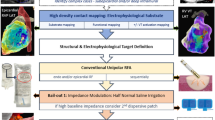
Abstract
Background
Several novel technologies allowing catheter ablation (CA) with a favorable safety/efficacy profile have been recently developed, but not yet extensively clinically tested in the setting of ventricular tachycardia CA.
Methods
In this technical report, we overview technical aspects and preclinical/clinical information concerning the application of three novel CA technologies in the ventricular milieu: a pulsed field ablation (PFA) generator (CENTAURI™, Galaxy Medical) to be used with linear, contact force-sensing radiofrequency ablation catheters; a contact force-sensing radiofrequency ablation catheter equipped with six thermocouples and three microelectrodes (QDOT Micro™, Biosense-Webster), allowing high-resolution mapping and temperature-controlled CA; and a flexible and mesh-shaped irrigation tip, contact force-sensing radiofrequency ablation catheter (Tactiflex, Abbott). We also report three challenging VT cases in which CA was performed using these technologies.
Results
The CENTAURI system was used with the Tacticath™ (Abbott) ablation catheter to perform ventricular PFA in a patient with advanced heart failure, electrical storm, and a deep intramural septal substrate. Microelectrode mapping using QDOT Micro™ helped to refine substrate assessment in a VT patient with congenitally corrected transposition of the great arteries, and allowed the identification of the critical components of the VT circuit, which were successfully ablated. Tactiflex™ was used in two challenging CA cases (one endocardial and one epicardial), allowing acute and mid-term control of VT episodes without adverse events.
Conclusion
The ideation and development of novel technologies initially intended to treat atrial arrhythmias and successfully implemented in the ventricular milieu is contributing to the progressive improvement in the clinical benefits derived from VT CA, making this procedure key for successful management of increasingly complex patients.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The year 2023 marks the fiftieth anniversary of the first catheter ablation procedure for the treatment of ventricular tachycardia (VT) [1, 2]. In fact, in 1983, a patient with recurrent VT episodes, despite a prior surgical ablation, was successfully treated with multiple 300 J-DC shocks delivered through a standard 7 Fr catheter placed at the site of earliest activation during VT [1]. Ever since, catheter ablation has evolved from an experimental solution to an important therapeutic option for patients with VT, and clinical practice guidelines now recommend the procedure in several clinical scenarios [3].
Continuous and tremendous technological advancements, as well as an increased understanding of mechanisms underpinning VT, have revolutionized our approach to the ablation procedure, and contributed to its improved efficacy/safety profile [4, 5]. Not only we currently have multipolar catheters with increasingly small electrodes, allowing high- and ultra-high density mapping, but also radiofrequency ablation catheter technology has evolved, with improved irrigation and temperature control, as well as with the possibility to map with microelectrodes on the catheter’s tip [2, 5, 6]. Furthermore, a novel non-thermal energy source—pulsed field ablation (PFA)—has now entered the clinical arena and holds promise for VT ablation in both idiopathic [7] and scar-related settings [8,9,10,11].
In this technical report, we provide an update on recent technological innovations in the field of VT catheter ablation—pulsed field ablation (PFA) via a linear radiofrequency catheter using the CENTAURI™ system (Galaxy Medical, USA); radiofrequency mapping and ablation with the QDOT Micro™ catheter (Biosense Webster, USA); and endocardial/epicardial VT ablation with the novel flexible tip Tactiflex™ catheter (Abbott Medical, USA)—by firstly overviewing the technical aspects and preclinical/clinical available data, and secondly illustrating the clinical application in a series of challenging VT ablation cases.
2 Pulsed field ablation for electrical storm ablation in non-ischemic cardiomyopathy
2.1 Overview
PFA is a non-thermal ablation modality which was recently introduced in clinical practice and is being extensively tested in the field of atrial fibrillation ablation [12]. Basically, PFA involves the application of electrical fields to the myocardial tissue, resulting in irreversible electroporation, which disrupts the membrane and cellular homeostasis, alters local tissue pH, and favors the generation of reactive oxygen species, leading to myocyte death [13]. The clinical use of PFA for VT ablation is currently limited by the paucity of available data in the ventricular milieu, as only isolated case reports have been reported so far [7,8,9,10,11]; furthermore, the 31-mm pentaspline catheter (Farawave, Boston Inc., USA) which is currently available in most clinical settings in Europe, was designed for pulmonary vein isolation and may have limited manoeuvrability in the ventricle. The recently developed CENTAURI PFA generator allows the delivery of unipolar, biphasic PFA through linear irrigated, contact force sensing radiofrequency ablation catheters (Tacticath [Abbott, USA], ThermoCool SmartTouch [Biosense Webster, USA], or Intellanav Stablepoint [Boston Scientific, USA]). Although the system was initially intended for atrial fibrillation ablation, its coupling with linear catheters may facilitate endocardial ventricular navigation. With CENTAURI, PFA pulses are synchronized to the cardiac cycle (with multiple brief pulse at the end of QRS) through a cardiac monitor, and three different energy settings may be chosen (19 A, 22 A, or 25 A; WAVE 1 waveform). The duration of complete energy delivery depends on the heart rate and the energy settings (4 s, 7 s, and 10 s for 19 A, 22 A, and 25 A, respectively, at a heart rate of 60/min) [14]. Recent preclinical studies showed that in swine models of myocardial infarction, the endocardial application of PFA using a linear catheter and the CENTAURI system in infarcted regions resulted in contraction band necrosis and myocytolysis extending deep into the epicardial borders of the scar, raising the possibility of ablating deeper intramural and/or epicardial arrhythmogenic substrates, which may be desirable in non-ischemic cardiomyopathy patients [11, 14].
2.2 Case report #1
A 57-year-old male patient with advanced heart failure due to dilated cardiomyopathy presented with electrical storm and heart failure worsening due to incessant monomorphic VT at a heart rate of 110 bpm. His past medical history was notable for multiple hospitalizations for heart failure worsening due to recurrent slow VT episodes despite the use of maximal doses of guideline-directed medical therapy, amiodarone, and mexiletine; the recent upgrading of his dual chamber implantable cardioverter defibrillator to cardiac resynchronization therapy defibrillator (CRT-D); and transcatheter mitral valve repair with Mitraclip implantation for functional mitral regurgitation [15]. Of note, during the 6 months preceding hospitalization, more than 50 anti-tachycardia pacing were delivered by the CRT-D to terminate VTs, indicating a highly unstable heart failure status [15]. The echocardiogram upon hospital admission showed a severely dilated and hypokinetic left ventricle, with an ejection fraction of 20%, a mildly dilated and hypokinetic right ventricle, and mild residual mitral regurgitation.
After medical stabilization, a decision was made to proceed to catheter ablation of VT. Due to the prior documentation of deep septal intramural fibrosis with a stria pattern by cardiac magnetic resonance imaging, and to the potentially preferable tissue penetration of PFA ablation lesions as compared to radiofrequency lesions in scarred tissue [13, 14, 16], biphasic PFA using an irrigated contact force sensing catheter (Tacticath™, Abbott Medical, USA) coupled with the CENTAURI PFA generator was performed. Intracardiac echocardiography (ICE) was used during the procedure, showing a hyperechogenic stria in the interventricular septum, consistent with intramural fibrosis (Fig. 1, panel A). The left ventricle was accessed with a retrograde aortic approach sequentially using the ablation catheter and a multipolar mapping catheter (Advisor HD Grid, Abbott Medical, USA); low-voltage electrograms with late abnormal ventricular activities (LAVAs, Fig. 1, panels B and C) were documented at the left interventricular septum. By programmed electrical stimulation, a sustained VT with left bundle branch block morphology could be reproducibly induced (Fig. 1, panel D), compatible with a septal origin but terminating spontaneously during activation mapping. Despite attempts at mapping from both the left and the right ventricular endocardium, the entire cycle of the VT could not be covered, consistent with an intramural circuit component; the earliest activation zone was recorded in the left mid-septum (Fig. 1, panel E). Multiple PFA pulses (n = 30) with high-energy settings (25 A) were delivered at that site (Fig. 1, panels F–H), eliminating LAVAs (Fig. 1, panels G–I), and the patient was rendered noninducible for VTs. Despite the procedural success, the patient died 1 month after the procedure due to refractory cardiogenic shock.
Pulsed field ablation of ventricular tachycardia with a linear, contact force-sensing catheter. A 57-year-old male patient with advanced heart failure due to dilated cardiomyopathy is referred for catheter ablation of electrical storm. (A–C) Substrate characterization using intracardiac echocardiography (A), bipolar voltage mapping (B), and local activation time (LAT) mapping in right ventricular paced rhythm. Note that a hyperechogenic stria is visible deep inside the interventricular septum (red arrows), consistent with an intramural septal substrate, which is also confirmed by bipolar voltage and LAT maps (RAO view), showing a heterogeneous septal low-voltage region (B) with late abnormal ventricular activities (LAVAs, red arrow in C); the red star in A denotes the MitraClip device. (D–F) Ventricular tachycardia (VT) induction (D), VT activation mapping (E), and ablation catheter (red arrow) positioning toward the interventricular septum via a retrograde aortic access to the left ventricle. Note that the entire VT cycle cannot be covered by endocardial mapping (E), consistent with intramural components of the circuit. (F–H) pulsed field ablation is performed using the CENTAURI™ system (see the artifact on intracardiac recordings), resulting in clearance of local abnormal ventricular activities (red circles in F and G). The septal area is covered by ablation lesions (red tags), resulting in complete LAVA elimination and scar homogenization (H). Abbreviations: Bip, bipolar voltage mapping; ICE, intracardiac echocardiography; LAT, local activation time mapping in sinus rhythm; PFA, pulsed field ablation; VT, ventricular tachycardia
To the best of our knowledge, this is the first report on the use of the CENTAURI PFA generator coupled with the Tacticath catheter for VT ablation. This case highlights that the use of PFA may be associated with procedural success in challenging patients with deep intramural substrates; nonetheless, catheter ablation should have probably been considered earlier in the patient’s clinical course to modify the adverse and relentless disease trajectory of advanced heart failure [17]. Further studies are urgently needed to identify the optimal PFA settings (power, number of stacked PFA applications at the same site) for dealing with the different arrhythmogenic ventricular substrates [14, 16].
3 Microelectrode voltage mapping and temperature-controlled ablation of electrical storm in an adult congenital heart disease patient
3.1 Overview
The assessment of the abnormal ventricular substrate underpinning VT is critically influenced by the dimensions of the mapping electrodes [18]. In recent years, the development of multipolar catheters with small mapping electrodes and narrow interelectrode spacing has allowed improved visualization of near-field bipolar electrograms, leading to a more accurate delineation of the arrhythmogenic substrate [18, 19]. The QDOT Micro catheter is a contact force sensing ablation catheter embedding six thermocouples for precise temperature control during ablation, a standard distal bipole (between the 3.5 mm tip and a 1-mm spaced 1-mm ring electrodes), as well as three microelectrodes on the distal tip (0.167 mm2 wide, 1.755 mm spaced), each recording microbipolar electrograms perpendicular to the standard bipolar electrogram [6]. As such, this catheter design may allow both high-resolution mapping and temperature-controlled ablation within a single device, eliminating the need to exchange catheter in case of VT induction during mapping and when desiring to rapidly start ablating [3]. Compared to the prior generation ablation catheter from the same constructor (Thermocool SmartTouch), QDOT Micro has higher proximal irrigation flow rate, which is automatically adjusted together with power to avoid tissue overheating as assessed by the hottest distal thermocouple when using power settings up to 50 W, i.e., QMODE ablation modality [6]. In a recent dual-center report, we demonstrated that the average microbipolar electrogram amplitude was 0.98 mV (95% CI, 0.93–1.04, p < 0.01) larger than standard bipolar electrogram amplitude, and 0.27 mV (95% CI, 0.16–0.37 mV) larger than minibipolar electrograms recorded by the multipolar (20 electrodes) PentaRay™ (Biosense Webster, USA) catheter, which carries 1 mm electrodes with 2–6–2 mm spacing. Furthermore, abnormal electrograms had higher amplitude, longer duration, and more peaks at microelectrode mapping compared to standard bipolar electrode mapping [6]. Whether this intriguing and long-awaited combination of high-resolution mapping and temperature-controlled ablation within a single catheter may improve outcomes for patients undergoing VT ablation is still to be determined.
3.2 Case #2
A 59-year-old male patient was admitted for recurrent nonsustained, well-tolerated VT episodes, symptomatic for palpitations. He had been diagnosed with congenitally corrected transposition of the great arteries at the adult age, and no surgical repair was performed; the patient received a dual-chamber pacemaker for complete atrioventricular block 1 month prior to the present hospitalization. Upon admission, the echocardiogram showed moderate dysfunction of the systemic right ventricle (fractional area change, 35%) associated with severe tricuspid regurgitation, which had both significantly worsened compared to the recent hospitalization for pacemaker implantation.
Due to the refractoriness of VT episodes to antiarrhythmic drug therapy with metoprolol and amiodarone, we decided to perform catheter ablation of VT. At electrophysiology study, a sustained VT with a heart rate of 156 bpm and right bundle branch block, superior axis morphology could be reproducibly induced by programmed electrical stimulation during isoproterenol infusion; the VT was well tolerated. The systemic right ventricle was accessed through a patent foramen ovale, and the VT activation map was reconstructed using the QDOT Micro catheter. Diastolic electrograms could be better visualized by microelectrodes as compared to the standard bipole in the basal lateral wall of the left ventricle (Fig. 2, panel A) and radiofrequency energy delivery (50 W, QMODE ablation modality) in the region rapidly led to VT termination (Fig. 2, panel B). The voltage and local activation time (LAT) maps were then reconstructed in right ventricular paced rhythm, showing widespread dense scar at standard bipolar maps in anterior and lateral regions (Fig. 2, panels C and D), where late and fragmented potentials were also recorded (Fig. 2, panel E). Notably, the microbipolar map showed a more heterogeneous low-voltage region in the lateral region, with multiple potential heterogeneous conducting channels (Fig. 2, panel D), and late/fragmented electrograms could be better detected by microelectrode mapping, compared to standard bipolar mapping (Fig. 2, panel E). Substrate modification was then performed (50 W, QMODE ablation modality; total number of radiofrequency energy pulses, 65; average lesion duration, 12 (7-17) s; average ablation index, 431 [352–505] [20]; target temperature set at 47 °C; maximum temperature set at 55 °C; programmed power not reached for excessive heating in only 3 pulses, 4.6%) by eliminating all late potentials in the lateral segment (Fig. 2, panel F), and the VT was noninducible at the end procedure, even with high-dose isoproterenol infusion. Fourteen months after ablation, the patient is free from VT recurrences, as confirmed by pacemaker interrogation, and amiodarone has been discontinued.
Mapping and ablation of ventricular tachycardia in a patient with congenitally corrected transposition of the great arteries using a novel catheter with microelectrodes and thermocouples. (A, B) Ventricular tachycardia (VT) activation mapping and VT termination with radiofrequency energy. Diastolic electrograms are recorded in the basal lateral region and have higher amplitudes by microelectrode mapping (green arrows in A) compared standard bipolar mapping (white arrow in A). Radiofrequency energy delivery in the region rapidly leads to VT termination. (C–E) Substrate characterization by standard bipolar, microbipolar, and local activation time (LAT) mapping (PA view) in right ventricular paced rhythm using a catheter with microelectrodes and thermocouples (QDOT Micro™). Note that the standard bipolar map (C) reveals a dense scar area in basal lateral region, but irregular low-voltage myocardium is detected by microelectrode mapping, with multiple heterogeneous conducting channels (D). In the same region, late/fragmented electrograms are recorded (E). Note that local abnormal ventricular electrograms are easier to be visualized by microelectrode mapping as compared to standard bipolar mapping (green boxes). (F) Final ablation lesion set (same view as C–E). Abbreviations: LAT, local activation time; MicroBip, microbipolar voltage mapping; RF, radiofrequency energy; Standard Bip, standard bipolar voltage mapping; VT, ventricular tachycardia
This case highlights that in case of widespread abnormal ventricular substrates, microelectrode voltage mapping may complement functional substrate mapping for selecting ablation targets [6, 21], and that temperature-controlled ablation may be successful even in the challenging setting of complex adult congenital heart disease patients, for which available evidence on the safety/efficacy profile of VT ablation is limited to isolated case reports [22].
4 First reported use of a novel flexible tip radiofrequency catheter for mapping and ablation of electrical storm
4.1 Overview
Recently, a novel catheter (Tactiflex) incorporating both contact-force technology (as opposed to FlexAbility™, Abbott, USA) and a mesh-shaped irrigation tip was developed and approved for atrial fibrillation ablation [23, 24]. This device is characterized by a flexible tip and a distal thermocouple, allowing temperature-controlled ablation even when high-power (i.e., 40 W or higher), short-duration ablation settings are chosen, and producing transmural atrial lesions in preclinical studies [23, 24]. The mesh-shaped irrigation tip and the distal thermocouple allow improved tip cooling with a lower irrigation flow rate (13 mL/min), as compared to the prior irrigation technology (that of Tacticath), which featured six irrigation holes and required a higher irrigation flow rate of 30 mL/min [25]. Accordingly, preclinical data showed that the mesh-shaped irrigation tip may be associated with lower risk of steam-pops, compared to the six-hole irrigation technology [25], while a clinical study reported a lower risk of procedure-related stroke with the mesh-shaped irrigation system, possibly due to a reduced risk of ablation-induced tissue overheating, char formation, and thrombosis [26].
As of today, the Tactiflex catheter has not been evaluated in the ventricular milieu, but due to its flexible and mesh-shaped irrigation tip, contact force sensing technology, and accurate temperature control, a favorable safety/efficacy profile may be hypothesized.
4.2 Case report #3: endocardial ablation in ischemic cardiomyopathy
A 78-year-old lady presented with electrical storm due to incessant VT, which was terminated with electrical cardioversion. The past medical history was notable for prior acute coronary syndrome due to three-vessel coronary artery disease, for which she underwent coronary artery bypass grafting, and sustained VT episodes, for which she received an implantable cardioverter defibrillator (ICD). The ECG revealed a sustained monomorphic VT at a heart rate of 147 bpm, with left bundle branch morphology and negative concordance in precordial leads, while the echocardiography showed a severely dilated and hypokinetic left ventricle (left ventricular ejection fraction, 20%), as well as a mildly hypokinetic right ventricle. After electrical cardioversion, coronary angiography showed a stable picture and patency of bypass grafts. Due to multiple VT recurrences despite maximally tolerated medical therapy, a decision was taken to perform catheter ablation.
Left ventricular high-density mapping was performed via both retrograde aortic and transseptal approaches, using the multipolar Advisor HD Grid (Abbott, USA), and the Tactiflex catheters together with the EnsiteX V2 electroanatomical mapping system (Abbott, USA). The omnipolar voltage map showed a central dense scar area surrounded by a low-voltage border zone in the posterior wall; the border zone was very irregular, containing potential heterogeneous conducting channels (Fig. 3, panel A). The LAT map in sinus rhythm disclosed very late, multi-component potentials in the same regions, covering a surface area of 11 cm2 (Fig. 3, panel B). The region of interest appeared thin and hyperechogenic by ICE, consistent with post-myocardial infarction scarring (Fig. 3, panel C).
First in human endocardial catheter ablation of VT using a novel flexible tip, contact force sensing catheter. Catheter ablation of electrical storm in a 78-year-old lady with ischemic cardiomyopathy. (A–C) Substrate characterization using omnipolar and local activation time (LAT) mapping in sinus rhythm (PA view), as well as intracardiac echocardiography (ICE). In the posterior wall, a dense scar region with heterogeneous surrounding border zone (A) and late/fragmented electrograms (B) are recorded; the posterior wall appears thinned and hyperechogenic by ICE (blue arrows in C). (D–E) Ventricular tachycardia (VT) induction, mapping, and ablation. A sustained monomorphic VT is induced by programmed electrical stimulation; note the slow intrinsicoid deflection and the large negative pseudodelta wave lead in DI, consistent with an epicardial exit site (D). The velocity map in VT shows slow conduction in the lateral wall of the left ventricle (E), where radiofrequency energy delivery allows VT termination (blue arrow, F). After scar homogenization, late potentials cannot be recorded any more by high-density mapping (G). Abbreviations: ICE, intracardiac echocardiography; LAT, local activation time; Omni, omnipolar voltage mapping; VT, ventricular tachycardia
Clinical VT was easily inducible by programmed electrical stimulation (Fig. 3, panel D); although it was not possible to cover the entire VT cycle by mapping the endocardium, consistent with a possible epicardial/deep intramural circuit component [27], the velocity map during VT showed maximum conduction slowing in the posterior wall of the left ventricle [28], where late potentials had been recorded in sinus rhythm (Fig. 3, panels E and F). Shortly after the beginning of radiofrequency energy delivery at that site (35 W-25 s pulse), VT termination was obtained (Fig. 3, panel F), and late/fragmented potentials were then successfully eliminated with multiple RF pulses (either 35 W-25 s or 40 W-20 s), as verified by remapping (Fig. 3, panel G). At the end of the procedure, the patient was noninducible for any VT, and no procedure-related complications were encountered. The patient has been free of VT recurrences ever since.
4.3 Case report #4: epicardial VT ablation
A 32-year-old man was referred for electrical storm due to several episodes of monomorphic VT requiring ICD intervention. The patient’s past medical history included a Kawasaki disease diagnosed at the age of 8 and recurrent VT episodes, for which he was already receiving metoprolol plus amiodarone. The ECG revealed a sustained monomorphic VT at a heart rate of 155 bpm, with right bundle branch morphology, superior axis, and precordial transition in V3. The echocardiography showed a slightly dilated left ventricle with a left ventricular ejection fraction of 40%; the basal and mid segments of the LV were hypo-akinetic. The patient had previously undergone an endocardial VT ablation, which was acutely unsuccessful. Due to the multiple VT recurrences despite maximally tolerated medical therapy and the prior failed endocardial ablation, the patient was scheduled for epicardial catheter ablation. The procedure was performed under general anesthesia, and the epicardial access was obtained by subxiphoid percutaneous pericardial puncture, as described elsewhere [29, 30]. The access to the epicardial space was granted by the Agilis EPI deflectable introducer sheath (Abbott, USA), and left ventricular epicardial high-density mapping was performed using the Advisor HD Grid catheter with the EnsiteX V2 electroanatomical mapping system. The epicardial omnipolar map showed a wide epicardial scar in the mid portion of the inferior wall and in the mid-apical lateral wall of the left ventricle (Fig. 4, panel A). An activation map was performed during fixed-cycle length RV pacing, showing an area of late potentials at the basal edge of the inferior wall scar (Fig. 4, panel B). After completion of the substate and activation map, a ventricular stimulation protocol was carried out to induce the clinical VT, which was then mapped using the HD Grid catheter (Fig. 4, panel C). A mid-diastolic activity was identified at the border between the mid and basal segments of the inferior wall, where late potentials had been tagged. During further mapping, the clinical VT spontaneously terminated. At this point, the mapping catheter was exchanged to insert the TactiFlex ablation catheter into the epicardial space. Ablation was carried out using 50 W radiofrequency pulses for 10 s, aiming at late potentials’ elimination, as confirmed by remapping (Fig. 4, panel D); steam pops did not occur. Although limited in-depth penetration in the ventricular myocardium may be expected when using high-power ablation, highlighting the need to adjust radiofrequency power and duration according to the specific myocardial substrate [31], these ablation settings were chosen for being recommended by the constructor. Furthermore, the endocardium had already been ablated during the previous endocardial procedure, and therefore deep intramural ablation was not targeted. At end of the procedure, a programmed electrical stimulation protocol was repeated and the patient was non-inducible. The procedure was terminated without any procedure-related complications, and the patient has been discharged without amiodarone and is free of VT recurrences since then.
First in human epicardial catheter ablation of VT using a novel flexible tip, contact force sensing catheter. Epicardial catheter ablation of recurrent ventricular tachycardia (VT) in a 32-year-old male patient with Kawasaki disease. (A, B) Substrate characterization with omnipolar and local activation time mapping (PA views) in right ventricular paced rhythm. A dense scar region is demonstrated in the inferior-lateral walls of the left ventricle (A), and late potentials are recorded in the same region (orange arrows in B). (C, D) VT activation mapping and ablation. During VT, diastolic electrograms (orange arrows in C) are recorded in the same region of interest, but the VT terminates spontaneously before obtaining a complete activation map. Late potentials are then completely eliminated with multiple radiofrequency energy pulses (D, final lesion set), and the patient is noninducible at end procedure. Abbreviations: LAT, local activation time map; Omni, omnipolar voltage map; VT, ventricular tachycardia
5 Conclusions
In this technical report, we have overviewed several novel catheter platforms which may soon become more and more commonly used for VT ablation. The relentlessly accumulating experience with catheter ablation of VT, as well as the expanding clinical indications for the procedure are contributing to increase the complexity of patients coming into the electrophysiology laboratory [2, 3], and the availability of novel energy sources (such as PFA) and/or more efficient catheter designs (such as QDOT Micro and Tactiflex) are more than welcomed to increase safety and efficacy. These advances will widen the landscape of treatment opportunities for patients with VT and help reduce its cumbersome health, social, and psychological burden. The high costs of these technologies will likely need to be renegotiated to make these devices cost-effective and accessible to larger patient cohorts in and beyond western countries.
Data availability
The data that support the findings of this study are available on request from the corresponding author, PC.
References
Hartzler GO. Electrode catheter ablation of refractory focal ventricular tachycardia. J Am Coll Cardiol. 1983;2(6):1107–13. https://doi.org/10.1016/s0735-1097(83)80337-4.
Guandalini GS, Liang JJ, Marchlinski FE. Ventricular tachycardia ablation: past, present, and future perspectives. JACC Clin Electrophysiol. 2019;5(12):1363–83. https://doi.org/10.1016/j.jacep.2019.09.015.
Cronin EM, Bogun FM, Maury P, Peichl P, Chen M, Namboodiri N, Aguinaga L, Leite LR, Al-Khatib SM, Anter E, Berruezo A, Callans DJ, Chung MK, Cuculich P, d’Avila A, Deal BJ, Bella PD, Deneke T, Dickfeld TM, Hadid C, Haqqani HM, Kay GN, Latchamsetty R, Marchlinski F, Miller JM, Nogami A, Patel AR, Pathak RK, Saenz Morales LC, Santangeli P, Sapp JL Jr, Sarkozy A, Soejima K, Stevenson WG, Tedrow UB, Tzou WS, Varma N, Zeppenfeld K. 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias. J Interv Card Electrophysiol. 2020;59(1):145–298. https://doi.org/10.1007/s10840-019-00663-3.
Compagnucci P, Volpato G, Falanga U, Cipolletta L, Conti M, Grifoni G, Verticelli L, Schicchi N, Giovagnoni A, Casella M, Guerra F, Dello Russo A. Recent advances in three-dimensional electroanatomical mapping guidance for the ablation of complex atrial and ventricular arrhythmias. J Interv Card Electrophysiol. 2021;61(1):37–43. https://doi.org/10.1007/s10840-020-00781-3.
Marashly Q, Najjar SN, Hahn J, Rector GJ, Khawaja M, Chelu MG. Innovations in ventricular tachycardia ablation. J Interv Card Electrophysiol. 2022. https://doi.org/10.1007/s10840-022-01311-z. Epub ahead of print.
Dello Russo A, Compagnucci P, Bergonti M, Cipolletta L, Parisi Q, Volpato G, Santarelli G, Colonnelli M, Saenen J, Valeri Y, Carboni L, Marchese P, Marini M, Sarkozy A, Natale A, Casella M. Microelectrode voltage mapping for substrate assessment in catheter ablation of ventricular tachycardia: a dual-center experience. J Cardiovasc Electrophysiol. 2023;34(5):1216–27. https://doi.org/10.1111/jce.15908.
Schmidt B, Chen S, Tohoku S, Bordignon S, Bologna F, Chun KRJ. Single shot electroporation of premature ventricular contractions from the right ventricular outflow tract. Europace. 2022;24(4):597. https://doi.org/10.1093/europace/euab212.
Krause U, Bergau L, Zabel M, Müller MJ, Paul T. Flowerpower: pulsed field ablation of ventricular tachycardia in a patient with Ebstein’s anomaly. Eur Heart J Case Rep. 2023;7(3):ytad093. https://doi.org/10.1093/ehjcr/ytad093.
Ouss A, van Stratum L, van der Voort P, Dekker L. First in human pulsed field ablation to treat scar-related ventricular tachycardia in ischemic heart disease: a case report. J Interv Card Electrophysiol. 2023;66(3):509–10. https://doi.org/10.1007/s10840-022-01407-6.
Weyand S, Löbig S, Seizer P. First in human focal pulsed field ablation to treat an epicardial VT focus with an endocardial approach in non-ischemic cardiomyopathy. J Interv Card Electrophysiol. 2023;66(5):1057–8. https://doi.org/10.1007/s10840-023-01534-8.
Martin CA, Zaw MT, Jackson N, Morris D, Costanzo P. First worldwide use of pulsed-field ablation for ventricular tachycardia ablation via a retrograde approach. J Cardiovasc Electrophysiol. 2023;34(8):1772–5. https://doi.org/10.1111/jce.16002.
Verma A, Haines DE, Boersma LV, Sood N, Natale A, Marchlinski FE, Calkins H, Sanders P, Packer DL, Kuck KH, Hindricks G, Onal B, Cerkvenik J, Tada H, DeLurgio DB. PULSED AF Investigators. Pulsed field ablation for the treatment of atrial fibrillation: PULSED AF pivotal trial. Circulation. 2023;147(19):1422–32. https://doi.org/10.1161/CIRCULATIONAHA.123.063988.
Verma A, Asivatham SJ, Deneke T, Castellvi Q, Neal RE 2nd. Primer on pulsed electrical field ablation: understanding the benefits and limitations. Circ Arrhythm Electrophysiol. 2021;14(9):e010086. https://doi.org/10.1161/CIRCEP.121.010086.
Sandhu U, Alkukhun L, Kheiri B, Hodovan J, Chiang K, Splanger T, Castellvi Q, Zhao Y, Nazer B. In vivo pulsed-field ablation in healthy vs. chronically infarcted ventricular myocardium: biophysical and histologic characterization. Europace. 2023;25(4):1503–9. https://doi.org/10.1093/europace/euac252.
Compagnucci P, Casella M, Bianchi V, Franculli F, Vitali F, Santini L, Savarese G, Santobuono VE, Chianese R, Lavalle C, Amellone C, Pecora D, Calvanese R, Stronati G, Santoro A, Ziacchi M, Campari M, Valsecchi S, Calò L, Guerra F, Dello Russo A. Implantable defibrillator-detected heart failure status predicts ventricular tachyarrhythmias. J Cardiovasc Electrophysiol. 2023;34(5):1257–67. https://doi.org/10.1111/jce.15898.
Im SI, Higuchi S, Lee A, Stillson C, Buck E, Morrow B, Schenider K, Speltz M, Gerstenfeld EP. Pulsed field ablation of left ventricular myocardium in a swine infarct model. JACC Clin Electrophysiol. 2022;8(6):722–31. https://doi.org/10.1016/j.jacep.2022.03.007.
Tzou WS, Tung R, Frankel DS, Vaseghi M, Bunch TJ, Di Biase L, Tholakanahalli VN, Lakkireddy D, Dickfeld T, Saliaris A, Weiss JP, Mathuria N, Tedrow U, Afzal MR, Vergara P, Nagashima K, Patel M, Nakahara S, Vakil K, Burkhardt JD, Tseng CH, Natale A, Shivkumar K, Callans DJ, Stevenson WG, Della Bella P, Marchlinski FE, Sauer WH. Ventricular tachycardia ablation in severe heart failure: an international ventricular tachycardia ablation center collaboration analysis. Circ Arrhythm Electrophysiol. 2017;10(1):e004494. https://doi.org/10.1161/CIRCEP.116.004494.
Josephson ME, Anter E. Substrate mapping for ventricular tachycardia: assumptions and misconceptions. JACC Clin Electrophysiol. 2015;1(5):341–52. https://doi.org/10.1016/j.jacep.2015.09.001.
Takigawa M, Kitamura T, Basu S, Bartal M, Martin CA, Martin R, Cheniti G, Vlachos K, Pillois X, Frontera A, Massoullié G, Thompson N, Bourier F, Lam A, Duchateau J, Pambrun T, Denis A, Derval N, Cochet H, Haïssaguerre M, Sacher F, Hocini M, Jaïs P. Effect of electrode size and spacing on electrograms: optimized electrode configuration for near-field electrogram characterization. Heart Rhythm. 2022;19(1):102–12. https://doi.org/10.1016/j.hrthm.2021.09.011.
Compagnucci P, Dello Russo A, Bergonti M, Anselmino M, Zucchelli G, Gasperetti A, Cipolletta L, Volpato G, Ascione C, Ferraris F, Valeri Y, Bongiorni MG, Natale A, Tondo C, De Ferrari GM, Casella M. Ablation index predicts successful ablation of focal atrial tachycardia: results of a multicenter study. J Clin Med. 2022;11(7):1802. https://doi.org/10.3390/jcm11071802.
Vlachos K, Letsas KP, Srinivasan NT, Frontera A, Efremidis M, Dragasis S, Martin CA, Martin R, Nakashima T, Bazoukis G, Kitamura T, Mililis P, Saplaouras A, Georgopoulos S, Sofoulis S, Kariki O, Koskina S, Takigawa M, Sacher F, Jais P, Santangeli P. The value of functional substrate mapping in ventricular tachycardia ablation. Heart Rhythm O2. 2022;4(2):134–46. https://doi.org/10.1016/j.hroo.2022.10.013.
González-Lozano J, Ballesteros G, Cano J, Osorio D, Urbano C. Endocardial substrate of ventricular tachycardia in an adult patient with congenitally corrected transposition of great arteries without surgical repair. Pacing Clin Electrophysiol. 2023. https://doi.org/10.1111/pace.14756.
Ptaszek LM, Koruth J, Santangeli P, Piccini JP, Ranjan R, Mahapatra S, Pipenhagen C, Fish JM, Moon LB, Ambrosius NM, Boudlali H, Jensen JA. Safe and effective delivery of high-power, short-duration radiofrequency ablation lesions with a flexible-tip ablation catheter. Heart Rhythm O2. 2022;4(1):42–50. https://doi.org/10.1016/j.hroo.2022.10.009.
Dello Russo A, D’Angelo L, Compagnucci P, Cipolletta L, Parisi Q, Valeri Y, Campanelli F, Volpato G, Carboni L, Ciliberti G, Stronati GE, Barbarossa A, La Piscopia V, Bondavalli B, Guerra F, Natale A, Casella M. High-power short-duration catheter ablation of atrial fibrillation: is it really a new era? Comparison between new and old radiofrequency contact force-sensing catheters. J Interv Card Electrophysiol. 2023. https://doi.org/10.1007/s10840-023-01612-x. Epub ahead of print.
Yamaguchi J, Takigawa M, Goya M, Martin C, Amemiya M, Yamamoto T, Nishimura T, Nakamura R, Shirai Y, Tao S, Miyazaki S, Takahashi Y, Sasano T. Impact of tip design and thermocouple location on the efficacy and safety of radiofrequency application. J Interv Card Electrophysiol. 2023;66(4):885–96. https://doi.org/10.1007/s10840-022-01219-8.
Kim YG, Shim J, Boo KY, Kim DY, Lee KN, Choi JI, Kim YH. Slit-based irrigation catheters can reduce procedure-related ischemic stroke in atrial fibrillation patients undergoing radiofrequency catheter ablation. PLoS ONE. 2020;15(10):e0239339. https://doi.org/10.1371/journal.pone.0239339.
Mohanty S, Trivedi C, Di Biase L, Burkhardt JD, Della Rocca DG, Gianni C, MacDonald B, Mayedo A, Shetty SS, Zagrodzky W, Baqai F, Bassiouny M, Gallinghouse GJ, Horton R, Al-Ahmad A, Natale A. Endocardial scar-homogenization with vs without epicardial ablation in VT patients with ischemic cardiomyopathy. JACC Clin Electrophysiol. 2022;8(4):453–61. https://doi.org/10.1016/j.jacep.2021.12.011.
Hawson J, Anderson RD, Al-Kaisey A, Chieng D, Segan L, Watts T, Campbell T, Morton J, McLellan A, Kistler P, Voskoboinik A, Pathik B, Kumar S, Kalman J, Lee G. Functional assessment of ventricular tachycardia circuits and their underlying substrate using automated conduction velocity mapping. JACC Clin Electrophysiol. 2022;8(4):480–94. https://doi.org/10.1016/j.jacep.2021.12.013.
Sosa E, Scanavacca M, D’Avila A, Pilleggi F. A new technique to perform epicardial mapping in the electrophysiology laboratory. J Cardiovasc Electrophysiol. 1996;7:531–6. https://doi.org/10.1111/j.1540-8167.1996.tb00559.x.
De Martino G, Compagnucci P, Mancusi C, Vassallo E, Calvanese C, Della Ratta G, Librera M, Franciulli M, Marino L, Russo AD, Casella M. Stepwise endo-/epicardial catheter ablation for atrial fibrillation: the Mediterranea approach. J Cardiovasc Electrophysiol. 2021;32(8):2107–15. https://doi.org/10.1111/jce.15151.
Garg L, Daubert T, Lin A, Dhakal B, Santangeli P, Schaller R, Hyman MC, Kumareswaran R, Arkles J, Nazarian S, Lin D, Riley MP, Supple GE, Frankel DS, Zado E, Callans DJ, Marchlinski FE, Dixit S. Utility of prolonged duration endocardial ablation for ventricular arrhythmias originating from the left ventricular summit. JACC Clin Electrophysiol. 2022;8(4):465–76. https://doi.org/10.1016/j.jacep.2021.12.010.
Acknowledgements
Michela Colonnelli, BE; Giulia Santarelli, BE; Valentina La Piscopia, BE; and Barbara Bondavalli, BE, for help in the preparation of images.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Each patient provided his/her written informed consent.
Conflict of interest
A.D.R. is a consultant for Abbott Medical. A.N. is a consultant for Biosense Webster, Stereotaxis, and Abbott, and has received speaker honoraria/travel from Medtronic, Atricure, Biotronik, and Janssen. All other authors declared no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Compagnucci, P., Valeri, Y., Conti, S. et al. Technological advances in ventricular tachycardia catheter ablation: the relentless quest for novel solutions to old problems. J Interv Card Electrophysiol 67, 855–864 (2024). https://doi.org/10.1007/s10840-023-01705-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-023-01705-7