Abstract
Purpose
To provide a brief overview of some relevant technological advances in the field of three-dimensional electroanatomical mapping (3D-EAM) that have recently entered the clinical arena and their role in guiding catheter ablation (CA) of complex atrial and ventricular arrhythmias.
Methods
In this technical report, we describe the general features of three novel algorithms featured in the updated CARTO PRIME™ mapping module for CARTO®3 version 7 3D-EAM system (Biosense Webster Inc., Diamond Bar, CA, USA): local activation time (LAT) hybrid, coherent mapping and map replay modules. We also report three challenging arrhythmia cases in which CA was successfully guided by these softwares.
Results
The LAT hybrid module was used in a case of premature ventricular complex originating from the right coronary cusp. This algorithm facilitated safe positioning of the ablation catheter away from the right coronary ostium, avoiding potential harm to this vital structure. The coherent mapping module helped to identify the critical as well as a bystander isthmus of an atrial macro-re-entrant tachycardia in a grown-up patient with congenital heart disease. The map replay module allowed rapid retrospective activation mapping of two unstable ventricular tachycardias in a case of nonischemic cocaine-associated cardiomyopathy.
Conclusion
3D-EAM systems offer significant advantages in the management of challenging arrhythmias, and the introduction of novel algorithms underpins improvements in patients’ outcomes. Given the increasing sophistication of these systems, however, a close collaboration among cardiac electrophysiologists, engineers and technicians is highly needed in order to get the best from the available technology.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Three-dimensional electroanatomical mapping (3D-EAM) systems have become indispensable tools to guide catheter ablation (CA) of complex arrhythmias, including premature ventricular complexes (PVCs) originating from unusual anatomic locations [1,2,3], scar-related atrial tachycardias (ATs) [4] and endocardial or epicardial ventricular tachycardias (VTs) [1]. The updated CARTO PRIME™ mapping module for CARTO ®3 version 7 (Biosense Webster Inc., Diamond Bar, CA, USA) 3D-EAM system features several novel algorithms that can add critical information during CA of these challenging arrhythmias. In this report, we describe the new LAT (local activation time) hybrid [5], coherent mapping [6] and map replay modules, firstly providing a brief technical overview and secondly reporting their use in a series of complex clinical cases.
2 LAT hybrid module
2.1 Overview
The new LAT hybrid module was designed to solve the problem of the displacement between the catheter’s spatial location during PVC and during sinus rhythm beat. This motion is the result of the different position of the heart in the chest during sinus rhythm and PVC. The LAT hybrid tool adapts the spatial location of the beat during sinus rhythm to the LAT information obtained during PVC, thus creating a more accurate map that allows more precise positioning of the catheter during PVCs’ ablation procedures.
2.2 Case report #1
A 73-year-old man was referred to our institution for drug-refractory palpitations. Electrocardiogram (ECG) showed frequent PVCs with left bundle branch block and inferior axis morphology with early transition in the precordial leads (Fig. 1a). Both echocardiography and cardiac magnetic resonance imaging (cMRI) were normal. Because of the high PVCs’ burden (15% on 24-h ECG-Holter monitoring) and patient’s symptoms, we decided to perform electrophysiology study and CA. We used the CARTO®3 version 7 mapping system integrated with intracardiac echocardiography (ICE).
a 12-lead surface ECG showing a left bundle branch block-type premature ventricular complex (PVC) with a small R wave in V2 and transition in V3, consistent with a left ventricular outflow tract origin. b Intracardiac echocardiography view of the aortic valve, showing the origins and proximal portions of the right (green double line) and left coronary arteries. c LAT (local activation time) hybrid activation map of the aortic root (87-degree left anterior oblique view with 54 degrees of caudal angulation). The red pin indicates the point of earliest activation as shown in the LAT hybrid map, and the rad tags and red arrow indicate the points where radiofrequency energy was delivered. The green point and arrow indicate the projection of the ablation catheter at the moment of PVC’s maximally anticipated potential recording in the LAT map, which overlies the right coronary artery ostium (green double line). Note that the point of earliest activation was relocated 3.3 mm away from the right coronary artery ostium in the LAT hybrid map, enabling successful ablation with no damage to the right coronary artery. The red star indicates the intracardiac echocardiography catheter. d Surface ECG tracings and unipolar signal showing the maximally anticipated potential with a unipolar QS configuration (red arrow). LA, left atrium; LCA, left coronary artery; LCC, left coronary cusp; RCA, right coronary artery; RCC, right coronary cusp
PVCs had a left bundle branch block morphology with a small and broad R wave in V2 and transition in V3 (Fig. 1a). Therefore, we hypothesized a left ventricular outflow tract origin [7] and performed a retro-aortic approach. Activation mapping localized the earliest activation zone in the right coronary cusp area, with local bipolar electrogram anticipating surface QRS by 38 ms and unipolar signal displaying QS configuration (Fig. 1d). An optimal pace mapping (99% PASO module correlation coefficient, Biosense Webster Inc., CA, USA) was also obtained in this point. The right coronary ostium was carefully identified by ICE (Fig. 1b, and, because of its proximity to the PVC’s site of origin, particular care was taken to optimize catheter’s position in order to avoid any potential damages (Fig. 1c).
In this case, we used the LAT hybrid software. As compared with the conventional LAT map, according to which the PVC’s site of origin would have been exactly located in the right coronary artery ostium, the LAT hybrid map showed that, in sinus rhythm, PVC’s site of origin was displaced by 3.3 mm away, enabling safer positioning of the ablation catheter (Fig. 1c). Few radiofrequency (RF) pulses up to 30 W were delivered up to a maximum ablation index value of 493 [8], and the PVC rapidly and permanently disappeared, with no adverse events reported.
Despite being a small distance from an absolute perspective, the 3.3 mm displacement was clinically relevant in this case, because it allowed a minimal safety margin from the right coronary artery ostium, avoiding potential damages to this vital anatomical structure.
3 Coherent mapping
3.1 Overview
Atrial tachyarrhythmias represent a common finding in grown-up patients with congenital heart disease (CHD) [9, 10]. These arrhythmias are often produced by re-entry around complex anatomical barriers which follow surgical repairs [9, 10]. In this setting, 3D-EAM systems have been shown to facilitate substrate characterization and tachyarrhythmia’s activation mapping [11,12,13]. The coherent mapping module is a novel tool which integrates local activation time (LAT) information with vector data and the likelihood of conduction in a single algorithm, thus helping to identify areas of conduction slowing or block and to characterize complex patterns of impulse propagation [6].
3.2 Case report #2
A 64-year-old man with recurrent symptomatic episodes of atrial tachyarrhythmia was referred to our institution. He had a history of infundibular pulmonary stenosis and atrial septal defect treated with two previous surgical reparative procedures (Blalock procedure in 1965; pulmonary valvotomy and right ventricular outflow tract repair with a Dacron patch in 1975).
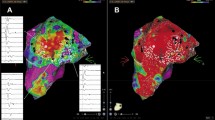
Because of lack of effectiveness of multiple antiarrhythmic drugs in maintaining sinus rhythm and patient intolerance to arrhythmic episodes, we scheduled CA. By using a multi-electrode high-density mapping catheter (PentaRay, Biosense Webster Inc., CA, USA), we were able to show, at the bipolar map, a large homogenous scar in the posterior wall of the right atrium between the superior and inferior venae cavae, and a smaller scar in the zone of previous atriotomy, close to the cavotricuspid isthmus (Fig. 2a). Although activation mapping with the high-density colouring software [14] showed a line of block along the whole posterior wall and a single critical slow-conducting area in the zone between previous atriotomy and inferior vena cava (Fig. 2b, the map obtained with the new CARTO prime coherent mapping module by setting the fill threshold at five and the internal points filtering at seven showed a critical isthmus in the region of prior atriotomy (Fig. 3a, movie 1) and, interestingly, a second bystander isthmus in the posterior wall (Fig. 2c and Fig. 3b). Entrainment manoeuvres confirmed these electroanatomical findings.
Posterior views of the right atrium (139-degree right anterior oblique projections with 13 degrees of caudal angulation). a Voltage map, showing a large scar in the posterior wall of the right atrium, in the zone of prior extracorporeal circuit cannulation; b high-density (HD) colouring map, showing a potential zone of conduction block (extended early meets late, white line) in the same zone as the scar; c coherent map, showing in brown a zone of non-conduction/low probability of conduction and arrows representing conduction vectors, with thicker vectors indicating slower conduction. ICV, inferior cava vein; SCV, superior cava vein
a Thirty-nine-degree right anterior oblique projection with 63 degrees of caudal angulation of the right atrium, showing the critical isthmus in the zone between previous atriotomy (PA) and inferior cava vein (ICV). The red tags indicate the points were radiofrequency pulses were delivered, and the tachycardia was interrupted in the critical isthmus; b posterior view of the right atrium (139-degree right anterior oblique projection with 13 degrees of caudal angulation). Coherent map showing a potential bystander isthmus in the posterior wall (big white arrow). SCV, superior cava vein; TV, tricuspid valve
Multiple pulses of RF were delivered in the critical isthmus by means of a 3.5-mm irrigated tip ablation catheter (SmartTouch, Biosense Webster Inc., CA, USA), firstly interrupting the tachycardia (Fig. 4) and then achieving a line of bidirectional block, as confirmed by differential site pacing manoeuvres. Pulses of RF were also delivered in the posterior wall in order to homogenize the scar and possibly eliminate a substrate for further tachycardias. After a follow-up of 3 months, the patient is free from recurrent arrhythmias.
Right atrium, 68-degree left anterior oblique projection with 65 degrees of caudal angulation. Interruption of atrial flutter by radiofrequency delivery in the critical isthmus between the inferior cava vein (ICV) and the zone of previous atriotomy (PA, brown area). The red tags indicate the points where radiofrequency energy pulses were delivered. a Coherent map clearly showing the critical isthmus. b Local activation time (LAT) map, in which the critical isthmus is not as clearly identifiable as in the coherent map. c ECG and electrograms at the moment of arrhythmia termination. TV, tricuspid valve
By means of its capability to integrate LAT information, vector data and probability of conduction, the coherent mapping module can provide accurate characterization of tachycardia substrates and circuits in an easy-to-interpret manner. In this case, it was able to comprehensively characterize the atrial substrate in a complex patient, guiding restoration of sinus rhythm as well as modification of the substrate itself, thus preventing arrhythmic recurrences. Therefore, the CARTO prime coherent mapping module has the potential to become a valuable tool in the management of complex macro-re-entrant tachycardias, which are frequently encountered in CHD patients.
4 Map replay module
4.1 Overview
Unstable VTs remain a major challenge for the cardiologist [15, 16]. Rapid hemodynamic decompensation prevents accurate definition of the tachycardia circuitry by activation or entrainment mapping in the majority of patients referred for CA, even when 3D-EAM systems are used [17, 18]. The map replay module allows rapid retrospective review and acquisition of signals in order to map different arrhythmias with the same catheter locations, even after brief arrhythmic events. Whether this novel system aids in activation mapping of unstable VTs is currently unknown.
4.2 Case report #3
A 63-year-old man presented with incessant palpitations and hypotension. ECG showed a VT with right bundle branch block (RBBB) morphology and right axis deviation (Fig. 5a), and the patient was rapidly cardioverted [15]. Coronary angiography excluded significant coronary artery disease, whereas cMRI showed a hypertrophic and mildly hypokinetic LV with a midmyocardial late gadolinium enhancement (LGE) area in the posterolateral wall (Fig. 6b). The patient admitted to prior cocaine abuse, supporting a diagnosis of cocaine-associated cardiomyopathy [19], and a subcutaneous defibrillator was implanted for secondary prevention of sudden death [20]. One month later, the patient experienced an appropriate defibrillator shock and was therefore referred for CA.
a First (clinical) unstable ventricular tachycardia (VT), with right bundle branch block morphology and right axis deviation, which was cardioverted after 42 s because of hemodynamic instability; b clinical VT’s activation and vector map (104-degree left anterior oblique projection with 23 degrees of cranial angulation) retrospectively and rapidly obtained with the map replay and coherent mapping modules; the red area is the zone of earliest activation, in the lateral left ventricular outflow tract. The red tags indicate the points where radiofrequency energy was delivered. Ao, aortic valve; MV, mitral valve
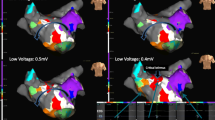
a Second induced ventricular tachycardia, with right bundle branch block morphology and left axis deviation; this tachycardia was cardioverted after 30 s due to hemodynamic instability; b cardiac magnetic resonance imaging (cMRI) short axis view, showing a midmyocardial late gadolinium enhancement (LGE) area in the posterolateral LV wall (white arrow). c Endocardial unipolar voltage map (174-degree left anterior oblique projection with 48 degrees of caudal angulation) showing a low voltage area (red) in the posterolateral wall of the left ventricle, consistent with the LGE area shown by cMRI; d local activation time (LAT) map obtained in sinus rhythm, from the same projection as c, showing late potentials in the posterior wall of the left ventricle; e second VT’s activation map as retrospectively and quickly drawn with the map replay module from the same projection as c and d, showing in red the zone of earliest activation in the posterolateral wall. Note the correspondence among the area where late potentials where recorded in panel b, the transition between normal and diseased myocardium in the endocardial unipolar map in panel c and the tachycardia’s zone of earliest activation in panel d (white arrows). The red tags indicate the points where radiofrequency energy was delivered. Ao, aortic valve; MV, mitral valve
Due to the presumed origin of the clinical VT, the left ventricle was mapped with a combined transseptal and retrograde endocardial approach, using a high-density multi-electrode mapping catheter (PentaRay, Biosense Webster Inc., CA, USA). The bipolar voltage map was completely normal, whereas the unipolar map showed a low voltage area in the posterolateral wall, consistent with the location of the LGE area on cMRI (Fig. 6c). Activation mapping during sinus rhythm showed a limited area of late potentials in the border zone between normal and diseased myocardium as identified by unipolar voltage mapping (Fig. 6d). During programmed electrical stimulation, two different rapid monomorphic RBBB VTs were reproducibly induced, the former with right axis deviation and similar characteristics to the clinical VT (Fig. 5a) and the latter with left axis deviation (Fig. 6a). Because of marked hemodynamic instability, both VTs had to be promptly cardioverted after 42 and 30 s, respectively, before any activation or entrainment mapping could be prospectively performed.
Nonetheless, retrospective review and acquisition of signals obtained during the VTs with the map replay module enabled activation mapping of both tachycardias. Intracavitary electrograms were recorded by gently moving the multi-electrode mapping catheter in the left ventricle while maintaining adequate contact with the endocardium, in order to acquire as many signals as possible during the brief VT episodes, and maps were obtained by setting both the fill threshold and internal points filtering at seven. Two different sites of earliest activation were revealed: for the former VT, the earliest activation point was located in the lateral LV outflow tract (Fig. 5b), whereas for the latter VT, activation wave spread from the same region where late potentials were found in sinus rhythm, in the posterior basal wall (Fig. 6e). These sites of precocious activation were confirmed by pace mapping manoeuvres during sinus rhythm. Multiple pulses of RF were delivered in both sites, and late potentials were eliminated. A programmed electrical stimulation confirmed loss of inducibility. The procedure was carried out with no complications, and, at 3-month follow-up, the patient experienced no arrhythmic recurrences.
5 Conclusions
Cardiac electrophysiologists have to face increasingly difficult challenges, dealing with complex arrhythmias in special patients. In this context, the introduction of novel algorithms, such as the ones described in the preceding sections, should be encouraged and applauded. The novel CARTO PRIME™ mapping module for CARTO®3 version 7 3D-EAM system offers a valuable opportunity to render CA procedures more accurate, effective and safe. Given the increasing sophistication of 3D-EAM systems, however, a close collaboration between different professionals (physicians, engineers, technicians) is highly needed in order to get the best from the available technology and to explore all of its potential. Larger clinical studies with higher number of patients and longer follow-up will tell whether the challenge of improving patients’ outcome will be won.
Data availability
Available data.
References
Cronin EM, Bogun FM, Maury P, Peichl P, Chen M, Namboodiri N, et al. 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias. Europace. 2019;21(8):1143–4.
Yamada T, McElderry HT, Doppalapudi H, Murakami Y, Yoshida Y, Yoshida N, et al. Idiopathic ventricular arrhythmias originating from the aortic root prevalence, electrocardiographic and electrophysiologic characteristics, and results of radiofrequency catheter ablation. J Am Coll Cardiol. 2008;52(2):139–47.
Sassone B, Muser D, Casella M, Luzi M, Virzì S, Balla C, Nucifora G; Task force on imaging and task force on ablation of ventricular tachycardia of the Italian Association of Arrhythmias and Cardiac Pacing (AIAC). Detection of concealed structural heart disease by imaging in patients with apparently idiopathic premature ventricular complexes: a review of current literature. Clin Cardiol 2019 Dec;42(12):1162–1169.
Coffey JO, d'Avila A, Dukkipati S, Danik SB, Gangireddy SR, Koruth JS, et al. Catheter ablation of scar-related atypical atrial flutter. Europace. 2013;15(3):414–9.
De Potter T, Iliodromitis K, Bar-On T, Silva Garcia E, Ector J. Premature ventricular contractions cause a position shift in 3D mapping systems: analysis, quantification, and correction by hybrid activation mapping. Europace. 2020;22:607. https://doi.org/10.1093/europace/euaa013.
Anter E, Duytschaever M, Shen C, Strisciuglio T, Leshem E, Contreras-Valdes FM, et al. Activation mapping with integration of vector and velocity information improves the ability to identify the mechanism and location of complex scar-related atrial tachycardias. Circ Arrhythm Electrophysiol. 2018;11(8):e006536.
Lin D, Ilkhanoff L. Twelve-lead electrocardiographic characteristics of the aortic cusp region guided by intracardiac echocardiography and electroanatomic mapping. Heart Rhythm. 2008;5(5):663–9.
Casella M, Gasperetti A, Gianni C, Zucchelli G, Notarstefano P, Al-Ahmad A, et al. Ablation index as a predictor of long-term efficacy in premature ventricular complex ablation: a regional target value analysis. Heart Rhythm. 2019;16(6):888–95.
Walsh EP, Cecchin F. Arrhythmias in adult patients with congenital heart disease. Circulation. 2007;115:534–45.
Hernández-Madrid A, Paul T, Abrams D, Aziz PF, Blom NA, Chen J, et al. ESC Scientific Document Group. Arrhythmias in congenital heart disease: a position paper of the European Heart Rhythm Association (EHRA), Association for European Paediatric and Congenital Cardiology (AEPC), and the European Society of Cardiology (ESC) Working Group on Grown-up Congenital heart disease, endorsed by HRS, PACES, APHRS, and SOLAECE. Europace. 2018;20(11):1719–53.
Drago F, Russo MS, Marazzi R, Salerno-Uriarte JA, Silvetti MS, De Ponti R. Atrial tachycardias in patients with congenital heart disease: a minimally invasive simplified approach in the use of three-dimensional electroanatomic mapping. Europace. 2011;13(5):689–95.
Klehs S, Schneider HE, Backhoff D, Paul T, Krause U. Radiofrequency catheter ablation of atrial tachycardias in congenital heart disease: results with special reference to complexity of underlying anatomy. Circ Arrhythm Electrophysiol. 2017;10(12):e005451.
Mantziari L, Butcher C, Shi R, Kontogeorgis A, Opel A, Chen Z, et al. Characterization of the mechanism and substrate of atrial tachycardia using ultra-high-density mapping in adults with congenital heart disease: impact on clinical outcomes. J Am Heart Assoc. 2019;8(4):e010535.
Asferg C, Chen X, Pehrson S, Jacobsen PK. Catheter ablation of atypical flutter using new 3-dimensional electroanatomic mapping software focusing on areas of conduction block. HeartRhythm Case Rep. 2019;5(4):225–8.
Nichol G, Sayre M, Guerra F, Poole J. Defibrillation for ventricular fibrillation: a shocking update. J Am Coll Cardiol. 2017;70(12):1496–150.
Guerra F, Palmisano P, Dell'Era G, Ziacchi M, Ammendola E, Bonelli P, et al. Implantable cardioverter-defibrillator programming and electrical storm: results of the OBSERVational registry On long-term outcome of ICD patients (OBSERVO-ICD). Heart Rhythm. 2016;13(10):1987–92.
Santangeli P, Marchlinski FE. Substrate mapping for unstable ventricular tachycardia. Heart Rhythm. 2016;13(2):569–83.
Kautzner J, Peichl P. Mapping and ablation of unmappable ventricular tachycardia, ventricular tachycardia storm, and those in acute myocardial infarction. Card Electrophysiol Clin. 2019;11(4):675–88.
Havakuk O, Rezkalla SH, Kloner RA. The cardiovascular effects of cocaine. J Am Coll Cardiol. 2017;70(1):101–13.
Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, et al. ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J. 2015;36(41):2793–867.
Acknowledgements
The authors acknowledge Matteo Mirarchi, BE, for technical support in image editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Yes.
Consent to participate
Yes.
Consent for publication
Yes.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
Propagation map of atrial macro-re-entrant tachycardia obtained with the coherent mapping module. Note the critical isthmus in the region between the inferior cava vein and the scar due to prior atriotomy and, also, a second bystander isthmus in the superior portion of the posterior wall, close to the superior cava vein. The representation of small moving vectors simplifies interpretation of arrhythmia’s circuitry. (MP4 44,322 kb)
Rights and permissions
About this article
Cite this article
Compagnucci, P., Volpato, G., Falanga, U. et al. Recent advances in three-dimensional electroanatomical mapping guidance for the ablation of complex atrial and ventricular arrhythmias. J Interv Card Electrophysiol 61, 37–43 (2021). https://doi.org/10.1007/s10840-020-00781-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-020-00781-3