Abstract
We synthesized an original reversible colorimetric chemosensor PDJ ((E)-9-((2-(6-chloropyridazin-3-yl)hydrazono)methyl)-2,3,6,7-tetrahydro-1H,5H-pyrido[3,2,1-ij]quinolin-8-ol) for the detection of F−. PDJ displayed a selective colorimetric detection to F− with a variation of color from colorless to yellow. Limit of detection of PDJ for F− was calculated as 12.1 µM. The binding mode of PDJ and F− turned out to be a 1:1 ratio using Job plot. Sensing process of F− by PDJ was demonstrated by 1H NMR titration and DFT calculation studies that suggested hydrogen bond interactions followed by deprotonation. Moreover, the practicality of PDJ was demonstrated via a reversible test with TFA (trifluoroacetic acid).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fluoride is a trace element present in our bodies, which helps to care tooth, build dental enamel and prevent osteoporosis [1,2,3,4,5]. However, even at low concentration, long-term consumption causes bone fluoridation, decreased thyroid activity, bone disease, and adversely affecting the immune system [6,7,8,9,10]. In addition, fluoride is widely applied in industries such as pesticide production containing fluoride and production of steel, aluminum and ceramics. By this industrial spread, fluoride is increasing irreversible pollution to the environment [11,12,13,14]. Thus, monitoring and sensing fluoride are of great importance to health care and environment.
So far, fluoride detection techniques can be classified into several types, such as electrode methods, 19F NMR analysis, fluorescence or colorimetric detection [15,16,17,18,19,20]. Among the various approaches, the most attractive is the colorimetric sensor that can detect fluoride via color changes visually without relying on expensive device use. In addition, colorimetric sensors have diverse advantages like low cost, easy method, quick response, and great selectivity [21,22,23,24,25,26].
Fluoride interacts with NH or OH groups through strong hydrogen bonds [27]. Therefore, a variety of colorimetric chemosensors which include NH or OH groups, have been designed to sense fluoride [28,29,30,31,32,33,34]. Julolidine moiety having an OH group is well known as a chromophore and great proton donor [35,36,37,38,39,40]. Pyridazine moiety acts as an electron withdrawing group and is also used in various biochemical and physicochemical applications [41]. Therefore, we predicted that the combination of the pyridazine group and the julolidine one may show deformation of energy transition via hydrogen bond interactions and unique sensing properties to fluoride.
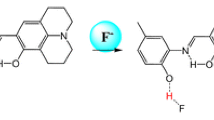
Herein, we illustrate a novel reversible chemosensor PDJ, which was produced in one step by coupling 3-chloro-6-hydrazinylpyridazine with 8-hydroxyjulolidine-9-carboxaldehyde. PDJ could sense F– by a color variation from colorless to yellow through the naked eye, show reversible reaction, and be reused by TFA (trifluoroacetic acid). Binding pattern and sensing mechanism of PDJ to F– were presented by Job plot, 1H NMR titration, ESI-mass spectral analyses and calculations.
Experiments
General Information
With a Varian spectrometer, 1H and 13C NMR data were afforded. Absorption and ESI-MS data were given with a Perkin Elmer spectrometer and a ACQUITY QDa, respectively.
Synthesis of PDJ ((E)-9-((2-(6-chloropyridazin-3-yl)hydrazono)methyl)-2,3,6,7-tetrahydro-1H,5H-pyrido[3,2,1-ij]quinolin-8-ol)
3-Chloro-6-hydrazinylpyridazine (0.9x10-3 mol, 0.133 g) and 8-hydroxyjulolidine-9-carboxaldehyde (1.2x10-3 mol, 0.272 g) were dissolved in methanol (5.0 mL). The mixture was stirred for 8 h after a few drops of CH3COOH were added. The yellowish-brown powder formed. Then, it was rinsed with CH3OH, filtered and dried (yield: 32%). 1H NMR: 11.32 (s, 1H), 10.77 (s, 1H), 8.08 (s, 1H), 7.60 (d, J =9.3 Hz, 1H), 7.21 (d, J =9.5 Hz, 1H), 6.71 (s, 1H), 3.15 (m, 4H), 2.60 (m, 4H), 1.85 (m, 4H). 13C NMR: 157.1(1C), 153.4(1C), 146.7(1C), 146.5(1C), 144.7(1C), 129.8(1C), 127.5(1C), 115.3(1C), 112.6(1C), 106.4(2C), 49.2(1C), 48.8(1C), 26.7(1C), 21.5(1C), 20.7(1C), 20.3(1C). ESI-MS for [PDJ + H+], calcd, 344.13 (m/z); found, 344.34.
UV–vis Titration
A PDJ stock (5.0x10–3 M) was provided in 1,000 μL of DMSO. 12 μL of PDJ (5.0x10–3 M) was diluted with 2.986 mL of CH3CN to produce 2.0x10–5 M. TEAF (tetraethylammonium fluoride, 1x10–4 mol) was dissolved in CH3CN (1,000 μL) and 3.0 - 33.0 μL of the F– (1x10–1 M) was added to 2.0x10–5 M of PDJ. UV-vis spectra were measured after 8 s.
Job Plot
Solutions having PDJ (100 μM) and TEAF (100 μM) were made. Amounts of PDJ and F– kept steady (3,000 μL) and acetonitrile as solvent was employed. UV-vis spectra were measured after 8 s. Job plot was drawn by plotting against the molar fraction of fluoride under the constant total concentration (100 μM). A is the absorbance of PDJ after addition of F–, and A0 is the absorbance of the free PDJ at 414 nm.
Competitive Test
A PDJ stock (5.0x10-3 M) was provided in 1,000 μL of DMSO. In cells containing 3,000 μL of CH3CN, 27 μL of other anion stocks (I−, NO2−, Br−, SCN−, OAc−, Cl−, H2PO4−, N3−, BzO−, CN− and S2−; 100 mM) was diluted to produce 45 equiv. 27 μL of TEAF (1x10-1 M) was added to each cell. 12 μL (5.0x10-3 M) of PDJ was added to the cell. UV-vis spectra were measured after 8 s.
1H NMR Titration
Five NMR tubes containing PDJ (4.8 mg, 1.4x10-5 mol) dissolved in DMSO-d6 (1,400 μL) were provided. Five varied equivalents (0, 0.5, 1, 2 and 5) of TEAF dissolved in DMSO-d6 were put into five NMR tubes. 1H NMR spectra were measured after 8 s.
Reversible UV–vis Titration
A PDJ stock (5.0x10–3 M) was provided in 1,000 μL of DMSO and a F- stock (100 mM) was provided in CH3CN (1 mL). 12 μL of PDJ (5x10–3 M) and 27 μL of F– were diluted with 2.961 mL of CH3CN. Then, 1.2 – 18.0 μL of TFA (5x10–2 M) were added to a mixture of PDJ and F–. UV-vis spectra were measured after 8 s.
Theoretical Studies
To apprehend geometry structures and energy transition states of PDJ and PDJ with F–, calculations were worked through Gaussian 16 program [42]. We used B3LYP and DFT calculations for geometry optimization, and applied the 6-31G(d,p) basis set to all atoms [43,44,45,46]. Imaginary frequencies were not displayed for optimized patterns of PDJ and PDJ with F–, indicating that the optimized geometry signified local minima. To consider the solvent interaction to PDJ, IEFPCM model was applied in all DFT calculations [47]. PDJ was placed into a small cavity surrounded by a dielectric continuum of given solvent CH3CN (ε = 35.688). Based on the optimized patterns of PDJ and PDJ with F–, TD-DFT calculations were performed and twenty of UV-vis transition states were investigated.
Results and Discussion
PDJ was synthesized by the coupling reaction between 3-chloro-6-hydrazinylpyridazine and 8-hydroxyjulolidine-9-carboxaldehyde (Scheme 1). PDJ was affirmed by 1H NMR, 13C NMR and ESI-MS (Figs. S1, S2 and S3).
Colorimetric Response of PDJ to F−
Colorimetric probing capabilities of receptor PDJ with varied anions in CH3CN were studied with UV-vis spectroscopy (Fig.1a). On addition of anions (45 equiv), PDJ exhibited little variation in absorption spectra except CN– and F–. The addition of CN– to PDJ displayed that the absorbance at 414 nm increased slightly. However, its solution color did not change. In contrast, the addition of F– to PDJ displayed that the absorbance at 414 nm remarkably increased and its solution color varied from colorless to yellow (Fig. 1b). This outcome suggested that PDJ can be a clearly selective colorimetric receptor for F–.
Binding characters of PDJ with fluoride were investigated through UV-vis titration (Fig. 2). On the addition of F–, the absorbance at 372 nm consistently decreased and that at 414 nm increased constantly with a saturation at 45 equiv of F–. Complete isosbestic point emerged at 388 nm, meaning that a species was formed from the interaction of PDJ and F–. The bathochromic shift drove us to presume the transition of intramolecular charge transfer (ICT) band via deprotonation of PDJ by F– [48].
Job plot was executed to comprehend the binding stoichiometry of PDJ and F– (Fig. S4). When the ratio ([F–]/([PDJ]+[F–])) was 0.5, the value of A-A0 at 424 nm was the largest, suggesting that PDJ reacted with F– through a 1:1 ratio. Binding constant of PDJ with F– was afforded to be 8.9×10 M–1 (R2 = 0.9914) with Li’s equation (Fig. S5) [49]. Detection limit of PDJ for F– was calculated 12.1 μM using 3σ/K (Fig. 3), which is low compared to those of colorimetric F– sensors (Table S1) [50].
A competing test was applied to extend the sensing ability of PDJ (Fig. 4a). S2- inhibited naked-eye sensing of F- by PDJ. The rest of the anions interfered little with absorbance (10 – 40%) at 414 nm. However, there was no problem observing color changes with the naked eye (Fig. 4b). These outcomes signified that PDJ may work as a clearly colorimetric sensor for fluoride with varied competing anions.
The 1H NMR titration further demonstrated the reaction between PDJ and fluoride (Fig. 5). The OH proton (H5) and the NH proton (H3) of PDJ were displayed, respectively, as a singlet at 11.3 ppm and 10.8 ppm. With addition of half equiv of F–, the H3 disappeared and the H5 was reduced owing to H-bonding between fluoride and H3 and H5. With addition of one equiv of F–, the H5 also disappeared. With excess addition of F– to PDJ, a new triplet peak at 16.2 ppm was displayed, signifying the generation of FHF– species through deprotonation of H5 in PDJ by F–. This presumed that the negative charge formed from the deprotonation of a hydroxyl group of PDJ by fluoride might be delocalized through the benzene ring and Schiff base. Deprotonation of H5 in PDJ by F– was further affirmed by an ESI-MS test (Fig. S6). Negative-ion data of PDJ with F– displayed the number of 342.19 (m/z), assignable to [PDJ – H+]– (calcd; 342.11). Based on Job plot, 1H NMR titrations and ESI-MS, the appropriate probing process of F– by PDJ was suggested in Scheme 2.
To examine the reversibility of PDJ to F–, TFA was put to the solution of PDJ and F–. (Fig. 6). Upon addition of TFA, absorbance at 414 nm constantly decreased and that at 372 nm continually increased. The last UV-visible spectrum was same as that of PDJ. On addition of F– again, the absorbance of 372 and 414 nm was returned. The variations of absorbance were reversible even in third cycles with the subsequently alternating addition of F– and TFA (Fig. S7). These results suggested that PDJ can be easily recycled through treatment with appropriate reagents like TFA.
Theoretical Calculations
With reference to the outcomes of ESI-MS and Job plot, optimized structures of PDJ and PDJ with F– were investigated (Fig. 7). Dihedral angle of PDJ was 179.632° and exhibited a planer structure (Fig. 7a). Dihedral angle of PDJ with F– was –2.246° and also showed a planer structure (Fig. 7b).
Based on energy-optimized patterns of PDJ and PDJ with F–, TD-DFT calculations were performed. For PDJ, the big absorption band occurred from the HOMO → LUMO+1 (372.37 nm, Fig. S8), indicating that ICT occurred from the julolidine to the pyridazine. For PDJ with F–, absorption band relevance with red-shift stemmed from HOMO → LUMO+1 transition (415.96 nm, Fig. S9) and exhibited π → π* transition. In the category of the major excited states of PDJ and PDJ-F–, their molecular orbitals and transition energies are shown in Fig. S10. With addition of F– to PDJ, the decrease of HOMO to LUMO+1 energy gap would be caused by the deprotonation of –OH proton and hydrogen bonding of –NH proton, which subsequently results in bathochromic shift. In addition, the red-shift recorded in the UV-visible experiment was well consistent with the calculated results. Based on diverse spectroscopic analyses and calculations, we envisioned the plausible detection process of PDJ to F– (Scheme 2).
Conclusion
We synthesized a reversible colorimetric chemosensor PDJ for detecting F–. PDJ exhibited selectivity only to F– by responding colorless to yellow. The limit of detection for F– was 12.1 μM. Especially, PDJ can detect F– with little interference in other anions except for S2–. Moreover, PDJ can be simply recycled through treatment with appropriate reagents such as TFA. The binding character and sensing process of PDJ with F– were demonstrated by Job plot, 1H NMR titration, DFT calculation and ESI-MS. We believe that a new reversible sensor PDJ may contribute to designing a useful fluoride probe.
References
Sahu S, Sikdar Y, Bag R et al (2019) Visual detection of fluoride ion based on ICT mechanism. Spectrochim Acta - Part A Mol Biomol Spectrosc 213:354–360
Gupta N, Singhal D, Singh AK et al (2017) A highly selective chromogenic sensor for Mn2+, turn-off fluorometric for Hg2+ ion, and turn-on fluorogenic sensor for F- ion with the practical application. Spectrochim Acta - Part A Mol Biomol Spectrosc 176:38–46
Singh A, Tom S, Trivedi DR (2018) Aminophenol based colorimetric chemosensor for naked-eye detection of biologically important fluoride and acetate ions in organo-aqueous medium: Effective and simple anion sensors. J Photochem Photobiol A Chem 353:507–520
Lim C, Seo H, Choi JH et al (2018) Highly selective fluorescent probe for switch-on Al3+ detection and switch-off F- detection. J Photochem Photobiol A Chem 356:312–320
Zhang YM, He JX, Zhu W et al (2019) Novel pillar[5]arene-based supramolecular organic framework gel for ultrasensitive response Fe3+ and F– in water. Mater Sci Eng C 100:62–69
Jeong HY, Lee SY, Kim C (2017) Furan and Julolidine-Based “Turn-on” Fluorescence Chemosensor for Detection of F– in a Near-Perfect Aqueous Solution. J Fluoresc 27:1457–1466
Ding S, Xu A, Li M et al (2020) Theoretical study on the sensing mechanism of an ON1-OFF-ON2 type fluoride fluorescent chemosensor. Spectrochim Acta - Part A Mol Biomol Spectrosc 237:118397
Das A, Dighe SU, Das N et al (2019) β-carboline-based turn-on fluorescence chemosensor for quantitative detection of fluoride at PPB level. Spectrochim Acta - Part A Mol Biomol Spectrosc 220:117099
Peng Y, Dong YM, Dong M, Wang YW (2012) A selective, sensitive, colorimetric, and fluorescence probe for relay recognition of fluoride and Cu(II) ions with “off-On-Off” switching in ethanol-water solution. J Org Chem 77:9072–9080
Yadav P, Kumari M, Jain Y et al (2020) Antipyrine based Schiff’s base as a reversible fluorescence turn “off-on-off” chemosensor for sequential recognition of Al3+ and F− ions: A theoretical and experimental perspective. Spectrochim Acta - Part A Mol Biomol Spectrosc 227:117596
Wu N, Zhao LX, Jiang CY et al (2020) A naked-eye visible colorimetric and fluorescent chemosensor for rapid detection of fluoride anions: Implication for toxic fluorine-containing pesticides detection. J Mol Liq 302:112549
Gowri A, Veeraragavan V, Kathiresan M, Kathiravan A (2019) A pyrene based colorimetric chemosensor for CO2 gas detection triggered by fluoride ion. Chem Phys Lett 719:67–71
Karuppiah K, Muniyasamy H, Sepperumal M, Ayyanar S (2020) Design and synthesis of new salicylhydrazone tagged indole derivative for fluorometric sensing of Zn2+ ion and colorimetric sensing of F– ion: Applications in live cell imaging. Microchem J 159:105543
Landge SM, Lazare DY, Freeman C et al (2020) Rationally designed phenanthrene derivatized triazole as a dual chemosensor for fluoride and copper recognition. Spectrochim Acta - Part A Mol Biomol Spectrosc 228:117758
Ma L, Leng T, Wang K et al (2017) A coumarin-based fluorescent and colorimetric chemosensor for rapid detection of fluoride ion. Tetrahedron 73:1306–1310
Fang H, Gan Y, Wang S, Tao T (2018) A selective and colorimetric chemosensor for fluoride based on dimeric azulene boronate ester. Inorg Chem Commun 95:17–21
Dong M, Peng Y, Dong YM et al (2012) A selective, colorimetric, and fluorescent chemodosimeter for relay recognition of fluoride and cyanide anions based on 1,1′-binaphthyl scaffold. Org Lett 14:130–133
Lin Q, Gong GF, Fan YQ et al (2019) Anion induced supramolecular polymerization: A novel approach for the ultrasensitive detection and separation of F–. Chem Commun 55:3247–3250
Rajasekhar K, Narayanaswamy N, Murugan NA et al (2016) A High Affinity Red Fluorescence and Colorimetric Probe for Amyloid β Aggregates. Sci Rep 6:1–10
Goswami S, Hazra A, Chakrabarty R, Fun HK (2009) Recognition of carboxylate anions and carboxylic acids by selenium-based new chromogenic fluorescent sensor: A remarkable fluorescence enhancement of hindered carboxylates. Org Lett 11:4350–4353
Lee HJ, Park SJ, Sin HJ et al (2015) A selective colorimetric chemosensor with an electron-withdrawing group for multi-analytes CN– and F–. New J Chem 39:3900–3907
Beneto AJ, Siva A (2017) A phenanthroimidazole based effective colorimetric chemosensor for copper(II) and fluoride ions. Sens Actuators B Chem 247:526–531
Moon KS, Singh N, Lee GW, Jang DO (2007) Colorimetric anion chemosensor based on 2-aminobenzimidazole: naked-eye detection of biologically important anions. Tetrahedron 63:9106–9111
Anbu Durai W, Ramu A (2020) Hydrazone Based Dual – Responsive Colorimetric and Ratiometric Chemosensor for the Detection of Cu2+/F– Ions: DNA Tracking, Practical Performance in Environmental Samples and Tooth Paste. J Fluoresc 30:275–289
Zabihi FS, Mohammadi A (2020) Synthesis and application of a new chemosensor based on the thiazolylazo-quinazolinone hybrid for detection of F− and S2− in aqueous solutions. Spectrochim Acta - Part A Mol Biomol Spectrosc 238:118439
Chatterjee C, Sethi S, Mukherjee V et al (2020) Triazole derived azo-azomethine dye as a new colorimetric anion chemosensor. Spectrochim Acta - Part A Mol Biomol Spectrosc 226:117566
Shyamaprosad Goswami RC (2012) An imidazole based colorimetric sensor for fluoride anion. Eur J Chem 3:455–460
Wang Q, Xie Y, Ding Y et al (2010) Colorimetric fluoride sensors based on deprotonation of pyrrole-hemiquinone compounds. Chem Commun 46:3669–3671
dos Santos CH, Uchiyama NM, Bagatin IA (2019) Selective azo dye-based colorimetric chemosensor for F−, CH3COO− and PO43−. Spectrochim Acta - Part A Mol Biomol Spectrosc 210:355–361
Li Z, Wang S, Xiao L et al (2018) An efficient colorimetric probe for fluoride ion based on schiff base. Inorg Chim Acta 476:7–11
Zang L, Wei D, Wang S, Jiang S (2012) A phenolic Schiff base for highly selective sensing of fluoride and cyanide via different channels. Tetrahedron 68:636–641
Wang X, Bai T, Chu T (2021) A molecular design for a turn-off NIR fluoride chemosensor. J Mol Model 27:104
Helal A, Thao NTT, Lee SW, Kim HS (2010) Thiazole-based chemosensor II: Synthesis and fluorescence sensing of fluoride ions based on inhibition of ESIPT. J Incl Phenom Macrocycl Chem 66:87–94
Lee JJ, Park GJ, Choi YW et al (2015) Detection of multiple analytes (CN– and F–) based on a simple pyrazine-derived chemosensor in aqueous solution: Experimental and theoretical approaches. Sens Actuators B Chem 207:123–132
Jo TG, Na YJ, Lee JJ et al (2015) A diaminomaleonitrile based selective colorimetric chemosensor for copper(II) and fluoride ions. New J Chem 39:2580–2587
Ganesan JS, Gandhi S, Radhakrishnan K et al (2019) Execution of julolidine based derivative as bifunctional chemosensor for Zn2+ and Cu2+ ions: Applications in bio-imaging and molecular logic gate. Spectrochim Acta - Part A Mol Biomol Spectrosc 219:33–43
Deepa A, Srinivasadesikan V, Lee SL, Padmini V (2020) Highly Selective and Sensitive Colorimetric and Fluorimetric Sensor for Cu2+. J Fluoresc 30:3–10
Yun D, Chae JB, Kim C (2019) A novel benzophenone-based colorimetric chemosensor for detecting Cu2+ and F–. J Chem Sci 131:1–10
Budzák Š, Jacquemin D (2018) Excited state intramolecular proton transfer in julolidine derivatives: An: ab initio study. Phys Chem Chem Phys 20:25031–25038
Ryu HH, Lee YJ, Kim SE et al (2016) A colorimetric F– chemosensor with high selectivity: experimental and theoretical studies. J Incl Phenom Macrocycl Chem 86:111–119
Koçak R, Dastan A (2021) Synthesis of dibenzosuberenone-based novel polycyclic π-conjugated dihydropyridazines, pyridazines and pyrroles. Beilstein J Org Chem 17:719–729
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09. Gaussian Inc, Wallingford CT
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Hariharan PC, Pople JA (1973) The influence of polarization functions on molecular orbital hydrogenation energies. Theor Chim Acta 28:213–222
Francl MM, Pietro WJ, Hehre WJ et al (1982) Self-consistent molecular orbital methods. XXIII. A polarization-type basis set for second-row elements. J Chem Phys 77:3654–3665
Klamt A, Moya C, Palomar J (2015) A Comprehensive Comparison of the IEFPCM and SS(V)PE Continuum Solvation Methods with the COSMO Approach. J Chem Theory Comput 11:4220–4225
Kumari N, Jha S, Bhattacharya S (2011) Colorimetric probes based on anthraimidazolediones for selective sensing of fluoride and cyanide ion via intramolecular charge transfer. J Org Chem 76:8215–8222
Yang R, Li K, Wang K et al (2003) Porphyrin assembly on β-cyclodextrin for selective sensing and detection of a zinc ion based on the dual emission fluorescence ratio. Anal Chem 75:612–621
Olivieri AC (2014) Analytical figures of merit: From univariate to multiway calibration. Chem Rev 114:5358–5378
Acknowledgements
We politely acknowledged National Research Foundation of Korea (2018R1A2B6001686).
Author information
Authors and Affiliations
Contributions
Dongkyun Gil (60% contributions), Boeon Suh (10% contributions), Cheal Kim (30% contributions).
Corresponding author
Ethics declarations
Ethical Approval
This article does not contain any studies with human or animal subjects.
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gil, D., Suh, B. & Kim, C. A New Reversible Colorimetric Chemosensor Based on Julolidine Moiety for Detecting F−. J Fluoresc 31, 1675–1682 (2021). https://doi.org/10.1007/s10895-021-02801-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-021-02801-5