted to 29.91 mL of DMSO-buffer solution (97:3, v/v, 10 mM bis–tris, pH 7.0) to make the concentration of 30 μM. 5.0, 4.5, 4.0, 3.5, 3.0, 2.5, 2.0, 1.5, 1.0, 0.5 and 0 mL of the receptor 1 solution were taken and transferred to vials. In the same way, (TEA)F (2.61 mg, 0.01 mmol) was dissolved in DMSO (1 mL) and 0.09 mL the (TEA)F solution was diluted to 29.91 mL of DMSO-buffer solution (97:3, v/v, 10 mM bis–tris, pH 7.0) to make the concentration of 30 μM. 0, 0.5, 1.0, 1.5, 2.0, 2.5, 3.0, 3.5, 4.0, 4.5 and 5.0 mL of the F− solution were added to each diluted 1 solution. Each vial had a total volume of 5 mL. After shaking them for a few seconds, UV–vis spectra were taken at room temperature.
Competition with other anions
The receptor 1 (3.22 mg, 0.01 mmol) was dissolved in DMSO (1 mL). Tetraethylammonium salts (F−, Br−, Cl−, I−, and CN−; 0.1 mmol) or tetrabutylammonium salts (SCN−, BzO−, N3 −, AcO− and H2PO4 −; 0.1 mmol) were separately dissolved in DMSO (1 mL). 75 μL of each anion solution (100 mM) was dilluted to 2.847 mL of DMSO-buffer solution (97:3, v/v, 10 mM bis–tris, pH 7.0). 75 μL of the F− solution (100 mM) was taken and added to the solutions prepared above. Then, 3 μL of the 1 (10 mM) was taken and added to the mixed solutions. Each vial had a total volume of 3 mL. After shaking them for a few seconds, UV–vis spectra were taken at room temperature.
1H NMR titration of 1 with F−
Four NMR tubes of 1 (0.644 mg, 0.002 mmol) dissolved in DMSO-d 6 (0.7 mL) were prepared, and four different equiv (0, 1, 5 and 10 equiv) of the (TEA)F dissolved in DMSO-d 6 (0.3 mL) were added to the 1 solution, respectively. After shaking them for a minute, their 1H NMR spectra were taken.
Theoretical calculation methods
All theoretical calculations were performed by DFT method with the hybrid exchange–correlation functional B3LYP [41, 42] applying the 6-31G** [43, 44] basis set without any symmetry restrictions. The energy-minimized structure of 1 was obtained in various geometric forms. In case of 1-F−, as the proton of the hydroxyl group in 4-methylphenol was deprotonated, 1 1− species was optimized by the same level of 1. In vibrational frequency calculations, there was no imaginary frequency for the optimized geometries of 1 and 1 1−, suggesting that these geometries represented local minima. For all calculations, the solvent effect of DMSO was considered by using the Cossi and Barone’s CPCM (conductor-like polarizable continuum model) [45, 46]. In order to investigate the transition energies for the optimized structures of 1 and 1 −, we calculated the lowest 20 singlet–singlet transition using their ground state geometry (S0) with TD-DFT (B3LYP) method. The GaussSum 2.1 was used to calculate the contribution of molecular orbital in electronic transitions [47]. All the calculations were performed with Gaussian 03 suite [48].
References
Park, J.J., Kim, Y.-H., Kim, C., Kang, J.: Naked eye detection of fluoride and pyrophosphate with an anion receptor utilizing anthracene and nitrophenyl group as signaling group. Tetrahedron Lett. 52, 2759–2763 (2011)
Kim, Y.-J., Kwak, H., Lee, S.J., Lee, J.S., Kwon, H.J., Nam, S.H., Lee, K., Kim, C.: Urea/thiourea-based colorimetric chemosensors for the biologically important ions: efficient and simple sensors. Tetrahedron 62, 9635–9640 (2006)
Noh, J.Y., Hwang, I.H., Kim, H., Song, E.J., Kim, K.B., Kim, C.: Salicylimine-based colorimetric and fluorescent chemosensor for selective detection of cyanide in aqueous buffer. Bull. Korean Chem. Soc. 34, 1985–1989 (2013)
Miyaji, H., Sato, W., Sessler, J.: Naked-eye detection of anions in dichloromethane: colorimetric anion sensors based on calix. Angew. Chem. Int. Ed. Engl. 39, 1777–1780 (2000)
Kim, S.K., Sessler, J.L.: Ion pair receptors. Chem. Soc. Rev. 39, 3784–3809 (2010)
Aaseth, J., Shimshi, M., Gabrilove, J.L., Birketvedt, G.S.: Fluoride: a toxic or therapeutic agent in the treatment of osteoporosis. J. Trace Elem. Exp. Med. 17, 83–92 (2004)
Chen, J.-S., Zhou, P.-W., Zhao, L., Chu, T.-S.: A DFT/TDDFT study of the excited state intramolecular proton transfer based sensing mechanism for the aqueous fluoride chemosensor BTTPB. RSC Adv. 4, 254–259 (2014)
Chen, J.-S., Zhou, P.-W., Li, G.-Y., Chu, T.-S., He, G.-Z.: Fluoride anion sensing mechanism of 2-ureido-4[1H]-pyrimidinone quadruple hydrogen-bonded supramolecular assembly: photoinduced electron transfer and partial configuration change. J. Phys. Chem. B. 117, 5212–5221 (2013)
Mattiwala, N.M., Kamal, R., Sahoo, S.K.: Schiff base bis(5-nitrosalycilaldehyde)ethylenediamine as colorimetric sensor for fluoride. Res. Chem. Intermed. 41, 391–400 (2015)
Kim, Y., Gabbaï, F.P.: Cationic boranes for the complexation of fluoride ions in water below the 4 ppm maximum contaminant level. J. Am. Chem. Soc. 131, 3363–3369 (2009)
Broomsgrove, A.E.J., Addy, D.A., Di Paolo, A., Morgan, I.R., Bresner, C., Chislett, V., Fallis, I.A., Thompson, A.L., Vidovic, D., Aldridge, S.: Evaluation of electronics, electrostatics and hydrogen bond cooperativity in the binding of cyanide and fluoride by Lewis acidic ferrocenylboranes. Inorg. Chem. 49, 157–173 (2010)
Madhu, S., Ravikanth, M.: Boron-dipyrromethene based reversible and reusable selective chemosensor for fluoride detection. Inorg. Chem. 53, 1646–1653 (2014)
Tayade, K., Sahoo, S.K., Singh, A., Singh, N., Mahulikar, P., Attarde, S., Kuwar, A.: Architecture of dipodal ratiometric motif showing discrete nanomolar response towards fluoride ion. Sens. Actuators B Chem. 202, 1333–1337 (2014)
Wang, L., Fang, G., Cao, D.: A reversible and reusable selective chemosensor for fluoride detection using a phenolic OH-containing BODIPY dye by both colorimetric “naked-eye” and fluorometric modes. J. Fluoresc. 24, 1757–1766 (2014)
Bamesberger, A., Schwartz, C., Song, Q., Han, W., Wang, Z., Cao, H.: Rational design of a rapid fluorescent approach for detection of inorganic fluoride in MeCN-H2O: a new fluorescence switch based on N-aryl-1,8-naphthalimide. New J. Chem. 38, 884–888 (2014)
Song, E.J., Kim, H., Hwang, I.H., Kim, K.B., Kim, A.R., Noh, I., Kim, C.: A single fluorescent chemosensor for multiple target ions: Recognition of Zn2+ in 100% aqueous solution and F− in organic solvent. Sens. Actuators B Chem. 195, 36–43 (2014)
Anand, T., Sivaraman, G., Iniya, M., Siva, A., Chellappa, D.: Aminobenzohydrazide based colorimetric and “turn-on” fluorescence chemosensor for selective recognition of fluoride. Anal. Chim. Acta. 876, 1–8 (2015)
Peng, X., Wu, Y., Fan, J., Tian, M., Han, K.: Colorimetric and ratiometric fluorescence sensing of fluoride: tuning selectivity in proton transfer. J. Org. Chem. 70, 10524–10531 (2005)
Na, Y.J., Choi, Y.W., Yun, J.Y., Park, K.M., Chang, P.S., Kim, C.: Dual-channel detection of Cu2+ and F− with a simple Schiff-based colorimetric and fluorescent sensor. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 136, 1649–1657 (2015)
Sreenivasa Rao, K., Balaji, T., Prasada Rao, T., Babu, Y., Naidu, G.R.K.: Determination of iron, cobalt, nickel, manganese, zinc, copper, cadmium and lead in human hair by inductively coupled plasma-atomic emission spectrometry. Spectrochim. Acta Part B At. Spectrosc. 57, 1333–1338 (2002)
Sturgeon, R.E., Berman, S.S., Desaulniers, A., Russell, D.S.: Determination of iron, manganese, and zinc in seawater by graphite furnace atomic absorption spectrometry. Anal. Chem. 51, 2364–2369 (1979)
Gulaboski, R., Mireski, V., Scholz, F.: An electrochemical method for determination of the standard Gibbs energy of anion transfer between water and n-octanol. Electrochem. commun. 4, 277–283 (2002)
Yoshino, J., Kano, N., Kawashima, T.: Fluorescence properties of simple N-substituted aldimines with a B–N interaction and their fluorescence quenching by a cyanide ion. J. Org. Chem. 74, 7496–7503 (2009)
Divya, K.P., Sreejith, S., Balakrishna, B., Jayamurthy, P., Anees, P., Ajayaghosh, A.: A Zn2+-specific fluorescent molecular probe for the selective detection of endogenous cyanide in biorelevant samples. Chem. Commun. (Camb) 46, 6069–6071 (2010)
Yu, H., Fu, M., Xiao, Y.: Switching off FRET by analyte-induced decomposition of squaraine energy acceptor: A concept to transform “turn off” chemodosimeter into ratiometric sensors. Phys. Chem. Chem. Phys. 12, 7386–7391 (2010)
Lv, X., Liu, J., Liu, Y., Zhao, Y., Chen, M., Wang, P., Guo, W.: Rhodafluor-based chromo and fluorogenic probe for cyanide anion. Sens. Actuators B Chem. 158, 405–410 (2011)
Noh, J.Y., Park, G.J., Na, Y.J., Jo, H.Y., Lee, S.A., Kim, C.: A colorimetric “naked-eye” Cu(II) chemosensor and pH indicator in 100 % aqueous solution. Dalton Trans. 43, 5652–5656 (2014)
Vilar, R.: Anion-templated synthesis. Angew. Chem. Int. Ed. 42, 1460–1477 (2003)
Hijji, Y.M., Barare, B., Kennedy, A.P., Butcher, R.: Synthesis and photophysical characterization of a Schiff base as anion sensor. Sens. Actuators B Chem. 136, 297–302 (2009)
Helal, A., Thao, N.T.T., Lee, S.W., Kim, H.-S.: Thiazole-based chemosensor II: synthesis and fluorescence sensing of fluoride ions based on inhibition of ESIPT. J. Incl. Phenom. Macrocycl. Chem. 66, 87–94 (2010)
Noh, J.Y., Kim, S., Hwang, I.H., Lee, G.Y., Kang, J., Kim, S.H., Min, J., Park, S., Kim, C., Kim, J.: Solvent-dependent selective fluorescence assay of aluminum and gallium ions using julolidine-based probe. Dye. Pigment. 99, 1016–1021 (2013)
Maity, D., Manna, A.K., Karthigeyan, D., Kundu, T.K., Pati, S.K., Govindaraju, T.: Visible-near-infrared and fluorescent copper sensors based on julolidine conjugates: selective detection and fluorescence imaging in living cells. Chem. Eur. J. 17, 11152–11161 (2011)
Kumari, N., Jha, S., Bhattacharya, S.: Colorimetric probes based on anthraimidazolediones for selective sensing of fluoride and cyanide ion via intramolecular charge transfer. J. Org. Chem. 76, 8215–8222 (2011)
Udhayakumari, D., Velmathi, S.: Azo linked thiourea based effective dual sensor and its real samples application in aqueous medium. Sens. Actuators B Chem. 209, 462–469 (2015)
Benesi, H.A., Hildebrand, J.H.: A spectrophotometric investigation of the interaction of iodine with aromatic hydrocarbons. J. Am. Chem. Soc. 71, 2703–2707 (1949)
Tsui, Y.K., Devaraj, S., Yen, Y.P.: Azo dyes featuring with nitrobenzoxadiazole (NBD) unit: a new selective chromogenic and fluorogenic sensor for cyanide ion. Sens. Actuators B Chem. 161, 510–519 (2012)
Shenderovich, I.G., Limbach, H.-H., Smirnov, S.N., Tolstoy, P.M., Denisov, G.S., Golubev, N.S.: H/D isotope effects on the low-temperature NMR parameters and hydrogen bond geometries of (FH)2F− and (FH)3F− dissolved in CDF3/CDF2Cl. Phys. Chem. Chem. Phys. 4, 5488–5497 (2002)
Lee, J.J., Park, G.J., Choi, Y.W., You, G.R., Kim, Y.S., Lee, S.Y., Kim, C.: Detection of multiple analytes ( CN− and F− ) based on a simple pyrazine-derived chemosensor in aqueous solution : experimental and theoretical approaches. Sens. Actuators B. Chem. 207, 123–132 (2015)
Jo, T.G., Na, Y.J., Lee, J.J., Lee, M.M., Lee, S.Y., Kim, C.: A diaminomaleonitrile based selective colorimetric chemosensor for copper(II) and fluoride ions. New J. Chem. 39, 2580–2587 (2015)
Becke, A.D.: Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993)
Lee, C., Yang, W., Parr, R.G.: Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37, 785–789 (1988)
Hay, P.J., Wadt, W.R.: Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J. Chem. Phys. 82, 270–283 (1985)
Hay, P.J., Wadt, W.R.: Ab initio effective core potentials for molecular calculations. Potentials for main group elements Na to Bi. J. Chem. Phys. 82, 284–298 (1985)
Barone, V., Cossi, M.: Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J. Phys. Chem. A. 102, 1995–2001 (1998)
Cossi, M., Barone, V.: Time-dependent density functional theory for molecules in liquid solutions. J. Chem. Phys. 115, 4708–4717 (2001)
O’Boyle, N.M., Tenderholt, A.L., Langner, K.M.: Cclib: a library for package-independent computational chemistry algorithms. J. Comput. Chem. 29, 839–845 (2008)
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Montgomery, J.A. Jr., Vreven, T., Kudin, K.N., Burant, J.C., Millam, J.M., Iyengar, S.S., Tomasi, J., Barone, V., Mennucci, B., Cossi, M., Scalmani, G., Rega, N., Petersson, G.A., Nakatsuji, H., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Klene, M., Li, X. Knox, J.E., Hratchian, H.P., Cross, J.B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R.E., Yazyev, O., Austin, A.J., Cammi, R., Pomelli, C., Ochterski, J.W., Ayala, P.Y., Morokuma, K., Voth, G.A., Salvador, P., Dannenberg, J.J., Zakrzewski, V.G., Dapprich, S., Daniels, A.D., Strain, M.C., Farkas, O., Malick, D.K., Rabuck, A.D., Raghavachari, K., Foresman, J.B., Ortiz, J.V., Cui, Q., Baboul, A.G., Clifford, S., Cioslowski, J., Stefanov, B.B., Liu, G., Liashenko, A., Piskorz, P., Komaromi, I., Martin, R.L., Fox, D.J., Keith, T., Al-Laham, M.A., Peng, C.Y., Nanayakkara, A., Challacombe, M., Gill, P.M.W., Johnson, B., Chen, W., Wong, M.W., Gonzalez, C., Pople, J.A.: Gaussian 03, Revision D.01, Gaussian, Inc., Wallingford CT (2004)
Acknowledgments
Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2014R1A2A1A11051794 and NRF-2015R1A2A2A09001301) are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ryu, H.H., Lee, Y.J., Kim, S.E. et al. A colorimetric F− chemosensor with high selectivity: experimental and theoretical studies. J Incl Phenom Macrocycl Chem 86, 111–119 (2016). https://doi.org/10.1007/s10847-016-0646-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-016-0646-8