Abstract
Catheter ablation of ventricular arrhythmias (VAs) has evolved significantly over the past decade and is currently a well-established therapeutic option. Technological advances and improved understanding of VA mechanisms have led to tremendous innovations in VA ablation. The purpose of this review article is to provide an overview of current innovations in VA ablation. Mapping techniques, such as ultra-high density mapping, isochronal late activation mapping, and ripple mapping, have provided improved arrhythmogenic substrate delineation and potential procedural success while limiting duration of ablation procedure and potential hemodynamic compromise. Besides, more advanced mapping and ablation techniques such as epicardial and intramyocardial ablation approaches have allowed operators to more precisely target arrhythmogenic substrate. Moreover, advances in alternate energy sources, such as electroporation, as well as stereotactic radiation therapy have been proposed to be effective and safe. New catheters, such as the lattice and the saline-enhanced radiofrequency catheters, have been designed to provide deeper and more durable tissue ablation lesions compared to conventional catheters. Contact force optimization and baseline impedance modulation are important tools to optimize VT radiofrequency ablation and improve procedural success. Furthermore, advances in cardiac imaging, specifically cardiac MRI, have great potential in identifying arrhythmogenic substrate and evaluating ablation success. Overall, VA ablation has undergone significant advances over the past years. Innovations in VA mapping techniques, alternate energy source, new catheters, and utilization of cardiac imaging have great potential to improve overall procedural safety, hemodynamic stability, and procedural success.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Ventricular arrhythmias (VAs) represent a heterogeneous spectrum ranging from single premature ventricular contractions to sustained ventricular tachycardia (VT) and ventricular fibrillation. VAs are a significant cause of morbidity and mortality [1]. VT ablation is currently an essential therapeutic option [1]. Despite a reduction in ICD shocks with VT ablation, no significant reduction in mortality was demonstrated in multiple randomized controlled studies [2,3,4]. The VANISH trial has demonstrated that in patients with ischemic cardiomyopathy, VT ablation results in a significant reduction in a composite outcome of death, VT storm, and appropriate ICD shocks compared to escalation of antiarrhythmic drugs [5]. This review article discusses recent innovations in VT ablation, including novel mapping techniques, ablation techniques, alternate energy sources, new catheters, and imaging modalities.
2 Novel VT mapping techniques
Sustained monomorphic ventricular tachycardia is mostly caused by scar-related re-entry [6]. The substrate for re-entry is fibrosis areas interspersed within surviving myocytes leading to regions of conduction blocks and regions of slow conduction [1]. Success of VT ablation depends on accurate mapping and elimination of the VT circuits. In general, the two main approaches for identifying VT circuits are (1) activation/entrainment or pace-mapping and (2) substrate-based mapping. Substrate-based mapping allows for the identification of areas supporting VT re-entry regardless of VT inducibility or hemodynamic intolerance with VT induction [1].
2.1 UHD mapping
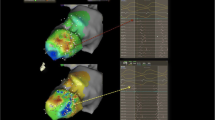
UHD mapping involves the use of multielectrode mapping (MEM) catheters with smaller electrodes with tight interelectrode spacing to acquire >1000 mapping points (Fig. 1). Compared to point-by-point mapping (PPM) catheters, MEM catheters have smaller electrodes with tight interelectrode distances, which improves spatial resolution [7, 8]. MEM has a lower sensitivity for far-field signals yet a higher sensitivity for local electrical activity [9, 8]. It has been shown to provide better electroanatomic mapping correlation with contrast-enhanced cardiac magnetic resonance scar assessment [9, 10], shorter radiofrequency ablation time [9], and shorter mapping time [11]. MEM can identify areas of preserved myocardial bundles within heterogeneous scar areas, which would otherwise be recognized as dense scar by mapping with standard ablation catheter (3.5–4 mm electrode) [8].
Diagram of the grid mapping catheter (the Advisor HD Grid Mapping Catheter). The 4 splines with 4 1-mm equidistanced (3 mm) electrodes allow parallel (along the splines) and perpendicular (across the splines) bipole recordings (reproduced from Okubu et al. [11] with permission)
2.2 RM
Ripple mapping (RM) represents endocardial activation in a dynamic 3-dimensional configuration conveying intracardiac electrograms timing, morphology, and location [12]. It is a feature of CARTO-3© electroanatomic mapping system (Biosense Webster Inc, Irvine, CA). RM has been used in humans to identify slow conduction channels within scar areas in ischemic and non-ischemic VT [13,14,15,16]. As RM displays all components of an electrogram by dynamic bars corresponding to the electrogram voltage, location, and timing, it allows differentiation between local activation signals originating from within the scar area and far-field signals from nearby myocardial tissue [13, 14]. RM-guided VT ablation has been shown to result in significant short-term and long-term freedom of VT [14, 16]. In addition, Xie et al. showed 97% of RM conduction channels localize within or adjacent to areas of late gadolinium enhancement on late gadolinium enhancement MRI (LGE-MRI) [16].
2.3 ILAM
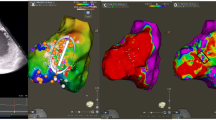
Isochronal late activation mapping (ILAM) is a voltage-independent functional mapping strategy that displays conduction velocity within the scar area visually; with conduction velocity being proportional to isochronal thickness [17]. Areas of slow conduction “deceleration zones” within scar have been recognized to represent critical sites for VT re-entry (Fig. 2) [18,19,20,21]. ILAM has demonstrated that areas of isolated late potentials could be geographically and functionally distant from areas of slow conduction [22]. Anter et al. have shown that areas of slowest conduction during tachycardia exhibited very slow conduction in sinus rhythm and corresponded to VT isthmus site. Compared to voltage and EGM criteria for identification of VT isthmus, areas of significant conduction slowing (>75%) during sinus rhythm had a significantly higher positive predictive value for identifying VT critical zones [23]. ILAM allows for identification of VT critical sites during sinus rhythm with a higher sensitivity and specificity compared of voltage and EGM mapping.
Area of slow conduction within scar indicated by long fractionated electrogram (red circle) corresponds to area of isochronal crowding -— “deceleration zone.” The critical isthmus site/termination site courses through the deceleration zone during VT (reproduced from Aziz et al. [22] with permission)
2.4 DEEP mapping
Decrement evoked potential (DEEP) mapping aims at identifying near-field potentials that demonstrate delayed or “decremental” conduction after a single “evoked” extra-stimulus [24]. DEEP mapping exhibits improved sensitivity and significantly higher specificity in detecting areas of VT diastolic pathways compared to late potentials [24, 25]. A multicenter study showed that DEEP mapping-guided VT radiofrequency ablation (RFA)–reduced VT inducibility after ablation by 80% and resulted in a significant reduction in number of shocks and VT burden within 6 months post-ablation as well as 75% freedom of VT at 6 months of follow-up [25].
2.5 Omnipolar mapping
Bipolar electrograms are relatively spared from far-field signal artifacts compared to unipolar electrograms. However, other factors such as angle and contact force of electrode, activation wavefront orientation relative to bipole pair, myocardial fiber orientation, electrode size, and interelectrode spacing can influence bipolar electrograms morphology [26, 27]. Omnipolar mapping aims at deriving electrograms that reflect wavefront direction and velocity independent of electrode orientation [28]. Omnipolar electrograms are obtained using the method described by Deno et al. [28]. An omnipolar electrogram represents a unifying electrogram created from simultaneous adjacent bipoles from an electric field caused by a passing activation wavefront [29]. Compared to unipole- and bipole-derived data, omnipole-derived conduction velocity and activation direction in swine models exhibited more consistent measurements as well as higher sensitivity for shifts in activation direction [30]. Omnipolar mapping has been shown to provide an enhanced delineation of VT substrate, increased identification of conduction channels, as well as a higher and more consistent peak-to-peak voltages compared to bipolar mapping [31, 32]. Besides, it provided a better correlation with evidence of scar on LGE-MRI [32]. No randomized clinical trials have compared omnipolar mapping to other forms of EGM recording [33]. Moreover, omnipolar mapping requires dedicated electro-anatomical mapping systems as well as more sophisticated computational efforts, which might be a limiting factor for its widespread use [33].
2.6 SAFE-T
The simultaneous amplitude frequency electrogram transformation (SAFE-T) mapping strategy is an automated process that recognizes high-frequency potentials during sinus rhythm using the Hilbert-Huang transform (HHT) processing tool for nonlinear and nonstationary signals [34, 35]. The SAFE-T value is a product of the instantaneous amplitude and instantaneous frequency of an electrogram. It is derived from the HHT processing tool and is expressed as (Hz mV). Louise et al. have shown that a SAFE-T cutoff value of >3 Hz mV provides 92% sensitivity and 78% specificity for predicting VT origin [34]. It has been shown to provide improved identification of VT isthmus site compared to unipolar and bipolar low-voltage zones and late potential mapping [35]. Besides, areas of scar identified via abnormal SAFE-T values were significantly smaller than area of unipolar low-voltage zones, bipolar low-voltage zones, and bipolar scar, which can potentially lead to a reduction in VT burden with a more limited area of ablation [35], which can limit procedural complications associated with larger areas of ablation as in full scar homogenization techniques.
2.7 Future directions
Targeting late potentials during sinus rhythm has been shown to reduce VT recurrence after ablation [36,37,38]. Novel VT mapping techniques, as highlighted above, allow for mapping and targeting sinus rhythm surrogates of VT critical isthmus sites, such as identification of slow conduction channels with RM, areas of slow conduction “deceleration zones” with ILAM, and near-field potentials with delayed or “decremental” conduction with DEEP mapping. This provides a significant advantage compared to conventional mapping techniques by evading hemodynamic instability associated with inducing VT. Novel mapping techniques also allow for identification of potential VT circuits in cases of inconsistent inducibility. Those mapping techniques can be utilized solely or complementary to conventional techniques. In addition to more effective and efficient mapping and VT ablation, we believe such novel techniques can also improve VT ablation procedural safety profile as it would limit peri-procedural hemodynamic compromise and would also allow for reducing the duration of the procedure.
Many trials (Table 1) are focused on evaluating new mapping technologies, as more precise identification of VT circuits could lead to improved ablation outcomes.
3 Innovations in ablation techniques
3.1 VT RFA
3.1.1 Bipolar RFA
Conventionally, VT RFA is performed in a unipolar configuration, in which an irrigated-tip catheter is used, and energy is applied between the catheter tip a reference electrode on the patient’s skin (Fig. 3A). However, despite the effectiveness of this approach in arrhythmia ablation, VT recurrence is still significant, which is in part related to the failure of transmural lesion creation [39]. Bipolar RFA ablation involves energy application between two closely placed catheter tips (Fig. 3B), which leads to a focused high-density energy delivery to the myocardial tissue between the two catheter tips, which improves lesion transmurality [40,41,42]. It has also been shown that bipolar ablation can reduce the time needed to create transmural lesions compared to unipolar ablation [40]. Bipolar RFA is particularly useful when targeting VT circuits deep within the tissue such as interventricular septum and intramyocardial tissue as unipolar RFA may not achieve sufficient energy delivery to create a transmural lesion [42,43,44]. Bipolar ablation is shown to be safe and effective when conventional unipolar ablation strategies fail to eradicate VT, even when performed in critical structures such as VA arising close to the His-bundle [45, 46].
3.1.2 Sequential and simultaneous unipolar RFA (Fig. 3C)
Sequential unipolar RFA involves the application of unipolar RFA energy in sequential order from endocardial then epicardial sites or vice versa, whereas simultaneous unipolar RFA involves the delivery of unipolar RFA simultaneously from endocardial and epicardial sites. Simultaneous RFA has been shown to be highly effective and safe in cases of failed sequential RFA [47, 48]. Compared to sequential ablation, in simultaneous ablation, radiofrequency energy is delivered from both sites of the myocardium leading to decreased temperature loss via convective cooling from the opposite side of the unipolar energy source as well as increased current density and heat load and delivery to intramyocardial tissue creating deeper lesions [47, 48]. It has been previously shown that unipolar RFA with an output of 30 W creates a lesion with a depth of 5 to 8 mm [49]. Thus, in cases where the distance between the endocardial and epicardial sites is >5 to 8 mm, simultaneous RFA should be considered over sequential RFA [48]. Compared to bipolar ablation, in simultaneous ablation, the two ablation catheters are independent of each other, which allows for individual control of power delivery at each side of the myocardial tissue, which theoretically can decrease the risk of surrounding tissue thermal damage (e.g., coronary arteries) and steam pop formation [44, 47, 48].
3.2 Epicardial VT ablation
Epicardial VT ablation has been clinically utilized in certain VT scenarios where a concealed VT circuit is present, such as arrhythmogenic right ventricular cardiomyopathy (ARVC), inferolateral non-ischemic cardiomyopathy VT, post-infarction transmural substrate, and in cases where endocardial ablation fails to control VT [50,51,52,53]. In patients with scar-related VT, a combined endocardial-epicardial ablation approach is associated with a decreased risk of VT recurrence and subsequent mortality compared to the endocardial-only ablation approach [52]. Moreover, using bipolar substrate mapping for scar identification, endocardial-epicardial VT ablation via scar homogenization is associated with a significantly higher success rate at 5 years of follow-up and reduced need for antiarrhythmic drugs compared to endocardial-only ablation [54]. Interestingly, epicardial adjunctive ablation was performed on all patients assigned to the endocardial-epicardial VT ablation treatment group despite VT being non-inducible following endocardial ablation [54]. Better outcomes with adjunctive epicardial ablations seem to be related to superior access to the epicardium and deep intramural tissue [54]. Recently, Matos et al. showed data from a multicentric observational registry demonstrating that endocardial-epicardial VT ablation whether done in a combined or sequential strategy to be associated with lower VT recurrence and lower all-cause death in patients with ischemic or nonischemic cardiomyopathy who underwent repeat VT ablations [55]. Despite reports of increased complications with epicardial access [52], complications can be significantly reduced with improved skill and experience [53, 54].
Besides the conventional subxiphoid epicardial access technique introduced by Sosa et al. [56], new techniques have been developed for improved epicardial access safety and success. Kumar et al. described the needle-in-needle technique in which subxiphoid access to the cardiac silhouette is performed via an 18-G Cook needle followed by the insertion of a 21-G micropuncture needle into the 18-G Cook needle [57], which allows pericardial access via a 21-G needle rather than a 17-G or 18-G needle. Epicardial access via a micropuncture needle is associated with decreased risk of large pericardial effusion when compared to epicardial access using a large-bore needle [57, 58]. Di Biase et al. described an epicardial access technique using the EpiAccess system (EpiEP, Inc., New Haven, CT) in which a fiber optic–equipped needle provides real-time confirmation of epicardial access via pressure waveform monitoring [59]. Moreover, real-time pressure waveform monitoring allows recognition of needle contact with the RV wall, which improves procedural safety [59]. Carbon dioxide (CO2) pericardial space insufflation has been utilized to aid with subxiphoid epicardial access, which can be performed via a right atrial appendage exit procedure (Fig. 4) [60] or via coronary venous system exit procedure [61]. Furthermore, advanced imaging modalities such as CT and CMR have been reported to guide epicardial access and improve procedural safety [62, 63].
Right atrial appendage exit procedure. An example of transatrial guidewire exit: Anteroposterior (A) and lateral (B) contrast angiograms of the right atrial appendage from a catheter positioned at its crest. (C) The back end of a 0.014-inch guidewire crosses the right atrial appendage. (D) A microcatheter is advanced over the guidewire into the pericardial space (reproduced from Greenbaum et al. [60] with permission)
3.3 Intramyocardial VT mapping and ablation
Endocardial and epicardial ablation and mapping techniques are limited to myocardial tissue close to the catheter and often fail to identify intramural excitable substrates [64, 65]. Sapp et al. demonstrated that intramural catheter ablation was feasible and resulted in 12 months free of VT in four out of eight patients with VT refractory to antiarrhythmic drugs and ablations [66]. In a larger multicenter study with 31 refractory VT patients, intramural catheter ablation resulted in 48% free of VT at 6 months of follow-up [65]. Stevenson et al. used an ablation catheter or a multi-electrode catheter for initial mapping, then a needle electrode was inserted into the myocardium for unipolar pacing to assess tissue excitability at potential VT target sites based on low-voltage areas [65]. Areas of IES had higher endocardial and intramural needle unipolar voltage as well as higher endocardial and intramural needle electrogram frequency compared to scar areas [67]. Coronary venous system (CVS) mapping and ablation have been proposed as an effective strategy for targeting VA originating from intramural or epicardial foci [68] and is particularly important when arrhythmogenic substrate delineating via endocardial and epicardial mapping techniques is suboptimal such as NICM VT arising from the septum [69,70,71]. Briceño et al. demonstrated that in patients with suspected intramural septal VA, CVS mapping can improve arrhythmogenic substrate identification and guide endocardial ablation [69]. In all cases, intramural activation and pace maps were better than endocardial and epicardial mapping [69]. CVS mapping is particularly important when epicardial approach is limited as in patients with prior history of CABG due to epicardial adhesions and scarring. Karimianpour et al. have demonstrated that CVS mapping is safe, feasible, and effective in patients with prior history of CABG [72]. Accurate delineation of CVS anatomy via advanced imaging, such as CT, is important when performing CVS mapping as identification of CVS branches within proximity of the arrhythmogenic substrate [72]. Romero et al. have described a novel technique for intramyocardial ablation using intracoronary wire for a patient with prior failed ablation attempts for VA arising from the LV summit [73].
3.4 Impedance modulation
RFA using irrigation catheters is a power-controlled procedure; thus, lesion formation is affected by power output and baseline impedance [74, 75]. Barkagan et al. have demonstrated a strong inverse correlation between baseline impedance and radiofrequency current output and lesion volume [74]. Shapira-Daniels et al. demonstrated that adding or adjusting return patches in patients with failed VT RFA reduces baseline impedance, increases in current output, and improves procedural success [75]. RFA with lower ionic concentration solutions, such as half-normal saline (HNS) and 5% dextrose in water (5DW) has been shown to result in increased energy delivery to tissue and larger lesion formation [76,77,78]. Nguyen et al. demonstrated that the use of HNS as the irrigant during VT ablation in patients who had failed standard ablation procedures with normal saline being the cooling RFA irrigant is safe and effective [79]. Half normal saline irrigation, compared to normal saline, exhibits a higher electrical impedance and thus is thought to result in less electrical current dispersion and more focused delivery of current to the cardiac tissue [79]. Steam pop incidence has been observed to be higher using HNS and D5W [76, 79]. Baseline impedance modulation is an important tool to maximize lesion formation and improve procedural success. However, more data regarding optimal baseline impedance values and safety profile is essential for better procedural optimization.
3.5 Contact force
Contact force has been utilized as an indicator of atrial fibrillation (AF) ablation efficacy. Using irrigated contact force-sensing catheters, Elsokkari et al. examined the contact force needed to achieve electrical unexcitability post-ablation [80]. Although contact force did not predict post-ablation electrical unexcitability, a contact force of 10 g or more within the scar region was associated with a significant association with post-ablation electrical unexcitability [80]. In addition, they have found that the retrograde aortic approach to the left ventricle resulted in better contact force with the anterior wall, whereas the transseptal approach had a better contact force with the lateral wall [80]. However, Hendriks et al. showed no difference in VT ablation procedural outcomes or safety profile between contact-force and non-contact force-sensing catheters [81]. Limited data exist regarding measures of VT ablation efficacy. More data is certainly needed to assess the role of contact force sensing catheters and the required contact force for effective and successful VT ablation.
3.6 ICE
Integration of intracardiac echocardiography (ICE) with VT ablation procedure has been shown to improve procedural overall safety and success by improving visualization of intracavitary structures, direct visualization of the ablation catheter, monitoring of the contact between the catheter and intracavitary structures, and better understanding of catheter positioning in relation to landmark structures [82, 83]. ICE-guided mapping and ablation is especially important in cases of papillary muscle VT/PVCs as anatomic visualization of PM and catheter is essential [82]. The use of ICE during VT ablation has been shown to significantly lower risk for 12-month VT-related admissions and repeat VT ablation compared to VT ablations without ICE [84].
Given the mixed procedural success of VT RFA as well as the risk of adverse effects, alternate modalities for cardiac ablation have become an area of increasing interest. Besides, many clinical trials are evaluating original strategies of VT ablation beyond the standard catheter ablation technique (Table 2).
3.7 Electroporation
Electroporation, also known as pulsed-field ablation (PFA), is achieved simply by applying a voltage between electrodes that contact tissue through various energy modalities including direct, alternating, or pulsed direct current [85]. Although the mechanism for electroporation is not completely understood, the general principle is that electric fields created contact cell membranes and cause pore formation and cell damage which can be reversible or permanent depending on the magnitude and duration of energy applied [86]. PFA is safe, effective, and results in durable ablation lesions in AF [87, 88]. In animal models, PFA has been shown to significantly reduce the window of vulnerability to ventricular fibrillation from baseline values with no evidence of underlying myocardial damage [89]. Ventricular endocardial PFA has been shown to result in homogenous focal fibrosis with preservation of nerves and vessels on histologic assessment [90, 91]. In addition, PFA has been shown to create ablative lesions rapidly (<10 s of PFA energy) [91]. Im et al. have shown that PFA produces greater histologic lesion depths in infarcted myocardial regions, which makes it an important choice for energy delivery in cases of post-infarction cardiomyopathy VT [91]. Recently, Tan et al. described a novel approach to PFA of ventricular myocardium using screw-in pacemaker leads [92]. Active fixation of ablation catheter improves stability and myocardial contact, which results in improved reliability [92]. Besides, it has also been shown that lesion durability improves with higher energy deliveries [92].
3.8 Ethanol ablation
Ethanol ablation is utilized as a last-resort option when radiofrequency ablation fails, especially with recent advances in mapping techniques and epicardial ablation [93,94,95,96,97]. Transarterial coronary ethanol ablation (TCEA) can be particularly useful in cases of septal VT with no endocardial origin and poor epicardial approach candidacy. In addition, TCEA can be considered in patients with a history of cardiac surgery as the epicardial approach may be challenging due to scarring [98]. Due to technical challenges involved with TCEA, retrograde coronary venous ethanol ablation (RCVEA) has become increasingly popular [93, 99]. This approach is thought to have less risk of canulating coronary veins as well as avoiding issues with coronary stenosis which can complicate the procedure significantly. Although ethanol ablation has been shown to improve VT control in the appropriate setting, it is associated with significant morbidity and mortality. Procedural complications occur in around 14% of cases, most commonly in the form of permanent or transient complete heart block, and risk of periprocedural mortality has been reported to be around 1% [100]. In addition, several anatomic variations can limit long-term durability of ethanol ablation such as the absence of a target vessel to deliver ethanol to the myocardial region of interest and the presence or development of vein-to-vein collaterals leading to ethanol bypassing the arrhythmogenic substrate [100].
3.9 STAR
Stereotactic arrhythmia radioablation (STAR) for VT was first performed in 2012 for a patient with sustained VT refractory to antiarrhythmic medications [101]. It involves the use of highly focused external beam radiotherapy for local concentrated ablative radiation doses with minimal injury to nearby tissues. The use of STAR for VT treatment has been shown to reduce initial VT recurrence, ICD therapies, and VT burden, in small studies [102,103,104]. In addition, STAR can reduce antiarrhythmic medication use and improve quality of life [103]. However, recurrences were observed in long-term follow-up [102]. STAR has been shown to be effective after one therapeutic session [102, 105, 106]. Although no significant adverse reactions were reported, low-grade pneumonitis has been reported [107]. Preliminary results from the ENCORE-VT study have shown two probable or definite related adverse events occurred 2 years after the procedure, including a pericardial effusion at 2.2 years and a gastropericardial fistula at 2.4 years [103]. The ideal location and size of the target volume for STAR remain uncertain [108]. On a molecular level, Zhang et al. have shown that STAR-treated tissue exhibited an increase in sodium channels expression (Nav1.5) and gap junctions (Cx43), which increase signal propagation leading to the prevention of re-entry [106]. Clinically, a significant reduction in QRS complex interval and a significant increase in conduction velocity were observed [106].
It is important to note that STAR depends on precise identification of arrhythmogenic substrate such as ischemic scar; thus, its application to other causes of VT such as NICM-related VT or infiltrative disease-related VT might be limited as the arrhythmogenic substrate is diffusely scattered throughout the myocardium. Early evidence of STAR therapy for refractory ventricular arrhythmias has been promising with regard to safety and efficacy outcomes; however, evidence is still limited. Larger randomized clinical trials are needed to make significant claims regarding the utility of this novel non-invasive cardiac ablation strategy [109].
4 Novel VT ablation catheters
Several new catheters and ablation approaches have been developed to provide more durable and ablation lesions and better identification and ablation of the arrhythmogenic substrate within the epicardium and deep intramural tissue (Table 3).
4.1 Lattice tip focal ablation catheter
While the lattice tip ablation catheter was not specifically designed for VT ablation and much of the supportive data are limited to atrial ablation studies, there is growing interest in this catheter’s utility for VT. The lattice catheter, Sphere-9 (Affera, Inc., MA, USA), has dual capacity for both radiofrequency (RF) as well as pulsed-field ablation (PF). Its design involves an expandable 8F irrigated spherical catheter with a lattice electrode surface that allows for higher current delivery at lower density compared to conventional catheters, thereby limiting the risk of tissue overheating and steam-pop formation [110,111,112]. In addition, its 9 mini-electrodes create a large area of conductive heating which provides deeper ablation lesions (Fig. 5) [110, 112, 113]. Additionally, it provides improved stability due to the compressibility of the lattice mesh and the complex lattice topography which attenuates sliding across the endocardial surface. The lattice catheter exhibits a similar safety profile in vivo studies compared to the conventional irrigated catheter in terms of steam-pop formation, char formation, and thrombus formation on the catheter [110]. Overall, although further studies on VT ablation in humans with the Sphere-9 lattice catheter are warranted, preliminary data on its use appear promising for the future of arrhythmia management [110].
The lattice catheter provides a larger effective surface area compared to standard linear irrigated electrodes, allowing higher current delivery at lower density, which reduces the risk of tissue overheating (reproduced from Shapira-Daniels et al. [112] with permission)
4.2 SERF catheter ablation
The saline-enhanced radiofrequency (SERF) ablation catheter uses a 25-G needle to deliver radiofrequency energy and degassed warmed saline injection into myocardial tissue [114]. The saline stabilizes the electrode temperature and acts as a medium to deliver energy deep into the tissue [114]. The SERF ablation catheter utilizes convective heating and reduces the chances of steam pops [114]. Animal models have shown that SERF ablation produces large, near-transmural lesions, without evidence of myocardial perforation, wall thinning, or aneurysm formation [114]. SERF catheters offer reproducible diameters, controllable volumes, less thrombolytic material, no steam pops, and can be reliably transmural when needed [114, 115]. High-power needle/saline lesions have been demonstrated to result in significantly deeper and larger lesions when compared to 4-mm-tip and irrigated-tip lesions [115]. Clinical data is limited but there are many promising active trials (Table 4) studying the SERF catheter among other novel ablation catheters and devices.
5 Mechanical circulatory support during VT ablation
Periprocedural hemodynamic decompensation occurs in 11% of patients [116]. IABPs provide relatively low cardiac output augmentation (~0.5 L/min) in comparison to other MCS devices, and their use is limited when used during VT ablation, as their mechanism of counterpulsation relies on a regular, stable, non-tachycardic rhythm to provide support [117, 118]. The TandemHeart (CardiacAssist Inc., Pittsburgh, PA) is a percutaneous left-atrial-to-femoral-artery bypass system and requires two large venous and arterial access sites. Its use is associated with vascular complications [117]. In addition, its trans-septal approach to the left atrium can interfere with transseptal mapping [117, 119]. TandemHert-assisted VT ablation has demonstrated its safety and feasibility [120, 121]. The Impella (2.5/CP/5.0) (Abiomed, Danvers, MA) is the most commonly used MCS device employed during VT ablation. Its axial flow pump unloads the LV and provides around 2.5–5 L/min of cardiac output, but its use can cause electromagnetic interference in magnetic-based mapping systems [117, 122]. Short-term MCS use has not been shown to decrease VT recurrence or increase VT-free survival long term [120,121,122,123,124,125].
Veno-arterial ECMO uses an external centrifugal pump and membrane oxygenator to extract blood from the venous system and shunt it back into the arterial system, which can provide biventricular support and augment cardiac output upwards of 4.5 L/min [119, 126]. Despite these advantages, outcomes associated with the use of ECMO for hemodynamic support during VT ablation are mixed. In a study of 64 patients undergoing VT ablation, pre-emptive use of EMCO was associated with improved survival and lower rates of VT recurrence, while another case series of 21 patients who required ECMO as “rescue therapy” following the development of hemodynamic compromise during VT ablation showed a higher rate of complications and decreased VT-free survival [127, 128].
Santangeli et al. have identified eight clinical factors associated with a high risk for acute hemodynamic decompensation during VT ablation: advanced age (>60 years), ischemic cardiomyopathy, advanced heart failure status with EF <25%, NYHA class III/IV, COPD, DM, VT storm at presentation, and use of general anesthesia [116]. Santangeli et al. have developed the PAINESD risk score to identify patients with a high risk for acute hemodynamic decompensation [129]. In a propensity score–matched analysis, Muser et al. have shown that the prophylactic use of MCS during VT ablation in high-risk patients, identified using the PAINESD score, is associated with a reduction in mortality [130, 131].
Despite mixed data regarding the use of generalized prophylactic use of MCS devices during VT ablation, we believe that selective prophylactic use of MCS during VT ablation for patients with a high risk of acute hemodynamic decompensation is critical for patients’ safety and procedural success. Larger randomized controlled clinical trials are needed for more accurate and precise identification of patients who would benefit from MCS during VT ablation.
6 Innovations in imaging
6.1 MRI
LGE-MRI has been shown to correlate well with the location and character of myocardial scarring [132]. Augmentation of electro-anatomical mapping (EAM) for the identification of arrhythmogenic foci with LGE-MRI has been an ongoing area of advancement, with well-established pre-clinical and preliminary evidence of feasibility (Fig. 6) [133, 134]. In small studies, MRI-guided ablation was shown to reduce VT recurrence [135] and radiofrequency applications [136]. Moreover, T1-weighted imaging have been studied for use in assessing necrosis at ablation sites [137, 138]. Lesion dimensions on T1-weighted imaging has been shown to correlate strongly with necrosis in pathology [139]. As inadequate ablation lesion formation is believed to be responsible for post-ablation VT recurrence, the acquisition of inversion-weighted T1 MRI during ablation may improve the durability of VT ablation.
(A) An example of ventricular tachycardia (VT) ablation performed using endocardial electroanatomical mapping without magnetic resonance imaging (MRI). (B) An example of VT ablation performed with electroanatomical mapping with MRI-derived scar integration. The scar detected on late-gadolinium enhancement MRI corresponded to areas of low-voltage, fractionated, and isolated potentials found on the electroanatomical mapping. Ao, aorta; PA, pulmonary artery (reproduced from Cuculich et al. [135] with permission)
6.2 Real-time MRI
Real-time MRI during ablation offers the benefit of increasing target precision as compared to traditional methods which are limited by errors in mapping registration [137]. Registration errors up to 3.5 mm are introduced by the integration of previously obtained cardiac imaging (CT or MRI) with EAM [140]. Real-time MR-guided procedures are hypothesized to be able to reduce targeting errors such that arrhythmogenic foci of 1–2 mm can be accurately targeted [141]. In porcine models, voltage mapping guided by real-time MRI was 57% sensitive for a scar on LGE-MRI and 96% specific [142].
7 ECGi
Electrocardiographic imaging (ECGi) is the use of a dense array of body-surface electrocardiograms to non-invasively reconstruct a digital 3D model of a patient’s heart [143]. A 252-electrode vest is used to collect the electrographic potentials which are then integrated with anatomical data from a high-resolution non-contrast cardiac CT [144]. Activation maps, voltage maps, isopotential maps, and phase maps can be created through post-processing algorithms (Fig. 7A) [144]. ECGi can accurately identify the origin of PVCs [145]. Simultaneous EAM and ECGi showed moderate concordance in activation-repolarization maps. The average spatial resolution of ECGi for sites of focal activation was 13.2 mm, which may not be sufficient for the guidance of VT ablation [146]. ECGi could be utilized to identify areas of low-voltage fractionated EGMs during sinus rhythm, which can aid in identifying the VT critical sites [147, 148]. However, current evidence is very limited and ECGi clinical validation in large patient cohorts has been a challenge [143].
(A) Electrocardiographic imaging (ECGi): Body surface electrical potentials from 250 electrodes are integrated with geometric data from computed tomography via ECGi software algorithms (reproduced from Rudy et al. [154] with permission). (B) View into ventricular onset (VIVO; Catheter Precision, Mt. Olive, NJ): Cardiac CT or MRI is integrated with a 3D image of the patient torso with ECG electrodes applied to register the position of the 12-lead ECG and produce an activation map (reproduced from Misra et al. [149] with permission). (C) Virtual heart arrhythmia ablation targeting (VAAT): Algorithmic reconstruction of the myocardium via MRI (a) allows prediction of ablation targets (c) (reproduced from Prakosa et al., [152] with permission). (D) InHEART: Preprocedural CT images are processed such that endocardium is segments, myocardial wall thinning is identified, a channelness filter is applied, and the Eikonal simulation predicts ablation targets (reproduced from Cedilnik et al. [153] with permission)
An example of ECGi is VIVO “View into Ventricular Onset” (Catheter Precision, Mt. Olive, NJ), which integrates standard 12-lead ECG, a 3D image of ECG lead placement, and myocardial model from pre-procedural imaging (CT/MRI) to localize ventricular arrhythmias (Fig. 7B) [149], which allows QRS vectors to be accurately calculated and localized by the anatomical region of the heart [150]. In a prospective observational study of VT ablation with standard EAM, VIVO ECGi predicted VT focus with 88% accuracy [149]. Correlation between ECGi electrograms and invasive epicardial recordings is overall well but exhibits significant variability [151]. However, even in cases with suboptimal correlation, improved electrogram correlation is found within 20 mm in the majority cases [151]. Thus, ECGi can potentially decrease mapping time during VT ablation by reducing the mapping area to a 2-cm radius area [151]. In addition, ECGi can be used to plan the strategy of VT ablation by distinguishing epicardial or endocardial VT origin based on EGM morphology [151].
8 Computational models
8.1 Virtual heart
Virtual heart arrhythmia ablation targeting (VAAT) involves an algorithmic reconstruction model of an individual patient’s myocardium from LGE-MRI data to identify optimal ablation targets for inducible reentrant VT pathways by simulation (Fig. 7C). As VAAT targeting predicts sites of arrhythmia due to underlying substrate, not solely the currently active culprit, it may decrease VT recurrence and reduce the need for repeat procedures [152].
VAAT-guided ablation matched EAM-based targets with smaller lesion sizes in both porcine models and retrospective human studies. In a five-patient prospective study, VAAT-guided ablation achieved acute lesion success, demonstrating clinical feasibility [152]. VAAT has been developed for scar-related VT and its utility outside of this setting is uncertain [152].
8.2 InHEART
A novel framework for the integration of computational modeling with pre-procedural CT has been advanced. A computational model applying an Eikonal model of wavefront propagation to thickness maps of the myocardial wall can create an individualized 3D simulated model of myocardial electrophysiology. Application of an image-based algorithm to the resultant model allows automatic prediction of VT isthmuses (Fig. 7D). Concordance with invasive EAM was seen in only 5 of 9 human subjects undergoing VT ablation. Such a model can be rapidly applied pre-procedurally to aid intervention planning and identify targets; however, data on clinical outcomes are lacking [153].
9 Conclusion
Approaches to VT ablations have developed rapidly to enhance its success, safety, and accuracy. Innovation in VT mapping techniques has lessened the requirement for mapping of poorly hemodynamically tolerated VTs to achieve satisfactory ablation efficacy. We believe that ILAM provides not only a novel approach to functional VT ablation during sinus rhythm but also a better understanding of VT by identifying area of slow conduction. In addition, we believe that epicardial access techniques as well as ICE are essential skills in the electrophysiologist’s toolbox when performing VT ablation. Mastering epicardial access allows for more efficient and effective ablation by targeting epicardial arrhythmogenic substrate as well as intramyocardial arrhythmogenic substrate via simultaneous unipolar RFA and bipolar RFA. New energy sources, such as electroporation and stereotactic radiation therapy, remain in experimental phases but are promising if current limitations can be overcome. New ablation catheters, such as the lattice-tip ablation catheter, are promising modalities that will potentially improve lesion creation and limit tissue overheating. Moreover, the introduction of various imaging modalities to assess arrhythmogenic substrate identification and ablation success evaluation will potentially enhance the overall success, safety, and accuracy of VT ablation. Despite existing small feasibility studies on electrocardiographic imaging and computational models, they are still not validated in large studies.
Abbreviations
- DEEP:
-
Decrement evoked potential
- ECMO:
-
Extracorporeal membrane oxygenation
- EGM:
-
Electrogram
- ICD:
-
Implantable Cardioverter Defibrillator
- IABP:
-
Intra-aortic balloon pump
- IES:
-
Intramural Excitable Substrate
- ILAM:
-
Isochronal late activation mapping
- LGE-MRI:
-
Late gadolinium enhancement on late gadolinium enhancement MRI
- MCS:
-
Mechanical circulatory support
- MEM:
-
Multielectrode mapping
- PFA:
-
Pulse field ablation
- PPM:
-
Point-by-point mapping
- PVAD:
-
Percutaneous ventricular assist device
- SAFE-T:
-
Simultaneous amplitude frequency electrogram transformation
- STAR:
-
Stereotactic arrhythmia radioablation
- UHD:
-
Ultra-high-density
- VA:
-
Ventricular arrhythmia
- VT:
-
Ventricular tachycardia
- VF:
-
Ventricular fibrillation
References
Cronin EM, Bogun FM, Maury P, Peichl P, Chen M, Namboodiri N, et al. HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias. Heart Rhythm. 2019;17(1):e2–e154. https://doi.org/10.1016/j.hrthm.2019.03.002.
Willems S, Tilz RR, Steven D, Kääb S, Wegscheider K, Gellér L, et al. Preventive or Deferred Ablation of Ventricular Tachycardia in Patients with Ischemic Cardiomyopathy and Implantable Defibrillator (BERLIN VT): a multicenter randomized trial. Circulation. 2020;141(13):1057–67. https://doi.org/10.1161/CIRCULATIONAHA.119.043400.
Reddy VY, Reynolds MR, Neuzil P, Richardson AW, Taborsky M, Jongnarangsin K, et al. Prophylactic catheter ablation for the prevention of defibrillator therapy. N Engl J Med. 2007;357(26):2657–65. https://doi.org/10.1056/NEJMoa065457.
Ravi V, Poudyal A, Khanal S, Khalil C, Vij A, Sanders D, et al. A systematic review and meta-analysis comparing radiofrequency catheter ablation with medical therapy for ventricular tachycardia in patients with ischemic and non-ischemic cardiomyopathies. J Interv Cardiac Electrophysiol: An Int J Arrhythmias Pac. 2022. https://doi.org/10.1007/s10840-022-01287-w.
Sapp JL, Wells GA, Parkash R, Stevenson WG, Blier L, Sarrazin JF, et al. Ventricular tachycardia ablation versus escalation of antiarrhythmic drugs. N Engl J Med. 2016;375(2):111–21. https://doi.org/10.1056/NEJMoa1513614.
Kumar S, Romero J, Mehta NK, Fujii A, Kapur S, Baldinger SH, et al. Long-term outcomes after catheter ablation of ventricular tachycardia in patients with and without structural heart disease. Heart Rhythm. 13(10):1957–63. https://doi.org/10.1016/j.hrthm.2016.07.001.
Stinnett-Donnelly JM, Thompson N, Habel N, Petrov-Kondratov V, Correa de Sa DD, Bates JH, Spector PS. Effects of electrode size and spacing on the resolution of intracardiac electrograms. Coron Artery Dis. 2012;23(2):126–32. https://doi.org/10.1097/MCA.0b013e3283507a9b.
Tschabrunn CM, Roujol S, Dorman NC, Nezafat R, Josephson ME, Anter E. High-resolution mapping of ventricular scar: comparison between single and multielectrode catheters. Circ Arrhythm Electrophysiol. 9(6):e003841. https://doi.org/10.1161/CIRCEP.115.003841.
Acosta J, Penela D, Andreu D, Cabrera M, Carlosena A, Vassanelli F, et al. Multielectrode vs. point-by-point mapping for ventricular tachycardia substrate ablation: a randomized study. Europace. 20(3):512–9. https://doi.org/10.1093/europace/euw406.
Viswanathan K, Mantziari L, Butcher C, Hodkinson E, Lim E, Khan H, et al. Evaluation of a novel high-resolution mapping system for catheter ablation of ventricular arrhythmias. Heart Rhythm. 14(2):176–83. https://doi.org/10.1016/j.hrthm.2016.11.018.
Okubo K, Frontera A, Bisceglia C, Paglino G, Radinovic A, Foppoli L, et al. Grid mapping catheter for ventricular tachycardia ablation. Circ Arrhythm Electrophysiol. 12(9):e007500. https://doi.org/10.1161/CIRCEP.119.007500.
Linton NW, Koa-Wing M, Francis DP, Kojodjojo P, Lim PB, Salukhe TV, et al. Cardiac ripple mapping: a novel three-dimensional visualization method for use with electroanatomic mapping of cardiac arrhythmias. Heart Rhythm. 2009;6(12):1754–62. https://doi.org/10.1016/j.hrthm.2009.08.038.
Jamil-Copley S, Vergara P, Carbucicchio C, Linton N, Koa-Wing M, Luther V, et al. Application of ripple mapping to visualize slow conduction channels within the infarct-related left ventricular scar. Circ Arrhythm Electrophysiol. 2015;8(1):76–86. https://doi.org/10.1161/CIRCEP.114.001827.
Luther V, Linton NW, Jamil-Copley S, Koa-Wing M, Lim PB, Qureshi N, et al. A prospective study of ripple mapping the post-infarct ventricular scar to guide substrate ablation for ventricular tachycardia. Circ Arrhythm Electrophysiol. 9(6):e004072. https://doi.org/10.1161/CIRCEP.116.004072.
Takamiya T, Inaba O, Nitta J, Goya M. Identification of an epicardial slow conduction channel using ripple mapping in ablation of postinfarct ventricular tachycardia. J Cardiovasc Electrophysiol. 2019;30(7):1156–8. https://doi.org/10.1111/jce.13955.
Xie S, Kubala M, Liang JJ, Yang J, Desjardins B, Santangeli P, et al. Utility of ripple mapping for identification of slow conduction channels during ventricular tachycardia ablation in the setting of arrhythmogenic right ventricular cardiomyopathy. J Cardiovasc Electrophysiol. 30(3):366–73. https://doi.org/10.1111/jce.13819.
Irie T, Yu R, Bradfield JS, Vaseghi M, Buch EF, Ajijola O, et al. Relationship between sinus rhythm late activation zones and critical sites for scar-related ventricular tachycardia: systematic analysis of isochronal late activation mapping. Circ Arrhythm Electrophysiol. 2015;8(2):390–9. https://doi.org/10.1161/CIRCEP.114.002637.
Marchlinski FE, Callans DJ, Gottlieb CD, Zado E. Linear ablation lesions for control of unmappable ventricular tachycardia in patients with ischemic and nonischemic cardiomyopathy. Circulation. 2000;101(11):1288–96. https://doi.org/10.1161/01.cir.101.11.1288.
Soejima K, Suzuki M, Maisel WH, Brunckhorst CB, Delacretaz E, Blier L, et al. Catheter ablation in patients with multiple and unstable ventricular tachycardias after myocardial infarction: short ablation lines guided by reentry circuit isthmuses and sinus rhythm mapping. Circulation. 2001;104(6):664–9. https://doi.org/10.1161/hc3101.093764.
Bogun F, Good E, Reich S, Elmouchi D, Igic P, Lemola K, et al. Isolated potentials during sinus rhythm and pace-mapping within scars as guides for ablation of post-infarction ventricular tachycardia. J Am Coll Cardiol. 2006;47(10):2013–9. https://doi.org/10.1016/j.jacc.2005.12.062.
Arenal A, Glez-Torrecilla E, Ortiz M, Villacastín J, Fdez-Portales J, Sousa E, et al. Ablation of electrograms with an isolated, delayed component as treatment of unmappable monomorphic ventricular tachycardias in patients with structural heart disease. J Am Coll Cardiol. 2003;41(1):81–92. https://doi.org/10.1016/s0735-1097(02)02623-2.
Aziz Z, Shatz D, Raiman M, Upadhyay GA, Beaser AD, Besser SA, et al. Targeted ablation of ventricular tachycardia guided by wavefront discontinuities during sinus rhythm: a new functional substrate mapping strategy. Circulation. 140(17):1383–97. https://doi.org/10.1161/CIRCULATIONAHA.119.042423.
Anter E, Kleber AG, Rottmann M, Leshem E, Barkagan M, Tschabrunn CM, et al. Infarct-related ventricular tachycardia. JACC: Clin Electrophysiol. 2018;4(8):1033–48. https://doi.org/10.1016/j.jacep.2018.04.007.
Jackson N, Gizurarson S, Viswanathan K, King B, Massé S, Kusha M, et al. Decrement evoked potential mapping: basis of a mechanistic strategy for ventricular tachycardia ablation. Circ Arrhythm Electrophysiol. 2015;8(6):1433–42. https://doi.org/10.1161/CIRCEP.115.003083.
Porta-Sánchez A, Jackson N, Lukac P, Kristiansen SB, Nielsen JM, Gizurarson S, et al. Multicenter study of ischemic ventricular tachycardia ablation with decrement-evoked potential (DEEP) mapping with extra stimulus. JACC Clin Electrophysiol. 4(3):307–15. https://doi.org/10.1016/j.jacep.2017.12.005.
Tung R, Josephson ME, Bradfield JS, Shivkumar K. Directional influences of ventricular activation on myocardial scar characterization: voltage mapping with multiple wavefronts during ventricular tachycardia ablation. Circ Arrhythm Electrophysiol. 9(8):e004155. https://doi.org/10.1161/CIRCEP.116.004155.
Josephson ME, Anter E. Substrate mapping for ventricular tachycardia: assumptions and misconceptions. JACC Clin Electrophysiol. 2015;1(5):341–52. https://doi.org/10.1016/j.jacep.2015.09.001.
Deno DC, Balachandran R, Morgan D, Ahmad F, Masse S, Nanthakumar K. Orientation-independent catheter-based characterization of myocardial activation. IEEE Trans Biomed Eng. 64(5):1067–77. https://doi.org/10.1109/TBME.2016.2589158.
van Schie MS, Kharbanda RK, Houck CA, Lanters EAH, Taverne YJHJ, Bogers AJJC, de Groot NMS. Identification of low-voltage areas: a unipolar, bipolar, and omnipolar perspective. Circ Arrhythm Electrophysiol. 2021;14(7):e009912. https://doi.org/10.1161/CIRCEP.121.009912.
Massé S, Magtibay K, Jackson N, Asta J, Kusha M, Zhang B, et al. Resolving myocardial activation with novel omnipolar electrograms. Circ Arrhythm Electrophysiol. 9(7):e004107. https://doi.org/10.1161/CIRCEP.116.004107.
Proietti R, Adlan AM, Dowd R, Assadullah S, Aldhoon B, Panikker S, et al. Enhanced ventricular tachycardia substrate resolution with a novel omnipolar high-density mapping catheter: the omnimapping study. J Interv Card Electrophysiol. 2020;58(3):355–62. https://doi.org/10.1007/s10840-019-00625-9.
Porta-Sánchez A, Magtibay K, Nayyar S, Bhaskaran A, Lai PFH, Massé S, et al. Omnipolarity applied to equi-spaced electrode array for ventricular tachycardia substrate mapping. Europace: European Pacing, Arrhythmias, and Cardiac Electrophysiology: Journal of the Working Groups on Cardiac Pacing, Arrhythmias, and Cardiac Cellular Electrophysiology of the European Society of. Cardiology. 2019;21(5):813–21. https://doi.org/10.1093/europace/euy304.
de Groot NMS, Shah D, Boyle PM, Anter E, Clifford GD, Deisenhofer I, et al. Critical appraisal of technologies to assess electrical activity during atrial fibrillation: a position paper from the European Heart Rhythm Association and European Society of Cardiology Working Group on eCardiology in collaboration with the Heart Rhythm Society, Asia Pacific Heart Rhythm Society, Latin American Heart Rhythm Society and Computing in Cardiology. Europace: European Pacing, Arrhythmias, and Cardiac Electrophysiology: Journal of the Working Groups on Cardiac Pacing, Arrhythmias, and Cardiac Cellular Electrophysiology of the European Society of Cardiology. 2022;24(2):313–30. https://doi.org/10.1093/europace/euab254.
Te AL, Higa S, Chung FP, Lin CY, Lo MT, Liu CA, et al. The use of a novel signal analysis to identify the origin of idiopathic right ventricular outflow tract ventricular tachycardia during sinus rhythm: simultaneous amplitude frequency electrogram transformation mapping. PLoS One. 2017;12(3):e0173189. https://doi.org/10.1371/journal.pone.0173189.
Lin CY, Silberbauer J, Lin YJ, Lo MT, Lin C, Chang HC, et al. Simultaneous amplitude frequency electrogram transformation (SAFE-T) mapping to identify ventricular tachycardia arrhythmogenic potentials in sinus rhythm. JACC Clin Electrophysiol. 2016;2(4):459–70. https://doi.org/10.1016/j.jacep.2016.01.013.
Jaïs P, Maury P, Khairy P, Sacher F, Nault I, Komatsu Y, et al. Elimination of local abnormal ventricular activities: a new end point for substrate modification in patients with scar-related ventricular tachycardia. Circulation. 2012;125(18):2184–96. https://doi.org/10.1161/CIRCULATIONAHA.111.043216.
Vergara P, Trevisi N, Ricco A, Petracca F, Baratto F, Cireddu M, et al. Late potentials abolition as an additional technique for reduction of arrhythmia recurrence in scar related ventricular tachycardia ablation. J Cardiovasc Electrophysiol. 2012;23(6):621–7. https://doi.org/10.1111/j.1540-8167.2011.02246.x.
Silberbauer J, Oloriz T, Maccabelli G, Tsiachris D, Baratto F, Vergara P, et al. Noninducibility and late potential abolition: a novel combined prognostic procedural end point for catheter ablation of postinfarction ventricular tachycardia. Circ Arrhythm Electrophysiol. 2014;7(3):424–35. https://doi.org/10.1161/CIRCEP.113.001239.
Stevenson WG, Wilber DJ, Natale A, Jackman WM, Marchlinski FE, Talbert T, et al. Irrigated radiofrequency catheter ablation guided by electroanatomic mapping for recurrent ventricular tachycardia after myocardial infarction. Circulation. 2008;118(25):2773–82. https://doi.org/10.1161/CIRCULATIONAHA.108.788604.
Soucek F, Caluori G, Lehar F, Jez J, Pesl M, Wolf J, et al. Bipolar ablation with contact force-sensing of swine ventricles shows improved acute lesion features compared to sequential unipolar ablation. J Cardiovasc Electrophysiol. 2020;31(5):1128–36. https://doi.org/10.1111/jce.14407.
Nagashima K, Watanabe I, Okumura Y, Ohkubo K, Kofune M, Ohya T, et al. Lesion formation by ventricular septal ablation with irrigated electrodes. Circ J. 2011;75(3):565–70. https://doi.org/10.1253/circj.CJ-10-0870.
Sivagangabalan G, Barry MA, Huang K, Lu J, Pouliopoulos J, Thomas SP, et al. Bipolar ablation of the interventricular septum is more efficient at creating a transmural line than sequential unipolar ablation. Pacing Clin Electrophysiol: PACE. 2010;33(1):16–26. https://doi.org/10.1111/j.1540-8159.2009.02602.x.
Teh AW, Reddy VY, Koruth JS, Miller MA, Choudry S, D’Avila A, Dukkipati SR. Bipolar radiofrequency catheter ablation for refractory ventricular outflow tract arrhythmias. J Cardiovasc Electrophysiol. 2014;25(10):1093–9. https://doi.org/10.1111/jce.12460.
Koruth JS, Dukkipati S, Miller MA, Neuzil P, d’Avila A, Reddy VY. Bipolar irrigated radiofrequency ablation: a therapeutic option for refractory intramural atrial and ventricular tachycardia circuits. Heart Rhythm. 2012;9(12):1932–41. https://doi.org/10.1016/j.hrthm.2012.08.001.
Futyma P, Ciąpała K, Sander J, Głuszczyk R, Futyma M, Kułakowski P. Bipolar radiofrequency ablation of ventricular arrhythmias originating in the vicinity of his bundle. Circ Arrhythm Electrophysiol. 2020;13(3):e008165. https://doi.org/10.1161/CIRCEP.119.008165.
Zhou B, Yu J, Ju W, Li X, Zhang F, Chen H, et al. Bipolar catheter ablation strategies for outflow tract ventricular arrhythmias refractory to unipolar ablation. J Cardiovasc Electrophysiol. 2022. https://doi.org/10.1111/jce.15579.
Yamada T, Maddox WR, McElderry HT, Doppalapudi H, Plumb VJ, Kay GN. Radiofrequency catheter ablation of idiopathic ventricular arrhythmias originating from intramural foci in the left ventricular outflow tract: efficacy of sequential versus simultaneous unipolar catheter ablation. Circ Arrhythm Electrophysiol. 2015;8(2):344–52. https://doi.org/10.1161/CIRCEP.114.002259.
Yang J, Liang J, Shirai Y, Muser D, Garcia FC, Callans DJ, et al. Outcomes of simultaneous unipolar radiofrequency catheter ablation for intramural septal ventricular tachycardia in nonischemic cardiomyopathy. Heart Rhythm. 2019;16(6):863–70. https://doi.org/10.1016/j.hrthm.2018.12.018.
Yokoyama K, Nakagawa H, Shah DC, Lambert H, Leo G, Aeby N, et al. Novel contact force sensor incorporated in irrigated radiofrequency ablation catheter predicts lesion size and incidence of steam pop and thrombus. Circ Arrhythm Electrophysiol. 2008;1(5):354–62. https://doi.org/10.1161/CIRCEP.108.803650.
Philips B, Madhavan S, James C, Tichnell C, Murray B, Dalal D, et al. Outcomes of catheter ablation of ventricular tachycardia in arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circ Arrhythm Electrophysiol. 2012;5(3):499–505. https://doi.org/10.1161/CIRCEP.111.968677.
Bai R, Di Biase L, Shivkumar K, Mohanty P, Tung R, Santangeli P, et al. Ablation of ventricular arrhythmias in arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circ Arrhythm Electrophysiol. 2011;4(4):478–85. https://doi.org/10.1161/CIRCEP.111.963066.
Romero J, Cerrud-Rodriguez RC, Di Biase L, Diaz JC, Alviz I, Grupposo V, et al. Combined endocardial-epicardial versus endocardial catheter ablation alone for ventricular tachycardia in structural heart disease: a systematic review and meta-analysis. JACC Clin Electrophysiol. 2019;5(1):13–24. https://doi.org/10.1016/j.jacep.2018.08.010.
Della Bella P, Brugada J, Zeppenfeld K, Merino J, Neuzil P, Maury P, et al. Epicardial ablation for ventricular tachycardia. Circ Arrhythm Electrophysiol. 2011;4(5):653–9. https://doi.org/10.1161/CIRCEP.111.962217.
Mohanty S, Trivedi C, Di Biase L, Burkhardt JD, Della Rocca DG, Gianni C, et al. Endocardial scar-homogenization with vs without epicardial ablation in vt patients with ischemic cardiomyopathy. JACC Clin Electrophysiol. 2022;S2405-500X(21):01095–1. https://doi.org/10.1016/j.jacep.2021.12.011.
Matos D, Adragão P, Pisani C, Hatanaka V, Freitas P, Costa F, et al. Outcomes of a combined vs non-combined endo-epicardial ventricular tachycardia ablation strategy. J Interv Card Electrophysiol. 2022. https://doi.org/10.1007/s10840-022-01175-3.
Sosa E, Scanavacca M, d’Avila A, Pilleggi F. A new technique to perform epicardial mapping in the electrophysiology laboratory. J Cardiovasc Electrophysiol. 1996;7(6):531–6. https://doi.org/10.1111/j.1540-8167.1996.tb00559.x.
Kumar S, Bazaz R, Barbhaiya CR, Enriquez AD, Helmbold AF, Chinitz JS, et al. “Needle-in-needle” epicardial access: preliminary observations with a modified technique for facilitating epicardial interventional procedures. Heart Rhythm. 2015;12(7):1691–7. https://doi.org/10.1016/j.hrthm.2015.03.045.
Gunda S, Reddy M, Pillarisetti J, Atoui M, Badhwar N, Swarup V, et al. Differences in complication rates between large bore needle and a long micropuncture needle during epicardial access: time to change clinical practice? Circ Arrhythm Electrophysiol. 2015;8(4):890–5. https://doi.org/10.1161/CIRCEP.115.002921.
Di Biase L, Burkhardt JD, Reddy V, Romero J, Neuzil P, Petru J, et al. Initial international multicenter human experience with a novel epicardial access needle embedded with a real-time pressure/frequency monitoring to facilitate epicardial access: feasibility and safety. Heart Rhythm. 2017;14(7):981–8. https://doi.org/10.1016/j.hrthm.2017.02.033.
Greenbaum AB, Rogers T, Paone G, Flynn SE, Guerrero ME, O’Neill WW, Lederman RJ. Intentional right atrial exit and carbon dioxide insufflation to facilitate subxiphoid needle entry into the empty pericardial space: first human experience. JACC Clin Electrophysiol. 2015;1(5):434–41. https://doi.org/10.1016/j.jacep.2015.06.007.
Silberbauer J, Gomes J, O’Nunain S, Kirubakaran S, Hildick-Smith D, McCready J. Coronary vein exit and carbon dioxide insufflation to facilitate subxiphoid epicardial access for ventricular mapping and ablation: first experience. JACC Clin Electrophysiol. 2017;3(5):514–21. https://doi.org/10.1016/j.jacep.2016.11.002.
Ebrille E, Killu AM, Anavekar NS, Packer DL, Munger TM, McLeod CJ, et al. Successful percutaneous epicardial access in challenging scenarios. Pacing Clin Electrophysiol: PACE. 2015;38(1):84–90. https://doi.org/10.1111/pace.12503.
Halabi M, Faranesh AZ, Schenke WH, Wright VJ, Hansen MS, Saikus CE, et al. Real-time cardiovascular magnetic resonance subxiphoid pericardial access and pericardiocentesis using off-the-shelf devices in swine. J Cardiovasc Magn Reson. 2013;15(1):61. https://doi.org/10.1186/1532-429X-15-61.
Koruth JS, Iwasawa J, Enomoto Y, Bar-Tal M, Ultchin Y, Sigal A, et al. Chamber-specific radiofrequency lesion dimension estimation using novel catheter-based tissue interface temperature sensing: a preclinical assessment. JACC Clin Electrophysiol. 3(10):1092–102. https://doi.org/10.1016/j.jacep.2017.08.008.
Stevenson WG, Tedrow UB, Reddy V, AbdelWahab A, Dukkipati S, John RM, et al. Infusion needle radiofrequency ablation for treatment of refractory ventricular arrhythmias. J Am Coll Cardiol. 73(12):1413–25. https://doi.org/10.1016/j.jacc.2018.12.070.
Sapp JL, Beeckler C, Pike R, Parkash R, Gray CJ, Zeppenfeld K, et al. Initial human feasibility of infusion needle catheter ablation for refractory ventricular tachycardia. Circulation. 2013;128(21):2289–95. https://doi.org/10.1161/CIRCULATIONAHA.113.003423.
Qian PC, Oberfeld B, Schaeffer B, Nakamura T, John RM, Sapp JL, et al. Frequency content of unipolar electrograms may predict deep intramural excitable substrate: insights from intramural needle catheter ablation of ventricular tachycardia. JACC Clin Electrophysiol. 2020;6(7):760–9. https://doi.org/10.1016/j.jacep.2020.03.003.
Liang JJ, Bogun F. Coronary venous mapping and catheter ablation for ventricular arrhythmias. Methodt DeBakey Cardiovasc J. 2021;17(1):13–8. https://doi.org/10.14797/HUZR1007.
Briceño DF, Enriquez A, Liang JJ, Shirai Y, Santangeli P, Guandalini G, et al. Septal coronary venous mapping to guide substrate characterization and ablation of intramural septal ventricular arrhythmia. JACC: Clin Electrophysiol. 2019;5(7):789–800. https://doi.org/10.1016/j.jacep.2019.04.011.
Haqqani HM, Tschabrunn CM, Tzou WS, Dixit S, Cooper JM, Riley MP, et al. Isolated septal substrate for ventricular tachycardia in nonischemic dilated cardiomyopathy: incidence, characterization, and implications. Heart Rhythm. 2011;8(8):1169–76. https://doi.org/10.1016/j.hrthm.2011.03.008.
Yokokawa M, Good E, Chugh A, Pelosi F, Crawford T, Jongnarangsin K, et al. Intramural idiopathic ventricular arrhythmias originating in the intraventricular septum: mapping and ablation. Circ Arrhythm Electrophysiol. 2012;5(2):258–63. https://doi.org/10.1161/CIRCEP.111.967257.
Karimianpour A, Badertscher P, Payne J, Field M, Gold MR, Winterfield JR. Epicardial mapping and ablation of ventricular tachycardia from the coronary venous system in post-coronary bypass patients. J Interv Card Electrophysiol. 2022. https://doi.org/10.1007/s10840-022-01250-9.
Romero J, Diaz JC, Hayase J, Dave RH, Bradfield JS, Shivkumar K. Intramyocardial radiofrequency ablation of ventricular arrhythmias using intracoronary wire mapping and a coronary reentry system: description of a novel technique. Heart Rhythm Case Rep. 2018;4(7):285–92. https://doi.org/10.1016/j.hrcr.2018.03.005.
Barkagan M, Rottmann M, Leshem E, Shen C, Buxton AE, Anter E. Effect of baseline impedance on ablation lesion dimensions. Circ Arrhythm Electrophysiol. 2018;11(10):e006690. https://doi.org/10.1161/CIRCEP.118.006690
Shapira-Daniels A, Barkagan M, Rottmann M, Sroubek J, Tugal D, Carlozzi MA, et al. Modulating the baseline impedance: an adjunctive technique for maximizing radio frequency lesion dimensions in deep and intramural ventricular substrate. Circ Arrhythm Electrophysiol. 2019;12(6):e007336. https://doi.org/10.1161/CIRCEP.119.007336
Nguyen DT, Gerstenfeld EP, Tzou WS, Jurgens PT, Zheng L, Schuller J, et al. Radiofrequency ablation using an open irrigated electrode cooled with half-normal saline. JACC: Clin Electrophysiol. 2017;3(10):1103–10. https://doi.org/10.1016/j.jacep.2017.03.006.
Nguyen DT, Olson M, Zheng L, Barham W, Moss JD, Sauer WH. Effect of irrigant characteristics on lesion formation after radiofrequency energy delivery using ablation catheters with actively cooled tips. J Cardiovasc Electrophysiol. 2015;26(7):792–8. https://doi.org/10.1111/jce.12682.
Bennett R, Campbell T, Byth K, Turnbull S, Kumar S. Catheter ablation using half-normal saline and dextrose irrigation in an ovine ventricular model. JACC: Clin Electrophysiol. 2021;7(10):1229–39. https://doi.org/10.1016/j.jacep.2021.05.002.
Nguyen DT, Tzou WS, Sandhu A, Gianni C, Anter E, Tung R, et al. Prospective multicenter experience with cooled radiofrequency ablation using high impedance irrigant to target deep myocardial substrate refractory to standard ablation. JACC Clin Electrophysiol. 2018;4(9):1176–85. https://doi.org/10.1016/j.jacep.2018.06.021.
Elsokkari I, Sapp JL, Doucette S, Parkash R, Gray CJ, Gardner MJ, et al. Role of contact force in ischemic scar-related ventricular tachycardia ablation; optimal force required and impact of left ventricular access route. J Interv Card Electrophysiol. 2018;53(3):323–31. https://doi.org/10.1007/s10840-018-0396-1.
Hendriks AA, Akca F, Dabiri Abkenari L, Khan M, Bhagwandien R, Yap S-C, et al. Safety and clinical outcome of catheter ablation of ventricular arrhythmias using contact force sensing: consecutive case series. J Cardiovasc Electrophysiol. 2015;26(11):1224–9. https://doi.org/10.1111/jce.12762.
Proietti R, Rivera S, Dussault C, Essebag V, Bernier ML, Ayala-Paredes F, et al. Intracardiac echo-facilitated 3D electroanatomical mapping of ventricular arrhythmias from the papillary muscles: assessing the ‘fourth dimension’ during ablation. Europace. 2016;euw099. https://doi.org/10.1093/europace/euw099.
Campbell T, Bennett RG, Kumar S. Intracardiac echocardiography to guide the ablation of parahisian arrhythmias. Cardiac Electrophysiol Clin. 2021;13(2S):e1–e16. https://doi.org/10.1016/j.ccep.2022.01.001.
Field ME, Gold MR, Reynolds MR, Goldstein L, Lee SHY, Kalsekar I, et al. Real-world outcomes of ventricular tachycardia catheter ablation with versus without intracardiac echocardiography. J Cardiovasc Electrophysiol. 2020;31(2):417–22. https://doi.org/10.1111/jce.14324.
Wittkampf FHM, van Es R, Neven K. Electroporation and its relevance for cardiac catheter ablation. JACC Clin Electrophysiol. 4(8):977–86. https://doi.org/10.1016/j.jacep.2018.06.005.
Weaver JC. Electroporation theory. Concepts and mechanisms. Methods Mol Biol. 1995;47:1–26. https://doi.org/10.1385/0-89603-310-4:1.
Reddy VY, Koruth J, Jais P, Petru J, Timko F, Skalsky I, et al. Ablation of atrial fibrillation with pulsed electric fields: an ultra-rapid, tissue-selective modality for cardiac ablation. JACC Clin Electrophysiol. 2018;4(8):987–95. https://doi.org/10.1016/j.jacep.2018.04.005.
Reddy VY, Neuzil P, Koruth JS, Petru J, Funosako M, Cochet H, et al. Pulsed field ablation for pulmonary vein isolation in atrial fibrillation. J Am Coll Cardiol. 2019;74(3):315–26. https://doi.org/10.1016/j.jacc.2019.04.021.
Livia C, Sugrue A, Witt T, Polkinghorne MD, Maor E, Kapa S, et al. Elimination of Purkinje fibers by electroporation reduces ventricular fibrillation vulnerability. J Am Heart Assoc. 7(15):e009070. https://doi.org/10.1161/JAHA.118.009070.
Koruth JS, Kuroki K, Iwasawa J, Viswanathan R, Brose R, Buck ED, et al. Endocardial ventricular pulsed field ablation: a proof-of-concept preclinical evaluation. Europace. 22(3):434–9. https://doi.org/10.1093/europace/euz341.
Im SI, Higuchi S, Lee A, Stillson C, Buck E, Morrow B, et al. Pulsed field ablation of left ventricular myocardium in a swine infarct model. JACC Clin Electrophysiol. 2022;8(6):722–31. https://doi.org/10.1016/j.jacep.2022.03.007.
Tan NY, Ladas TP, Christopoulos G, Sugrue AM, van Zyl M, Ladejobi AO, et al. Ventricular nanosecond pulsed electric field delivery using active fixation leads: a proof-of-concept preclinical study. J Interv Cardiac Electrophysiol: An Int J Arrhythmias Pac. 2022. https://doi.org/10.1007/s10840-022-01268-z.
Kreidieh B, Rodríguez-Mañero M, Schurmann P, Ibarra-Cortez SH, Dave AS, Valderrábano M. Retrograde coronary venous ethanol infusion for ablation of refractory ventricular tachycardia. Circ Arrhythm Electrophysiol. 9(7):e004352. https://doi.org/10.1161/CIRCEP.116.004352.
Delacrétaz E, Seiler J, Tanner H, Hess OM. Ablation of ventricular tachycardia: neither inside nor out, thus back to alcohol. Heart Rhythm. 2006;3(10):1230–1. https://doi.org/10.1016/j.hrthm.2006.06.014.
Brugada P, de Swart H, Smeets J, Wellens HJ. Transcoronary chemical ablation of atrioventricular conduction. Circulation. 1990;81(3):757–61. https://doi.org/10.1161/01.cir.81.3.757.
Schurmann P, Peñalver J, Valderrábano M. Ethanol for the treatment of cardiac arrhythmias. Curr Opin Cardiol. 2015;30(4):333–43. https://doi.org/10.1097/HCO.0000000000000183.
Kay GN, Epstein AE, Bubien RS, Anderson PG, Dailey SM, Plumb VJ. Intracoronary ethanol ablation for the treatment of recurrent sustained ventricular tachycardia. J Am Coll Cardiol. 1992;19(1):159–68. https://doi.org/10.1016/0735-1097(92)90068-x.
Tokuda M, Sobieszczyk P, Eisenhauer AC, Kojodjojo P, Inada K, Koplan BA, et al. Transcoronary ethanol ablation for recurrent ventricular tachycardia after failed catheter ablation: an update. Circ Arrhythm Electrophysiol. 2011;4(6):889–96. https://doi.org/10.1161/CIRCEP.111.966283.
Baher A, Shah DJ, Valderrabano M. Coronary venous ethanol infusion for the treatment of refractory ventricular tachycardia. Heart Rhythm. 2012;9(10):1637–9. https://doi.org/10.1016/j.hrthm.2012.06.003.
Creta A, Earley MJ, Schilling RJ, Finlay M, Sporton S, Dhinoja M, et al. Ethanol ablation for ventricular arrhythmias: a systematic review and meta-analysis. J Cardiovasc Electrophysiol. 2022;33(3):510–26. https://doi.org/10.1111/jce.15336.
Loo BW, Soltys SG, Wang L, Lo A, Fahimian BP, Iagaru A, et al. Stereotactic ablative radiotherapy for the treatment of refractory cardiac ventricular arrhythmia. Circ Arrhythm Electrophysiol. 2015;8(3):748–50. https://doi.org/10.1161/CIRCEP.115.002765.
Gianni C, Rivera D, Burkhardt JD, Pollard B, Gardner E, Maguire P, et al. Stereotactic arrhythmia radioablation for refractory scar-related ventricular tachycardia. Heart Rhythm. 2020;17(8):1241–8. https://doi.org/10.1016/j.hrthm.2020.02.036.
Robinson CG, Samson PP, Moore KMS, Hugo GD, Knutson N, Mutic S, et al. Phase I/II trial of electrophysiology-guided noninvasive cardiac radioablation for ventricular tachycardia. Circulation. 139(3):313–21. https://doi.org/10.1161/CIRCULATIONAHA.118.038261.
Cuculich PS, Schill MR, Kashani R, Mutic S, Lang A, Cooper D, et al. Noninvasive cardiac radiation for ablation of ventricular tachycardia. N Engl J Med. 2017;377(24):2325–36. https://doi.org/10.1056/NEJMoa1613773.
Neuwirth R, Cvek J, Knybel L, Jiravsky O, Molenda L, Kodaj M, et al. Stereotactic radiosurgery for ablation of ventricular tachycardia. Europace. 2019;21(7):1088–95. https://doi.org/10.1093/europace/euz133.
Zhang DM, Navara R, Yin T, Szymanski J, Goldsztejn U, Kenkel C, et al. Cardiac radiotherapy induces electrical conduction reprogramming in the absence of transmural fibrosis. Nat Commun. 2021;12(1):5558. https://doi.org/10.1038/s41467-021-25730-0.
Lloyd MS, Wight J, Schneider F, Hoskins M, Attia T, Escott C, et al. Clinical experience of stereotactic body radiation for refractory ventricular tachycardia in advanced heart failure patients. Heart Rhythm. 2020;17(3):415–22. https://doi.org/10.1016/j.hrthm.2019.09.028.
Krug D, Blanck O, Andratschke N, Guckenberger M, Jumeau R, Mehrhof F, et al. Recommendations regarding cardiac stereotactic body radiotherapy for treatment refractory ventricular tachycardia. Heart Rhythm. 2021;18(12):2137–45. https://doi.org/10.1016/j.hrthm.2021.08.004.
Blanck O, Buergy D, Vens M, Eidinger L, Zaman A, Krug D, et al. Radiosurgery for ventricular tachycardia: preclinical and clinical evidence and study design for a German multi-center multi-platform feasibility trial (RAVENTA). Clin Res Cardiol. 2020;109(11):1319–32. https://doi.org/10.1007/s00392-020-01650-9.
Barkagan M, Leshem E, Rottmann M, Sroubek J, Shapira-Daniels A, Anter E. Expandable lattice electrode ablation catheter: a novel radiofrequency platform allowing high current at low density for rapid, titratable, and durable lesions. Circ Arrhythm Electrophysiol. 12(4):e007090. https://doi.org/10.1161/CIRCEP.118.007090.
Anter E, Neužil P, Rackauskas G, Peichl P, Aidietis A, Kautzner J, et al. A lattice-tip temperature-controlled radiofrequency ablation catheter for wide thermal lesions: first-in-human experience with atrial fibrillation. JACC Clin Electrophysiol. 6(5):507–19. https://doi.org/10.1016/j.jacep.2019.12.015.
Shapira-Daniels A, Barkagan M, Yavin H, Sroubek J, Reddy VY, Neuzil P, Anter E. Novel irrigated temperature-controlled lattice ablation catheter for ventricular ablation: a preclinical multimodality biophysical characterization. Circ Arrhythm Electrophysiol. 12(11):e007661. https://doi.org/10.1161/CIRCEP.119.007661.
Kitamura T, Hocini M, Bourier F, Martin R, Takigawa M, Frontera A, et al. Larger and deeper ventricular lesions using a novel expandable spherical monopolar irrigated radiofrequency ablation catheter. J Cardiovasc Electrophysiol. 30(9):1644–51. https://doi.org/10.1111/jce.14089.
John RM, Connell J, Termin P, Houde-Walter H, Eberl G, Stohlman KM, Curley MG. Characterization of warm saline-enhanced radiofrequency ablation lesions in the infarcted porcine ventricular myocardium. J Cardiovasc Electrophysiol. 2014;25(3):309–16. https://doi.org/10.1111/jce.12307.
Sapp JL, Cooper JM, Zei P, Stevenson WG. Large radiofrequency ablation lesions can be created with a retractable infusion-needle catheter. J Cardiovasc Electrophysiol. 2006;17(6):657–61. https://doi.org/10.1111/j.1540-8167.2006.00439.x.
Santangeli P, Muser D, Zado ES, Magnani S, Khetpal S, Hutchinson MD, et al. Acute hemodynamic decompensation during catheter ablation of scar-related ventricular tachycardia: incidence, predictors, and impact on mortality. Circ Arrhythm Electrophysiol. 2015;8(1):68–75. https://doi.org/10.1161/CIRCEP.114.002155.
Palaniswamy C, Miller MA, Reddy VY, Dukkipati SR. Hemodynamic support for ventricular tachycardia ablation. Cardiac Electrophysiol Clin. 2017;9(1):141–52. https://doi.org/10.1016/j.ccep.2016.10.011.
Naidu SS. Novel percutaneous cardiac assist devices: the science of and indications for hemodynamic support. Circulation. 2011;123(5):533–43. https://doi.org/10.1161/CIRCULATIONAHA.110.945055.
Virk SA, Keren A, John RM, Santageli P, Eslick A, Kumar S. Mechanical circulatory support during catheter ablation of ventricular tachycardia: indications and options. Heart, Lung Circulation. 2019;28(1):134–45. https://doi.org/10.1016/j.hlc.2018.10.006.
Bunch TJ, Mahapatra S, Madhu Reddy Y, Lakkireddy D. The role of percutaneous left ventricular assist devices during ventricular tachycardia ablation. Europace: European Pacing, Arrhythmias, and Cardiac Electrophysiology: Journal of the Working Groups on Cardiac Pacing, Arrhythmias, and Cardiac Cellular Electrophysiology of the European Society of. Cardiology. 2012;14(Suppl 2):ii26–32. https://doi.org/10.1093/europace/eus210.
Friedman PA, Munger TM, Torres N, Rihal C. Percutaneous endocardial and epicardial ablation of hypotensive ventricular tachycardia with percutaneous left ventricular assist in the electrophysiology laboratory. J Cardiovasc Electrophysiol. 2007;18(1):106–9. https://doi.org/10.1111/j.1540-8167.2006.00619.x.
Panuccio G, Neri G, Macrì LM, Salerno N, De Rosa S, Torella D. Use of Impella device in cardiogenic shock and its clinical outcomes: a systematic review and meta-analysis. Int J Cardiol Heart Vasc. 2022;40:101007. https://doi.org/10.1016/j.ijcha.2022.101007.
Aryana A, Gearoid O’Neill P, Gregory D, Scotti D, Bailey S, Brunton S, et al. Procedural and clinical outcomes after catheter ablation of unstable ventricular tachycardia supported by a percutaneous left ventricular assist device. Heart Rhythm. 2014;11(7):1122–30. https://doi.org/10.1016/j.hrthm.2014.04.018.
Aryana A, d’Avila A, Cool CL, Miller MA, Garcia FC, Supple GE, et al. Outcomes of catheter ablation of ventricular tachycardia with mechanical hemodynamic support: an analysis of the Medicare database. J Cardiovasc Electrophysiol. 2017;28(11):1295–302. https://doi.org/10.1111/jce.13312.
Kusa S, Miller MA, Whang W, Enomoto Y, Panizo JG, Iwasawa J, et al. Outcomes of ventricular tachycardia ablation using percutaneous left ventricular assist devices. Circ Arrhythm Electrophysiol. 2017;10(6):e004717. https://doi.org/10.1161/CIRCEP.116.004717.
Gilotra NA, Stevens GR. Temporary mechanical circulatory support: a review of the options, indications, and outcomes. Clin Med Insights Cardiol. 2014;8(Suppl 1):75–85. https://doi.org/10.4137/CMC.S15718.
Baratto F, Pappalardo F, Oloriz T, Bisceglia C, Vergara P, Silberbauer J, et al. Extracorporeal membrane oxygenation for hemodynamic support of ventricular tachycardia ablation. Circ Arrhythm Electrophysiol. 2016;9(12):e004492. https://doi.org/10.1161/CIRCEP.116.004492.
Enriquez A, Liang J, Gentile J, Schaller RD, Supple GE, Frankel DS, et al. Outcomes of rescue cardiopulmonary support for periprocedural acute hemodynamic decompensation in patients undergoing catheter ablation of electrical storm. Heart Rhythm. 2018;15(1):75–80. https://doi.org/10.1016/j.hrthm.2017.09.005.
Santangeli P, Frankel DS, Tung R, Vaseghi M, Sauer WH, Tzou WS, et al. Early membrane oxygenation for hemodynamic support of ventricular tachycardia ablation. J Am Coll Cardiol. 2017;69(17):2105–15. https://doi.org/10.1016/j.jacc.2017.02.044.
Muser D, Liang JJ, Castro SA, Hayashi T, Enriquez A, Troutman GS, et al. Outcomes with prophylactic use of percutaneous left ventricular assist devices in high-risk patients undergoing catheter ablation of scar-related ventricular tachycardia: a propensity-score matched analysis. Heart Rhythm. 2018;15(10):1500–6. https://doi.org/10.1016/j.hrthm.2018.04.028.
Muser D, Castro SA, Liang JJ, Santangeli P. Identifying risk and management of acute haemodynamic decompensation during catheter ablation of ventricular tachycardia. Arrhythmia Electrophysiol Rev. 2018;7(4):282–7. https://doi.org/10.15420/aer.2018.36.3.
Mukherjee RK, Whitaker J, Williams SE, Razavi R, O’Neill MD. Magnetic resonance imaging guidance for the optimization of ventricular tachycardia ablation. Europace. 20(11):1721–32. https://doi.org/10.1093/europace/euy040.
Andreu D, Berruezo A, Ortiz-Pérez JT, Silva E, Mont L, Borràs R, et al. Integration of 3D electroanatomic maps and magnetic resonance scar characterization into the navigation system to guide ventricular tachycardia ablation. Circ Arrhythm Electrophysiol. 2011;4(5):674–83. https://doi.org/10.1161/CIRCEP.111.961946.
Fernández-Armenta J, Berruezo A, Mont L, Sitges M, Andreu D, Silva E, et al. Use of myocardial scar characterization to predict ventricular arrhythmia in cardiac resynchronization therapy. Europace. 2012;14(11):1578–86. https://doi.org/10.1093/europace/eus104.
Zghaib T, Ipek EG, Hansford R, Ashikaga H, Berger RD, Marine JE, et al. Standard ablation versus magnetic resonance imaging-guided ablation in the treatment of ventricular tachycardia. Circ Arrhythm Electrophysiol. 11(1):e005973. https://doi.org/10.1161/CIRCEP.117.005973.
Andreu D, Penela D, Acosta J, Fernández-Armenta J, Perea RJ, Soto-Iglesias D, et al. Cardiac magnetic resonance-aided scar dechanneling: influence on acute and long-term outcomes. Heart Rhythm. 14(8):1121–8. https://doi.org/10.1016/j.hrthm.2017.05.018.
Campbell-Washburn AE, Tavallaei MA, Pop M, Grant EK, Chubb H, Rhode K, Wright GA. Real-time MRI guidance of cardiac interventions. J Magn Reson Imaging. 46(4):935–50. https://doi.org/10.1002/jmri.25749.
Guttman MA, Tao S, Fink S, Kolandaivelu A, Halperin HR, Herzka DA. Non-contrast-enhanced T. Magn Reson Med. 79(2):879–89. https://doi.org/10.1002/mrm.26750.
Tao S, Guttman MA, Fink S, Elahi H, Patil KD, Ashikaga H, et al. Ablation lesion characterization in scarred substrate assessed using cardiac magnetic resonance. JACC Clin Electrophysiol. 5(1):91–100. https://doi.org/10.1016/j.jacep.2018.11.001.
Roujol S, Basha TA, Tan A, Khanna V, Chan RH, Moghari MH, et al. Improved multimodality data fusion of late gadolinium enhancement MRI to left ventricular voltage maps in ventricular tachycardia ablation. IEEE Trans Biomed Eng. 2013;60(5):1308–17. https://doi.org/10.1109/TBME.2012.2233738.
Radau PE, Pintilie S, Roey F, Biswas L, Oduneye SO, Ramanan V, et al. VURTIGO: visualization platform for real-time, mri-guided cardiac electroanatomic mapping. In: In International Workshop on Statistical Atlases and Computational Models of the Heart. Berlin: Springer; 2012. p. 244–53. https://doi.org/10.1007/978-3-642-28326-0_25.
Mukherjee RK, Costa CM, Neji R, Harrison JL, Sim I, Williams SE, et al. Evaluation of a real-time magnetic resonance imaging-guided electrophysiology system for structural and electrophysiological ventricular tachycardia substrate assessment. Europace. 2019;21(9):1432–41. https://doi.org/10.1093/europace/euz165.
Cluitmans M, Brooks DH, MacLeod R, Dössel O, Guillem MS, van Dam PM, et al. Validation and opportunities of electrocardiographic imaging: from technical achievements to clinical applications. Front Physiol. 2018;9:1305. https://doi.org/10.3389/fphys.2018.01305.
Cheniti G, Puyo S, Martin CA, Frontera A, Vlachos K, Takigawa M, et al. Noninvasive mapping and electrocardiographic imaging in atrial and ventricular arrhythmias (cardioinsight). Card Electrophysiol Clin. 11(3):459–71. https://doi.org/10.1016/j.ccep.2019.05.004.
Jamil-Copley S, Bokan R, Kojodjojo P, Qureshi N, Koa-Wing M, Hayat S, et al. Noninvasive electrocardiographic mapping to guide ablation of outflow tract ventricular arrhythmias. Heart Rhythm. 2014;11(4):587–94. https://doi.org/10.1016/j.hrthm.2014.01.013.
Graham AJ, Orini M, Zacur E, Dhillon G, Daw H, Srinivasan NT, et al. Simultaneous comparison of electrocardiographic imaging and epicardial contact mapping in structural heart disease. Circ Arrhythm Electrophysiol. 12(4):e007120. https://doi.org/10.1161/CIRCEP.118.007120.
Wang L, Gharbia OA, Nazarian S, Horácek BM, Sapp JL. Non-invasive epicardial and endocardial electrocardiographic imaging for scar-related ventricular tachycardia. Europace: European Pacing, Arrhythmias, and Cardiac Electrophysiology: Journal of the Working Groups on Cardiac Pacing, Arrhythmias, and Cardiac Cellular Electrophysiology of the European Society of Cardiology. 2018;20(FI2):f263–72. https://doi.org/10.1093/europace/euy082.
Zhang J, Cooper DH, Desouza KA, Cuculich PS, Woodard PK, Smith TW, Rudy Y. Electrophysiologic scar substrate in relation to VT: noninvasive high-resolution mapping and risk assessment with ECGI. Pacing Clin Electrophysiol: PACE. 2016;39(8):781–91. https://doi.org/10.1111/pace.12882.
Misra S, van Dam P, Chrispin J, Assis F, Keramati A, Kolandaivelu A, et al. Initial validation of a novel ECGI system for localization of premature ventricular contractions and ventricular tachycardia in structurally normal and abnormal hearts. J Electrocardiol. 2018;51(5):801–8. https://doi.org/10.1016/j.jelectrocard.2018.05.018.
van Dam PM, Gordon JP, Laks MM, Boyle NG. Development of new anatomy reconstruction software to localize cardiac isochrones to the cardiac surface from the 12 lead ECG. J Electrocardiol. 2015;48(6):959–65. https://doi.org/10.1016/j.jelectrocard.2015.08.036.
Cluitmans MJM, Bonizzi P, Karel JMH, Das M, Kietselaer BLJH, de Jong MMJ, et al. In vivo validation of electrocardiographic imaging. JACC: Clin Electrophysiol. 2017;3(3):232–42. https://doi.org/10.1016/j.jacep.2016.11.012.
Prakosa A, Arevalo HJ, Deng D, Boyle PM, Nikolov PP, Ashikaga H, et al. Personalized virtual-heart technology for guiding the ablation of infarct-related ventricular tachycardia. Nat Biomed Eng. 2018;2(10):732–40. https://doi.org/10.1038/s41551-018-0282-2.
Cedilnik N, Duchateau J, Dubois R, Sacher F, Jaïs P, Cochet H, Sermesant M. Fast personalized electrophysiological models from computed tomography images for ventricular tachycardia ablation planning. Europace. 2018;20(suppl_3):iii94–iii101. https://doi.org/10.1093/europace/euy228.
Rudy Y, Lindsay BD. Electrocardiographic imaging of heart rhythm disorders: from bench to bedside. Cardiac Electrophysiol Clin. 2015;7(1):17–35. https://doi.org/10.1016/j.ccep.2014.11.013.
Author information
Authors and Affiliations
Contributions
All authors contributed to the review article conception, data collection, and writing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Marashly, Q., Najjar, S.N., Hahn, J. et al. Innovations in ventricular tachycardia ablation. J Interv Card Electrophysiol 66, 1499–1518 (2023). https://doi.org/10.1007/s10840-022-01311-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-022-01311-z