Abstract
Butterfly species often synchronize their life cycles to seasonality, as increasing temperature and rainfall act as clues of resource availability. Nevertheless, human-made forest edges cause major changes in the microclimatic conditions that may jeopardize the synchrony between insects and favorable conditions for their emergence, conversely to natural ecotones. Here, the distribution of fruit-feeding butterflies was studied over one year in three different habitats (forest interior, forest ecotone, forest edge) to examine if: (i) species richness and abundance varies among habitats and subfamily/tribe over the year; (ii) temperature and rainfall affect the abundance and temporal distribution of species richness; and (iii) the beta diversity and its monthly partition are similar among habitats. The present study was carried out in the Rio Doce State Park, Brazil, a 36,000 ha forest reserve. In total, 11,594 individuals representing 98 butterfly species were collected. The butterflies presented a nonuniform distribution of abundance in all habitats, with greater abundance, richness and species diversity during the wet season. Butterfly abundance increased with high temperatures in all habitats. The contribution of species turnover and nestedness varied over the months, overlapping with the seasonal changes. Understanding how rates of species turnover vary over time in different habitats can help explain the vulnerability of species to environmental changes, allowing comparison of assemblages over time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most tropical insects have some degree of response to seasonality, which can be measured in temporal variation in abundance throughout the year (Wolda 1988; Kishimoto-Yamada and Itioka 2015), as many insect populations synchronize their life cycles at or about the beginning of the wet seasons (Wolda 1989). During the wet season, the conditions for the development of most tropical organisms, such as high temperature and rainfall and resource availability, reach their optimum levels (Kishimoto-Yamada and Itioka 2015). The span and number of demographic peaks in a year are determined by the interactions of the environmental conditions, including the diversity of natural enemies (Didham and Springate 2003; Ribeiro et al. 2005) and the overlapping generations of the populations (uni-, bi- and multi-voltinism for one, two and three or more overlapping generations in a year, respectively, Gullan and Cranston 2010), with multivoltinism being the most common strategy for many tropical insects (Wolda 1988; Kishimoto-Yamada and Itioka 2015). Rainfall is recognized as a main predictor of insect emergence, and both the onset and the cessation of the rains play important role in the regulation of insect activity pulses (Wolda 1988, 1989). Therefore, wet and dry seasons impose ecological filters that affect both species abundance and detectable richness (Grøtan et al. 2012). However, although rainfall might be the cue hatching and breaking diapause for tropical organisms, other factors such as temperature, resource availability and the abundance of enemies may actually affect growth, survival and reproduction of herbivore insects (Didham and Springate 2003).
A temporal increase in temperature, along with constancy in rainfall, seems to act as clues of resource availability, triggering behaviors such as flight/dispersal, foraging and reproduction of tropical insects (Torres-Vila and Rodríguez-Molina 2002; Didham and Springate 2003; Kishimoto-Yamada and Itioka 2015). Even though some resources (e.g. leaves) are available all year round, their quality may vary over time generating some favorable periods throughout (Hunter and Lechowicz 1992; Aide 1993). For instance, herbivorous insects prefer young, tender, leaves to mature leaves (Coley 1983; Aide 1993; Ribeiro et al. 1994). Specifically, for butterflies, leaf availability and new plant tissues regulate the optimum period for caterpillar development (Murakami et al. 2008). The temporal variation in the availability of resources may regulate the activity patterns of adults butterflies, as previous data have suggested (Hamer et al. 2006; Ribeiro et al. 2010). Therefore, the temperature and rainfall may indicate favorable times for insect development.
The advance of the agricultural frontier and the increasing fragmentation of tropical habitats contributes to the formation of many human-made forest edges where microclimatic conditions change abruptly compared to the closed canopy forest (Murcia 1995; Steffen et al. 2015). This change in environmental conditions in anthropic forest edges may alter the synchrony between local environmental conditions and the emergence and growth of insect population (Hamer et al. 2005; Ribeiro and Freitas 2011). For instance, the rise in temperature in forest edges locally increase the activity of butterflies, with possible consequences for their life cycles (Ribeiro and Freitas 2010). In addition, for many phytophagous insects, the loss and reduction in size and quality of breeding areas and availability of larval host plants may contribute to population declines and changes in the community structure (Basset et al. 2015; Thomas 2016). Consequently, it is expected that the loss of synchrony with the favorable period for the emergence of several butterfly species may affect the demography and diversity among the different habitats. Lourenço et al. (2019) in a recent study showed that the structure of fruit-feeding butterfly assemblages (i.e. those whose adults primarily obtain resources by feeding on rotten fruits or fermenting sap; DeVries 1987) in the natural transitions (ecotones) was more similar to the forest interior than to the anthropic edges. Although the ecotones and the forest anthropic edges were richer and more diverse than the forest interior, the pattern of species distribution in the anthropic edges was distinct from the ecotone in various aspects, suggesting a lower predictability in the latter (Lourenço et al. 2019). Hence, the temporal variation in the fruit-feeding butterfly assemblages in contrasting habitats in the same forest ecosystem offers an opportunity to investigate how the variation in climatic and micro-climatic conditions throughout the year influence butterfly species and ultimately the whole butterfly assemblage.
Historically, the Brazilian Atlantic rainforest has experienced strong anthropic impacts since the early colonization in the Atlantic coast. Thereby, it became one of the most fragmented tropical forest biomes in South America with a consequent increase of forest edges in the last four centuries (Ribeiro et al. 2009). Fruit-feeding butterflies are considered an excellent model for studies of assemblage structure and temporal variation in diversity, as they are ecologically diverse, sensitive to seasons and to habitat fragmentation, which allows simultaneous and standardized sampling in several areas (Bonebrake et al. 2010; Freitas et al. 2014; Sant’Anna et al. 2014; DeVries et al. 2016). The present study examines the temporal variation in abundance of the fruit-feeding butterfly assemblages through one year in different habitats of the Atlantic forest (forest interior, ecotone and anthropic forest edge) with marked differences in their environmental conditions. The following hypotheses were tested: (i) the abundance of adult fruit-feeding butterflies is concentrated at certain periods of the year, with predictions that demographic peaks when temperature and rainfall increase; (ii) the increase in temperature and rainfall positively affect the species richness and abundance of butterflies, with the predictions that these yearly variation act as clues for resource availability and low enemy pressure; and (iii) the temporal beta diversity and its partition are similar between natural habitats (forest interior and ecotone) and distinct to the anthropic edges, because the natural habitats present similar conditions for the different species to develop, resulting in similar patterns of species substitution through time.
Material and methods
Study site
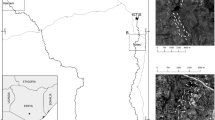
The study was carried out in the Rio Doce State Park (hereafter PERD, following the Portuguese abbreviation) (19° 48′–19° and 42° 38′–42° 28′ W), in the municipalities of Marliéria, Timóteo and Dionísio, state of Minas Gerais, southeastern Brazil. The PERD covers an area of approximately 36,000 ha of Atlantic rainforest with an elevational range from 200 up to 500 m encompassing a complex lake system that includes over 42 lakes of different sizes. These lakes were formed by tectonic movements during the Holocene around 10–8 thousand years ago, and the surrounding rainforest arose more recently (about 4500 years old) replacing a more xeric ecosystem (Fonseca-Silva et al. 2015). The current prevailing conditions correspond to Aw climate (tropical seasonal) on the Köppen classification, with a wet season from October to April and a dry season from May to September. The average annual temperature and precipitation are 21.9 ºC and 1480 mm, respectively (Alvares et al. 2014; CBH-Doce 2009).
Sampling methods
The butterflies were sampled in three different habitats in the PERD (for more details see, Lourenço et al. 2019): (i) the interior of the forest (hereafter called “forest interior”), at least 50 m distant from any border, with a canopy up to 10–25 m in height; (ii) natural contacts of forest with lakes or flooded grasslands (hereafter “ecotone”), with high light availability, resulting in the formation of a “brought-low canopy” (5–15 m high, sensu Lourenço et al. 2019) where the main branches naturally bent towards the lakes at 1–3 m above the ground, and have similar characteristics of forest canopy (Barbosa 2014); and (iii) anthropic edges (hereafter “edge”), a result of planned cut within the park, as in borders of dirt roads and facilities, with a canopy higher than the ecotone (between 10–30 m), but dominated by saplings and young trees close to the ground, right on the edge.
The sample design follows DeVries et al. (1999), modified after Ribeiro and Freitas (2012). Three transects of approximately 250 m in length were selected per habitat. Each habitat transect was separated by at least 1 km in distinct locations, constituting independent samples (Ribeiro and Freitas 2012). At each transect (considered a sampling unit) 10 portable traps (Van Someren-Rydon—VSR) were installed, spaced 25 m between each other, and alternating between canopy (1–3 m below the canopy surface) and understory (1.5 m above ground). Traps were baited with a mix of banana and sugar cane juice at a 3:1 ratio, fermented for 48 h. Fieldwork was carried out monthly, from August 2015 to July 2016 (n = 12 months). Every month the traps were activated and were left open for four consecutive days with visits and bait refreshment every 48 h totaling 4320 trap-days (10 traps × 3 transects × 3 habitats × 4 sampling days × 12 months). All captured butterflies were recorded and marked with a sequential number on the right posterior wing, in order to avoid overestimating butterfly abundance, thus those eventually recaptured were not counted as new individuals. Those individuals not identified in the field and those that died in the traps (n = 5958; 51.4%) were taken to the lab for later identification. For every captured species, three individuals (whenever possible) were spread and deposited at the Museu de Zoologia of the Universidade Estadual de Campinas, São Paulo, Brazil (ZUEC), as well as in the Laboratório de Ecohealph, Ecologia de Insetos de Dossel e Sucessão Natural of the Universidade Federal de Ouro Preto, Minas Gerais, Brazil.
Data analyses
For comparative purposes with previous studies, in all analyses the Nymphalidae taxonomy followed Freitas and Brown (2004) modified after Wahlberg et al. (2009) (subfamilies Biblidinae, Charaxinae, Satyrinae and the Nymphalinae tribe Coeini). The subfamily Satyrinae was subdivided into three tribes (Satyrini, Morphini and Brassolini) since they are distinct in several morphological, ecological and behavioral traits (see Freitas et al. 2014). Only a single individual of the tribe Haeterini (Satyrinae) was captured, therefore, it was excluded from the analyzes.
To test whether the abundance of fruit-feeding butterflies was distributed evenly throughout the year, a circular statistically approaches was implemented (Jammalamadaka and SenGupta 2001). Periodic changes such as those occurring on daily and yearly basis are cyclical in nature and, thus, it is possible to consider that month to month data over a year resemble circular data. For example, by using yearly data, the first data is the January 1st and the last data is the December 31st, however, December 31st is separated from January first in just one day. The previous example shows that in yearly data there is just one unit of difference between the first and the last data, note that this is one of the most important properties of circular statistics; because a circle starts at 0° and ends in 360°, however 0° is equal to 360°. Therefore, for the description of yearly data of butterfly abundance and species richness, it was tested whether the data were evenly distributed along the year or if they were clustered around some specific time periods (Jammalamadaka and SenGupta 2001). For this, data was divided by subfamily or tribe (Biblidinae, Charaxinae, Nymphalinae, Satyrinae: Brassolini, Morphini and Satyrini). Monthly samplings were coded as intervals of 30° of circumference (12 moths/360° of circumference) and then expressed as radians (by multiplying the degrees by π/180), that when transformed in sine and cosine are truly circular. Under this approach each observation is represented as a vector defined by the sine and cosine of the bearings in radians, therefore the bearings of the overall resulting vector represent the mean orientation (µ) and the average length of the resulting vector is used to test whether the orientation, in this case occurrence through the year, is random or nonrandom. The average length of the resulting vector ranges from zero (random orientation) to one (if all records occurred in the same sampling period). Specifically, Rayleigh test of uniformity was used to asses if the data presented a preferred direction (i.e., concentration of data towards a cardinal point; Ruxton 2017) and this was performed with the R software 3.4.0 (R Core Team 2017) using the package “circular” (Agostinelli and Lund 2017). In addition, to better describe the data, the concentration parameter of the distribution (which ranges from 0 and 1/pi), the mean circular angle and its circular standard deviance were calculated (according to Ruxton 2017).
Rarefaction curves were used to compare species richness for each month over one year in each habitat. Rarefaction is a statistical method of estimating the number of species expected in a random sample of individuals taken from a collection (Colwell and Coddington 1994). Given the number of individuals in each species for the collection, one can calculate how many species would be expected in a smaller sample of n individuals (Colwell and Coddington 1994); in this case, the total number of samples were 12, as it is the total number of the sampled months. To test if the mean temperature predicts the temporal distribution of species richness and abundance of butterflies, two mixed linear models were fitted. In which the dependent variable were abundance or species richness (one model for each variable), the fixed factor was the mean temperature of the transects for each month (measured in the field during of traps visits), the type of habitat as a categorical factor (forest interior, ecotone and edge) and each transect was considered as a random factor. The statistical model structure corresponds to an ANCOVA with three categorical factors. Temporal autocorrelation (time-lag) was examined to detect if there was independence between the values observed in the sampling months.
To assess the variation in butterfly species composition through time, the pooled data from all transects were aggregated by habitat and months (12 units per habitat). The scales analyzed were the accumulated diversity among the three transects of each habitat per months alpha diversity (α), and the beta diversity (hereafter β diversity) represents the dissimilarities of butterfly species composition between consecutive months. The additive partitioning of species β diversity is an approach that allows to disentangle the temporal variation in the turnover and richness differences (nestedness) between communities, as well as its variation over space (Baselga et al. 2015). In this study, the framework proposed by Baselga et al. (2015) was used and thereby the additive partition of beta diversity was performed using the “vegan” and “betapart” packages in R. Three dissimilarity matrices were also computed based on the Bray–Curtis index to consider the species abundance for β diversity calculations (Baselga et al. 2015). This analysis results in three dissimilarity matrices based on the Bray–Curtis index: (i) temporal turnover (i.e. replacement of some species by others from time to time), (ii) nestedness (i.e. species found on one site represent a subset of another site from time to time, richness differences between habitats), and (iii) total β diversity. It is important to stress that temporal changes in species composition can be related to both temporal turnover and richness differences (nestedness) from time to time (Baselga et al. 2015). All the statistical analyses were performed using the software R 3.4.0 (R Core Team 2017).
Results
In total, 11,594 individuals belonging to 98 fruit-feeding butterfly species were captured in 12 months of study. The captured specimens belonged to four subfamilies (Online Resource 1 and 2): Biblidinae, represented by 5339 individuals (46.05%), Satyrinae 3650 individuals (31.48%), Charaxinae 2495 individuals (21.52%) and Nymphalinae 110 individuals (0.95%). Both, species richness and abundance, varied over the months (Fig. 1, Table 1). Rarefaction analysis revealed that the wet months (January to March) had lower species richness than the remaining months in all three habitats (Online Resource 3). The monthly variation in the proportion of subfamilies/tribes was similar among the three habitats, with abundance peaks of each taxon highly coincident among habitats (Fig. 2).
The circular analysis showed that the distribution of abundance of fruit-feeding butterflies throughout the year were nonuniform and nonrandom in all habitats for the total sampled assemblage and for each subfamily/tribe tested (Fig. 3). The mean vector (µ) and the circular standard deviation of the total sample were similar among habitats, with 73.7% of the total abundance in the forest interior concentrated from August to March. Whereas, the period from September to March concentrated 66.3% and 68.9% of the total abundance in the ecotone and the edge, respectively. Considering the abundance distribution of each subfamily/tribe, the mean vector of Biblidinae was in December in the forest interior, while in the ecotone and in the edge, it was in January. Both Charaxinae and Morphini had the mean vector and the higher abundances in November in all habitats. However, the Morphini (here represented basically by Morpho helenor) showed a clearly bimodal distribution for all habitats, with a large peak in November and lower peak April. Nymphalinae were concentrated in December in the ecotone, while in the forest interior it occurred in February and in the edge in March, but the small mean vector and distributions clearly shows that the group was in general evenly distributed along the year. The Brassolini presented the higher abundances in November in all three habitats, but the mean vectors were slightly displaced to December. Although the peaks of abundance were not synchronous in all three habitats, the Satyrini were in general evenly distributed along the year, as indicated by the poorly defined mean vectors (Fig. 3).
Circular diagrams of the number of individuals observed for the total sampled assemblage and for subfamily/tribe of fruit-feeding butterflies in each habitat throughout the year, in Rio Doce State Park, Brazil. The arrows represent the average vector length (r) and indicate the average dates, the red area represent standard deviation
Considering the 10 most abundant species [more than 300 individuals captured, namely Taygetis rufomarginata (1405 individuals), Hamadryas amphinome (1164), H. epinome (950), Fountainea ryphea (883), H. feronia (694), H. laodamia (539), Zaretis strigosus (438), Memphis moruus (400), Callicore astarte (398), Paulograma pygas (316)], for most of them, the variation in abundance was congruent among the three habitats (Fig. 4). For three species, however, the patterns of variation in abundance were different in specific habitat types. In a first case, T. rufomarginata presented constant lower abundances in the edge compared with the natural habitats (forest interior and ecotone). In a second case, Hamadryas feronia presented overall low abundances in the forest interior and edge (less than 20 individuals per month), while presenting higher abundances in the ecotone. Finally, for Paulograma pygas the abundance was low in all three habitats during most of the year, except during the late wet season, when the abundance in the edge was about twice or more than in the natural habitats.
Most abundant fruit-feeding butterfly species per habitats throughout a year (more than 300 individuals across 12 months), in Rio Doce State Park, Brazil. The symbols represent the habitats: forest interior dark grey circle, ecotone light grey square, edge white triangle; the shaded area represents the wet season
The linear regression analysis showed that the species richness of fruit-feeding butterfly increased with mean temperature (F = 5.28, df = 2, P < 0.05), but this result was only significant in the forest interior (Fig. 5). The mean temperature also explained fruit-feeding butterfly abundance (F = 21.2, df = 2, P < 0.05), and this result was observed for all habitats (Fig. 5). There was no statistical interaction among mean temperature and habitat (abundance F = 0.10, df = 2, P = 0.9; richness F = 0.21, df = 2, P = 0.8). Rainfall was not related to either abundance or species richness.
Fruit-feeding butterfly abundance (above) and richness (below) per habitat corresponding to mean temperature (ºC), in Rio Doce State Park, Brazil. The symbols represent the habitats: circle forest interior, square ecotone and triangle edge; the solid lines represent the tendency and dashed lines represent the non-significant slopes for each habitat
Overall, the temporal partition of the β diversity of all months was similar across the studied habitats, with monthly variations overlapping with seasonal changes. However, the variation of the β diversity among months was clearer in the forest interior (Online Resource 4). For example, at the transition from the wet to the dry season (March to April), the forest interior has a greater species nestedness, that is, the months presented similar species richness. While, in this same period, in the ecotone and edge prevailed species turnover, that is, species composition changed over the months. During the following period, the early dry season (April and May), nestedness remained as the main process in the forest interior, but not in the edge, where seasonal turnover remains as a main process driving species variation, nor in the ecotone where a growth of nestedness contribution is noted. In the beginning of the wet season (October and November) the turnover process prevails in all the habitats, followed again by changes due to richness differences in the following period (November and December).
Discussion
It’s usually suggested that butterfly life cycles synchronize with the periods of greater resource availability, mating season and low enemy pressure (Brown 1992; Ribeiro et al. 2010). Following a quantitative temporal approach, the results of the present study demonstrated that the peaks of butterfly species richness, abundance and diversity observed during the wet season started with the seasonal changes occurring during the transition from dry to rainy season. Additionally, the intense leaf production after the first rains would be important for the success of immatures, as well as the following greater availability of fleshy fruits, essential for adults (Aide 1993; Morellato et al. 2000; Fischer et al. 2004). Although there are very few studies that investigated the yearly variation of butterfly diversity, the peaks of species richness and abundance during the transition from dry to wet season appear to be a recurrent pattern for several sites in the tropical regions (Brown 1992; DeVries et al. 1999; Ribeiro et al. 2010; Grøtan et al. 2012, 2014). Similar patterns have been also reported for other tropical insects, including beetles, bees and mosquitoes (see Kishimoto-Yamada and Itioka 2015), supporting the hypothesis that the onset of wet season triggers pulses of productivity influencing the emergence of several tropical organisms (Wolda 1989; Kishimoto-Yamada and Itioka 2015).
Seasonal changes are accompanied by variation in average temperature that plays an important role for diversification and species coexistence in plant and animal communities (Wolda 1988; Peters et al. 2016). In fact, temperature is an important driver in insect metabolism and thus an accelerator of development rates and adult reproductive activity (Wolda 1988). Higher temperatures, combined with high water availability, are known to trigger primary productivity and can predict species richness and other diversity components (e.g., species interactions) for taxonomically broad communities of both, plants and animals (Wolda 1988; Peters et al. 2016; Dáttilo and Vasconcelos 2019). In the present study, higher temperatures increased the butterfly abundance and richness, as reported by other studies with fruit-feeding butterfly (Ribeiro and Freitas 2010; Ribeiro et al. 2010; Grøtan et al. 2012; Santos et al. 2017). However, while a clear positive effect of mean temperature on abundance was observed in all three habitat types, the effect on species richness was detected only in the forest interior. It is possible that other factors such as vegetation structure, host plant availability and microclimatic conditions, all very important for the maintenance of viable butterfly populations (Saunders et al. 1991; Shahabuddin and Terborgh 1999; Hamer et al. 2006; Beirão et al. 2017), are playing an important role to predict the species richness in the transitional habitats (ecotones and forest edges) and overcoming the effects of the mean temperature.
The ecotones and forest edges studied here were generally richer and presented higher abundance of butterflies when compared to the forest interior throughout the year. This was expected because it is largely known that transitions (both natural and anthropic) are usually richer and more diverse than adjacent habitats, since they present characteristics of two nearby environments (Holland 1988). Additionally, the subfamily/tribes abundance and their proportions varied similarly among the habitats throughout the year. Seasonal patterns were evident for some subfamilies/tribes that coincide with that described in the literature. For example, the bimodal pattern of Morpho helenor (Morphini), with two distinct population peaks in all three habitat types is similar to the pattern reported for this species in other localities (Carreira 2015; Freire et al. 2014; Ribeiro et al. 2010; Santos et al. 2017) and should be associated with availability of fleshy fruits and with the regrowth period that are similar in the different studied sites (Morellato and Leitão-Filho 1992; Morellato et al. 2000). Additionally, the variation of resource availability and distinct microclimatic conditions among habitat type can trigger different species responses. Some butterfly species such as Taygetis rufomarginata (Satyrini) is a good example of this; in the present study, this species had highlighted peaks of abundance in natural habitats (forest interior and ecotone) over the year, while had few individuals in the edge. This result corroborated the pattern described by Uehara-Prado et al. (2007) that showed that large Satyrinae species prefer well preserved habitats.
In general, the monthly fluctuation of beta diversity was similar among habitats, although the variation was more evident in the forest interior. This variability can be related to the presence of a favorable season (hot and wet) with great resource abundance and other unfavorable (cold and dry) with little available resources (Brown 1992). In the transition between dry and wet seasons (September to November) the higher temperatures and the constant rainfall lead to the regrowth of many plants representing a period of bonanza promoting the development of the Nymphalidae family first generation (see Brown 1992). The next period (November–December) overlaps with the adult emergence peak of the species already present in the butterfly assemblage, which explains the greater diversities observed in all three habitats The changes in assemblage composition decrease significantly at the end of the wet season and at the beginning of the dry season, although in this period the transitional habitats change the species composition more than the forest interior. Finally, during the dry season there was an increase in diversity and a large change in butterfly assemblage structure in all habitats, in contrast to previous reports for the Atlantic Forest (Brown 1992; Ribeiro et al. 2010). This difference is likely related to the local climatic conditions; while the previous studies were in areas of where the dry season is also cold and present a lower productivity (Brown 1992; Ribeiro et al. 2010), the PERD has a comparatively higher mean monthly temperature, which favors the butterfly development and activity (Wolda 1988; Ribeiro and Freitas 2010).
As a final point, the importance of the present results goes beyond a better understanding of insect ecology in the tropics. The information here presented may help provide guidance for environmental diagnostics and monitoring, regardless of the aspects to be investigated, including anthropic changes (e.g. effects of forest logging and fire incidence, among other). Due to time and financial constraints, monitoring programs rarely include long term sampling. Therefore, knowing the best sampling period of an increasingly used indicator group such as butterflies helps to focus the samplings at the best periods (in this case, dry–wet transition and/or only wet season), combining the periods of highest richness and highest abundant of the group (Santos et al. 2016). The present results showed yet that transitional habitats (natural or anthropic transitions) are more variable in terms of diversity than the forest interior throughout the year. Thus, the results suggested that the maintenance of viable butterfly populations is more difficult in these transitional habitats, and events of local extinctions may occur over time, affecting species turnover and community composition. Determining how the rates of species turnover vary over time in different habitats is important to understand the sensitivity of the ecological systems to environmental change, a quite relevant task in a scenario of future climate change. All the above points should be taken into account for assessing the effectiveness of the tropical protected areas in maintaining diversity in the face of changing world.
References
Agostinelli C, Lund U (2017) R package ‘circular’: Circular statistics. Version 0.4–93. https://r-forge.r-project.org/projects/circular/. Accessed 28 August 2017
Aide M (1993) Patterns of leaf development and herbivory in a tropical understory community. Ecology 74:455–466
Alvares CA, Stape JL, Sentelhas PC, Gonçalves JLM, Sparovek G (2014) Köppen’s climate classification map for Brazil. Meteorol Z 22:128–711
Barbosa BC (2014) Arquitetura de ramos, alocação de biomassa e herbivoria em duas espécies arbóreas com diferentes histórias de vida em uma Floresta Tropical Semidecidual. M.Sc. Thesis, Universidade Federal de Minas Gerais. Available from: http://www.bibliotecadigital.ufmg.br/dspace/handle/1843/BUOS-9K5HF7
Baselga A, Bonthoux S, Balent G (2015) Temporal beta diversity of bird assemblages in agricultural landscapes: land cover change vs. stochastic processes. PLoS ONE 10:1–14
Basset Y, Barrios H, Segar S, Srygley RB, Aiello A, Warrens AD, Delgado F, Coronado J, Lezcano J, Arizala S, Rivera M, Perez F, Bobadilla R, Lopez Y, Ramirez JA (1930s) The butterflies of Barro Colorado Island, Panama: local extinction since the 1930s. PLoS ONE 10:1–22
Beirão MV, Neves FS, Penz CM, Devries PJ, Fernandes GW (2017) High butterfly beta diversity between Brazilian cerrado and cerrado-caatinga transition zones. J Insect Conserv 21:1–12
Bonebrake TC, Ponisio LC, Boggs CL, Ehrlich PR (2010) More than just indicators: a review of tropical butterfly ecology and conservation. Biol Conserv 143:1831–1841
Brown KS (1992) Borboletas da Serra do Japi: diversidade, habitats, recursos alimentares e variação temporal. In: Morellato LPC (ed) História Natural da Serra do Japi: Ecologia e Preservação de uma Área Florestal no Sudeste do Brasil. Unicamp, Campinas, pp 142–186
Carreira JYO (2015) Dinâmica temporal e sazonalidade de borboletas frugívoras na Mata Atlântica. Dissertation, Universidade Estadual de Campinas
Cbh-Doce—Comitê da Bacia Hidrográfica do Rio Doce (2009) Parque Estadual do Rio Doce. https://www.riodoce.cbh.gov.br/. Accessed 26 September 2013
Coley PD (1983) Herbivory and defensive characteristics of tree species in a lowland tropical forest. Ecol Monogr 53:209–234
Colwell RK, Coddington JA (1994) Terrestrial biodiversity through extrapolation. Phil Trans R Soc Lond 345:101–118
Dáttilo W, Vasconcelos HL (2019) Macroecological patterns and correlates of ant-tree interaction networks in Neotropical savannas. Global Ecol Biogeogr 00:1–12
Devries PJ (1987) The butterflies of Costa Rica and their natural history. Princeton University Press, Princeton
Devries PJ, Hamm CA, Fordyce JA (2016) A standardized sampling protocol for fruit-feeding butterflies (Nymphalidae). In: Larsen TH (ed) Core Standardized Methods for Rapid Biological Field Assessment. Conservation International, Arlington, pp 140–148
Devries PJ, Walla TR, Greeney HF (1999) Species diversity in spatial and temporal dimensions of fruit-feeding butterflies from two Ecuadorian rainforests. Biol J Linn Soc 68:333–353
Didham RK, Springate ND (2003) Determinants of temporal variation in community structure. In: Basset Y et al. (eds) Arthropods of tropical forests: spatio-temporal dynamics and resource use in the canopy. Cambridge University Press, Cambridge, pp 28–39
Fischer K, DM, O’brien, CL, Boggs (2004) Allocation of larval and adult resources to reproduction in a fruit-feeding butterfly. Funct Ecol 18:656–663
Fonseca-Silva FM, Carvalho MA, Ribeiro SP (2015) Caracterização da matéria orgânica particulada dos últimos 10 mil anos a partir de um testemunho do Parque Estadual do Rio Doce, Mg, Brasil: implicações paleoambientais. Rev Bras Paleontol 18:161–170
Freire GBJ, Nascimento AR, Malinov IK, Diniz IR (2014) Temporal occurrence of two Morpho butterflies (Lepidoptera: Nymphalidae): influence of weather and food resources. Environ Entomol 43:274–282
Freitas AVL, Brown KS (2004) Phylogeny of Nymphalidae (Lepidoptera). Syst Biol 53:363–383
Freitas AVL, Iserhard CA, Santos JP, Carreira JYO, Ribeiro DB, Melo DHA, Rosa AHB, Marini-Filho OJ, Accacio GM, Uehara-Prado M (2014) Studies with butterfly bait traps: an overview. Rev Colomb Entomol 40:203–212
Grøtan V, Lande R, Chacon IA, Devries PJ (2014) Seasonal cycles of diversity and similarity in a Central American rainforest butterfly community. Ecography 37:509–516
Grøtan V, Lande R, Engen S, Sæther BE, Devries PJ (2012) Seasonal cycles of species diversity and similarity in a tropical butterfly community. J Anim Ecol 81:714–723
Gullan PJ, Cranston PS (2010) The insects: an outline of entomology. Wiley-Blackwell Ltd, Oxford
Hamer KC, Hill JK, Mustaffa N, Benedick S, Sherratt TN, Chey VK, Maryati M (2005) Temporal variation in abundance and diversity of butterflies in Bornean rain forests: opposite impacts of logging recorded in different seasons. J Trop Ecol 21:417–425
Hamer KC, Hill JK, Benedick S, Mustaffa N, Chey VK, Maryati M (2006) Diversity and ecology of carrion- and fruit-feeding butterflies in Bornean rain forest. J Trop Ecol 22:25–33
Hunter AF, Lechowicz MJ (1992) Foliage quality changes during canopy development of some northern hardwood trees. Oecologia 89:316–323
Holland MM (1988) Scope/Mab Technical Consultations on Landscape Boundaries. Report of a Scope/Mab Workshop on Ecotones. In: Castri F, Hansen AJ, Holland MM (ed) A new look at Ecotones: Emerging International Projetcts on Landscape Boundaries. Special inssue 17. The Internacional Uni on of Biological Scienses, pp 47–106
Jammalamadaka SR, Sengupta A (2001) Topics in circular statistics Series on multivariate analysis. World Scientific publishing, Singapore
Kishimoto-Yamada K, Itioka T (2015) How much have we learned about seasonality in tropical insect abundance since Wolda (1988)? Entomol Sci 18:407–419
Lourenço GM, Soares GR, Santos TP, Dáttilo W, Freitas AVL, Ribeiro SP (2019) Equal but different: natural ecotones are dissimilar to anthropogenic edges. PLoS ONE 14:1–18
Magurran AE (2007) Species abundance distributions over time. Ecol Lett 10:347–354
Morellato LPC, Leitão-Filho HF (1992) Padrões de frutificação e dispersão na Serra do Japi. In: Morellato LPC (ed) História Natural da Serra do Japi: Ecologia e Preservação de uma Área Florestal no Sudeste do Brasil. Unicamp, Campinas, pp 112–141
Morellato LPC, Talora DC, Takahasi A, Bencke CC, Romera EC, Zipparro VB (2000) Phenology of Atlantic Rain Forest trees: a comparative study. Biotropica 32:811–823
Murakami M, Ichie T, Hirao T (2008) Beta-diversity of lepidopteran larval communities in a Japanese temperate forest: effects of phenology and tree species. Ecol Res 23:179–187
Murcia C (1995) Edge effects in fragmented forests: implications for conservation. TREE 10:58–62
Peters MK, Hemp A, Appelhans T, Behler C, Classen A, Detsch F, Ensslin A, Ferger SW, Frederiksen SB, Gebert F, Haas M, Helbig-Bonitz M, Hemp C, Kindeketa WJ, Mwangomo E, Ngereza C, Otte I, Röder J, Rutten G, Costa DS, Tardanico J, Zancolli G, Deckert J, Eardley CD, Peters RS, Rödel MO, Schleuning M, Ssymank A, Kakengi V, Zhang J, Böhning-Gaese K, Brandl R, Kalko EKV, Kleyer M, Nauss T, Tschapka M, Ficher M, Steffan-Dewenter I (2016) Predictors of elevation biodiversity gradients change from single taxa to the multi-taxa community level. Nat Commun 7:1–11
R Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org/. Accessed 08 June 2017
Ribeiro DB, Freitas AVL (2010) Differences in thermal responses in a fragmented landscape: temperature affects the sampling of diurnal, but not nocturnal fruit-feeding Lepidoptera. J Res Lepid 42:1–4
Ribeiro DB, Freitas AVL (2011) Large-sized insects show stronger seasonality than small-sized ones: a case study of fruit-feeding butterflies. Biol J Linn Soc 104:820–827
Ribeiro DB, Freitas AVL (2012) The effect of reduced-impact logging on fruit-feeding butterflies in Central Amazon, Brazil. J Insect Conserv 16:733–744
Ribeiro SP, Borges PP, Gaspar C, Melo C, Serrano ARM, Amaral J, Aguiar C, Andre G, Quartau JA (2005) Canopy insect herbivores in the Azorean Laurisilva forests: key host plant species in a highly generalist insect community. Ecography 28:315–330
Ribeiro MC, Metzger JP, Martensen AC, Ponzoni FJ, Hirota MM (2009) The Brazilian Atlantic Forest: how much is left, and how is the remaining forest distributed? Implications for conservation. Biol Conserv 142:1141–1153
Ribeiro DB, Prado PI, Brown KS, Freitas AVL (2010) Temporal diversity patterns and phenology in fruit-feeding butterflies in the Atlantic Forest. Biotropica 42:710–716
Ribeiro SP, Pimenta HR, Fernandes GW (1994) Herbivory by chewing and sucking insects on Tabebuia ochracaea. Biotropica 26:302–307
Ruxton GD (2017) Testing for departure from uniformity and estimating mean direction for circular data. Biol Lett 13:20160756
Santos JP, Marini-Filho OJ, Freitas AVL, Uehara-Prado M (2016) Monitoramento de borboletas: o papel de um indicador biológico na gestão de Unidades de Conservação. Biodiversidade Brasileira 6:87–99
Santos JP, Iserhard CA, Carreira JYO, Freitas AVL (2017) Monitoring fruit-feeding butterfly assemblages in two vertical strata in seasonal Atlantic Forest: temporal species turnover is lower in the canopy. J Trop Ecol 33:345–355
Sant’anna CLB, Ribeiro DB, Garcia LC, Freitas AVL (2014) Fruit-feeding butterfly communities are influenced by restoration age in tropical forests. Restor Ecol 22:480–485
Saunders DA, Hobbs RJ, Margules CR (1991) Biological consequences of ecosystem fragmentation: a review. Conserv Biol 5:18–32
Steffen W, Richardson K, Rockstrom J, Cornell SE, Fetzer I, Bennett EM, Biggs R, Carpenter SR, Vries W, Wit CA, Folke C, Gerten D, Heinke J, Mace GM, Persson LM, Ramanathan V, Reyers B, Sörlin S (2015) Planetary boundaries: guiding human development on a changing planet. Science 347:1259855
Torres-Vila LM, Rodríguez-Molina MC (2002) Egg size variation and its relationship with larval performance in the Lepidoptera: the case of the European grapevine moth Lobesia botrana. Oikos 99:272–283
Thomas JA (2016) Butterfly communities under threat. Science 353:216–218
Uehara-Prado M, Brown KS, Freitas AVL (2007) Species richness, composition and abundance of fruit-feeding butterflies in the Brazilian Atlantic Forest: comparison between a fragmented and a continuous landscape. Global Ecol Biogeogr 16:43–54
Wolda H (1988) Insect seasonality: why? Ann Rev Ecol Evol Syst 19:1–18
Wolda H (1989) Seasonal cues in tropical organisms. Rainfall? Not necessarily! Oecologia 80:437–442
Wahlberg N, Leneveu J, Kodandaramaiah U, Peña C, Nylin S, Freitas AVL, Brower AVZ (2009) Nymphalid butterflies diversify following near demise at the Cretaceous/Tertiary boundary. Proc R Soc B 276:4295–4302
Acknowledgements
The authors thank Talita P. Santos, Gloria R. Soares, Sidimario Freitas, Christiano Rocha, Leila Shirai, Tamara M. C. Aguiar, Patrícia A. Machado, Jessie P. Santos, Luiz Rocha, Caio Muniz, Simeão Moraes for assistance with fieldwork. To Jack Longino for suggesting the term “brought low canopy” to describe the ecotone habitat. Mathias M. Pires, Marina Beirão, Yves Basset, Danilo B. Ribeiro, Flávio A.M. dos Santos, Júlio N.C. Louzada carefully read and criticized the last version of the manuscript. We gratefully acknowledge the staff of the Instituto Estadual de Florestas (IEF-MG) and Parque do Rio Doce (PERD) for allowing us to work in the park and provide logistical support. We thank the transport sector of the Universidade Federal de Ouro Preto for providing the transportation in all fieldwork. Butterfly specimens are registered at SISGEN (Sistema Nacional de Gestão do Patrimônio Genético e do Conhecimento Tradicional Associado)—#A4156F5. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001, Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq (478481/2013-6) and Fundação de Amparo à Pesquisa de Minas Gerais—FAPEMIG (APQ-01184-15). GML thanks for a research scholarship from CNPq (155895/2014-1) and CAPES (88881.133074/2016-01). PL has a student fellowship (CVU 771366 from the Consejo Nacional de Ciencia y Tecnología (CONACYT), Mexico. AVLF thanks the CNPq (302585/2011-7, 303834/2015-3) and SISBIOTABrasil/CNPq (563332/2010-7), the National Science Foundation (DEB-1256742) and the BIOTA-FAPESP Program (2011/50225-3, 2013/50297-0). SPR was granted by the CNPq (478481/2013-6, 304024/2015-5) and FAPEMIG (APQ-01184-15).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Permits for the field studies
Permits for the field studies were issued by the state authority Instituto Estadual de Florestas (IEF) and the national authority Sistema de Autorização e Informação em Biodiversidade/Instituto Chico Mendes de Conservação da Biodiversidade (SISBIO/ ICMBio).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10841_2019_207_MOESM1_ESM.pdf
ESM 1 Fruit-feeding butterflies species recorded in the wet season in each habitat (forest interior, ecotone, edge), in Rio Doce State Park, state of Minas Gerais, Brazil. Some species absent in these months (wet season) were maintained here because this continues in the following table (PDF 285 kb)
10841_2019_207_MOESM2_ESM.pdf
Fruit-feeding butterflies species recorded in the dry season in each habitat (forest interior, ecotone, edge), in Rio Doce State Park, state of Minas Gerais, Brazil. Note that due to the temporal window of the study the dry season was broken between the end of dry season of 2015 and the early of dry season of 2016. Some species absent in these months (dry season) were maintained here because this is a continuity of previous table (PDF 226 kb)
10841_2019_207_MOESM3_ESM.pdf
ESM 3 Rarefaction analyses of fruit-feeding butterfly species richness for habitat (forest interior, ecotone, edge), in Rio Doce State Park, Brazil. Actual monthly species richness plotted against an individual-based accumulation curve for the total assemblage for habitat; The numbers correspond to the 12 months of the year (PDF 179 kb)
10841_2019_207_MOESM4_ESM.pdf
Temporal partitioning of beta-diversity of fruit-feeding butterflies among months (β) of a year by habitat, in Rio Doce State Park, Brazil. The dark grey color represents Turnover, the light grey color represents Nestedness and the shaded area represents wet season (PDF 133 kb)
Rights and permissions
About this article
Cite this article
Lourenço, G.M., Luna, P., Guevara, R. et al. Temporal shifts in butterfly diversity: responses to natural and anthropic forest transitions. J Insect Conserv 24, 353–363 (2020). https://doi.org/10.1007/s10841-019-00207-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-019-00207-0