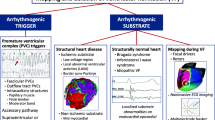
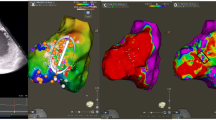
Abstract
Ventricular tachycardia (VT) ablation has become the gold standard for curing idiopathic VT and dramatically reducing VT burden and implantable cardioverter-defibrillator (ICD) shocks in patients with scar-related VT. This chapter identifies appropriate patient selection for patients undergoing catheter-based ablation. The techniques used in identifying the best target sites for ablation of mappable VT using the 12-lead electrocardiogram (ECG) of VT and activation and entrainment mapping are detailed. Many VTs are not hemodynamically tolerated, and targets and strategies for ablating mappable VT are provided. Appropriate ablation end points and considerations pre- and post-procedure to optimize outcomes are described.
Supported by: Mark S Marchlinski EP Research Fund and Katherine Miller EP Research Fund at the University of Pennsylvania.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Ventricular Tachycardia
- Ventricular Fibrillation
- Catheter Ablation
- Entrainment Mapping
- Pace mapping
- Substrate Mapping
- 12-Lead Electrocardiogram
- Bundle Branch Reentry
- Idiopathic VT
Introduction
Ventricular tachycardia (VT) is associated with significant morbidity and mortality in patients with structural heart disease. In structurally normal heart, idiopathic VT can be associated with increased morbidity. The most common cause of sustained monomorphic VT in patients with structural heart disease is myocardial scar from a prior infarct, whereas ventricular fibrillation (VF) is usually seen in the context of acute coronary ischemia [1].
Despite the introduction of implantable cardioverter-defibrillators (ICDs) which reliably terminate episodes of VT and VF and prevent sudden cardiac death, 20% of high-risk patients with primary prevention ICDs have recurrent shocks within 3–5 years [2]. Recurrent VT develops in 40–60% of patients who receive ICD therapy after an episode of spontaneous sustained VT. Antiarrhythmic therapy with amiodarone or sotalol decreases the number of VT episodes but with a high incidence of adverse effects and limited efficacy [3].
Catheter ablation remains the gold standard in managing patients with recurrent VT and a potentially lifesaving procedure in patients with VT storm. In this chapter, we will review the approach to catheter ablation in the management of ventricular tachycardia.
Definitions
The definitions of various VTs are outlined below as per the 2019 Heart Rhythm Society (HRS) expert consensus statement on catheter ablation of ventricular arrhythmias by Cronin et al. [1].
-
1.
VT Clinical Characteristics
VT: a tachycardia (rate >100 bpm) with three or more consecutive beats that originate from the ventricles.
VT storm: three or more separate episodes of sustained VT within 24 h, each requiring termination by an intervention.
Nonsustained VT: VT that terminates spontaneously within 30 s.
Idioventricular rhythm: three or more consecutive beats at a rate of up to 100 or less per minute that originate from the ventricles.
Clinical VT: VT that has occurred spontaneously based on analysis of 12-lead electrocardiogram (ECG) QRS morphology.
Hemodynamically unstable VT: causes hemodynamic compromise requiring prompt termination.
Idiopathic VT: VT that is known to occur in the absence of clinically apparent structural heart disease.
Incessant VT: continuous sustained VT that recurs promptly despite repeated intervention for termination.
Nonclinical VT: VT induced by program ventricular stimulation but has not been documented previously.
Repetitive monomorphic VT: continuously repeating episodes of self-terminating nonsustained VT.
-
2.
VT Morphologies (Fig. 19.1)
Monomorphic VT: a similar QRS configuration from beat to beat. Some variability in QRS morphology at initiation is not uncommon, followed by stabilization of the QRS morphology.
Monomorphic VT with indeterminate QRS morphology: preferred over ventricular flutter; it is a term that has been applied to rapid VT that has a sinusoidal QRS configuration that prevents identification of the QRS morphology.
Multiple monomorphic VTs: more than one morphologically distinct monomorphic VT occurring as different episodes or induced at different times.
Pleomorphic VT: has more than one morphologically distinct QRS complex occurring during the same episode of VT, but the QRS is not continuously changing.
Bidirectional VT: demonstrates beat-to-beat alternation in QRS morphology, usually seen in the frontal plane axis (Fig. 19.1d); right bundle branch block (RBBB) QRS morphology seen in patients with digoxin toxicity.
Polymorphic VT: has a continuously changing QRS configuration from beat to beat, indicating a changing ventricular activation sequence and functional reentrant mechanism.
Right bundle branch block- and left bundle branch block-like VT configurations: terms used to describe the dominant deflection in V1, with a dominant R wave described as “RBBB-like” and a dominant S wave with a negative final component in V1 described as “LBBB-like” configurations.
Torsade de pointes: a form of polymorphic VT with continually varying QRS complexes that appear to spiral around the baseline of the ECG lead in a sinusoidal pattern. It is associated with QT prolongation.
Unmappable VT: does not allow interrogation of multiple sites to define the activation sequence or perform entrainment mapping; this could be due to hemodynamic intolerance that necessitates immediate VT termination, spontaneous or pacing-induced transition to other morphologies of VT, or repeated termination during mapping.
Ventricular fibrillation (VF): a chaotic rhythm defined on the surface ECG by undulations that are irregular in both timing and morphology, without discrete QRS complexes.
-
3.
VT Mechanism
Focal VT: a point source of earliest ventricular activation with a spread of activation away in all directions from that site. The mechanism can be automaticity, triggered activity, or microreentry.
Scar-related reentry: arrhythmias that have characteristics of reentry that originate from an area of myocardial scar identified from electrogram characteristics or myocardial imaging. Large reentry circuits that can be defined over several centimeters are commonly referred to as “macroreentry.”
Various forms of ventricular arrhythmias. (a) Monomorphic VT with LBBB morphology. (b) Pleomorphic VT with more than one VT QRS morphology occurring at the same episode. (c) Monomorphic VT degenerating to polymorphic VT/VF. (d) Bidirectional VT in patient with digoxin toxicity originates from the LV Purkinje network and is thought to be triggered rhythm alternating from the anterior and posterior fascicles
VT Mechanism
Catheter ablation for ventricular arrhythmias has an important role in reducing and eliminating VTs in patients with heart disease as well as patients with idiopathic VTs and structurally normal heart. The approach to VT ablation is determined by the characteristics of the arrhythmia and location of the arrhythmia substrate, which can be predicted by the preprocedural 12-lead ECG of the clinical VT. Focal VTs are usually treated with ablation with discrete radiofrequency (RF) lesions [4,5,6]. Ventricular arrhythmias (VAs), which are associated with structural heart disease such as ischemic heart disease or nonischemic cardiomyopathy, often have a relatively large scar substrate and require typically more extensive ablation.
Focal VT spreads in a centrifugal pattern from a point of earliest ventricular activation. The mechanism can be automaticity, triggered activity, or microreentry. Focal nature of the VA is confirmed by mapping, showing spread of activation away from a site of earliest activation relative to the QRS onset. Unipolar electrograms at the site of origin (SOO) typically display a QS configuration denoting spread of ventricular activation away from the SOO. Pacing from the site of origin typically replicates the VT/PVC QRS morphology. However, good pace maps are usually seen up to 1 cm from the site of earliest activation. Pace mapping is particularly less reliable for VTs originating from the aortic sinuses, where strand-like fibers extend into the aortic root and are marked by late potentials in sinus rhythm, and more marked presystolic activity with arrhythmias may be difficult to pace with large-tip ablation catheters [7,8,9,10,11].
Triggered Activity and Automaticity
Triggered activity develops from oscillations in membrane potential during action potential phase 2 and 3 (early afterdepolarizations) or following an action potential (delayed afterdepolarizations) and can give rise to focal VA. Experimental evidence implicates early afterdepolarizations in the initiation of polymorphic tachycardias in long QT syndromes [12]. However, the mechanism of the premature ventricular beats targeted for ablation in these syndromes remains uncertain [13]. Delayed afterdepolarizations can be caused by intracellular calcium overload, which activates the Na1+/Ca2+ exchanger, resulting in the transient inward current Iti. Increases in heart rate, beta-adrenergic stimulation, and digitalis are known factors that increase intracellular calcium and potentiated arrhythmias due to triggered activity [14].
Beta-adrenergic effects are mediated through a cyclic adenosine monophosphate (cAMP)-induced increase in intracellular calcium and are antagonized by adenosine, which results in a decrease in cAMP. Termination of idiopathic Right Ventricular Outflow Tract (RVOT) tachycardias by an intravenous adenosine, by infusion of calcium channel blockers, or by vagotonic maneuvers is consistent with triggered activity as the likely mechanism for some of these tachycardias [6]. These tachycardias can be difficult to induce at electrophysiology testing, and rapid burst pacing and/or isoproterenol infusion is often required. Aminophylline, calcium, and atropine administration may also be useful but have not been systematically evaluated clinically [5].
Less commonly, focal VT can be due to automaticity provoked by adrenergic stimulation that is not triggered [14]. This type of VT can become incessant under stress or during isoproterenol administration. These arrhythmias cannot be initiated or terminated by programmed electrical stimulation (PES); however, they can sometimes be suppressed by calcium channel blockers or beta blockers. In contrast to its effects on triggered RVOT tachycardia, adenosine transiently suppresses, but does not terminate, the arrhythmia.
Automaticity from damaged Purkinje fibers has been suggested as the mechanism for some catecholamine-sensitive, focal origin VTs [15, 16]. Whether these VTs are due to abnormal automaticity, originating from partially depolarized myocytes, as has been shown for VTs during the early phase of myocardial infarction (MI), has not been determined with certainty [17]. Although automaticity is frequently considered as a mechanism of VT in the absence of overt structural heart disease (SHD), processes that diminish cell-to-cell coupling are more likely to facilitate automaticity. Automatic VTs can definitely occur in SHD, and automatic premature beats can also initiate reentrant VTs, making the distinctions related to arrhythmia mechanism more difficult [18].
Scar-Related Reentry
In patients with structurally abnormal heart, scar-related reentry is the most common cause for sustained monomorphic VT [19]. Evidence supporting reentry includes initiation and termination by ventricular programmed stimulation (although this does not exclude triggered activity), demonstrable entrainment or resetting with fusion, and continuous electrical activity that cannot be dissociated from VT by extrastimuli [20, 21].
Prior myocardial infraction is the most common cause of the substrate, but scar-related VT also occurs in other myocardial diseases, including Arrhythmogenic Right Ventricular Cardiomyopathy (ARVC), sarcoidosis, Chagas disease, and dilated cardiomyopathy (DCM) including laminopathies, and after cardiac surgery for congenital heart disease (CHD) (particularly, tetralogy of Fallot) or valve replacement [22,23,24,25,26,27]. Regions of fibrosis with surviving myocyte bundles create fixed and/or functional conduction block and disrupted or slow conduction that are the substrate for reentry. Stable circuits can be modeled as having an isthmus or channel comprised of a small mass of tissue that does not contribute to the surface ECG. QRS onset occurs when the excitation wave front emerges from an exit along the border of the scar and spreads across the ventricles [28, 29]. Scars associated with VT that are close to valve annuli may incorporate the valve annuli as one of the fixed boundaries of the VT isthmus. The annulus together with the scar can form the borders of a channel which creates the isthmus of the VT circuit [30, 31].
The 3D structure of the reentrant VT circuit and substrate can be entirely subendocardial, intramural, or subepicardial, or it can span the width of the entire ventricular wall. Thus, the entire circuit or only a portion of it might be accessible to endocardial and/or epicardial mapping and ablation [32, 33]. The substrate supporting scar-related reentry is characterized by the following: (1) regions of slow conduction and (2) unidirectional conduction block at some point in the activation path that allows initiation of reentry. Some of the substrate might exhibit functional rather than fixed conduction block that may only be manifest in response to an increase in rate, change in activation wave front with pacing, or with the introduction of premature extrastimuli [34,35,36].
VT after MI has been extensively studied in canine models and in humans [37]. Reentry occurs through surviving muscle bundles, commonly located in the subendocardium; however, the circuit can also extend to the midmyocardium and epicardium. Evidence has shown that ongoing ion channel remodeling within scar, at least early after MI, results in regional reductions in ionized sodium and ionized calcium currents [38], although action potential characteristics of surviving myocytes late after infarction can be normal or near normal [37]. Coupling between myocyte bundles and myocytes is reduced by increased collagen and connective tissue, diminished gap junction density, and alterations in gap junction distribution, composition, and function [39]. Surviving fibers can be connected by side-to-side connections in regions where the collagenous sheaths are interrupted, resulting in a “zig-zag” pattern of transverse conduction along a pathway lengthened by branching and merging bundles of surviving myocytes meandering through the collagen [40].
The fibrosis pattern might be important in determining the degree of conduction delay; patchy fibrosis between strands of surviving muscle produces greater delay than diffuse or consolidated fibrosis which produced block [35]. These aspects of scar remodeling contribute to the formation of channels and regions, in which conduction time is prolonged, facilitating reentry [41]. Unidirectional conduction block can occur after a properly timed Premature Ventricular Contraction (PVC) and is often functional [42]; it can present only during tachycardia, when the refractory period of the tissue exceeds the tachycardia cycle length (TCL) or is maintained by collision of excitation waves. Regions of conduction block can also be anatomically fixed such that they are present during tachycardia and sinus rhythm; dense, non-excitable fibrosis, calcifications, surgical scars, or valve annuli create these types of anatomical boundaries for reentry [43].
Multiple VTs with various QRS morphologies can be due to multiple exits from the same region of scar or to changes in activation remote from the circuit due to functional regions of block. Ablation at one region can abolish more than one VT. Multiple reentry circuits from widely separated areas can also occur.
It is possible that other reentry mechanisms cause some VTs. Spiral wave reentry can be induced in excitable tissue in the absence of tissue discontinuities and could cause VF or polymorphic VT [44]; a spiral anchoring to a discontinuity or to a region of slow conduction could theoretically cause monomorphic VT [45]. These alternative mechanisms have not yet been proven to be important clinically in relation to mapping and ablating VT.
Reentry in the Purkinje System and Ventricular Fibrillation
Reentry within the Purkinje fibers and the specialized conduction system is a particular form of reentry accounting for 5% of all MMVT encountered during catheter ablation. Other nonreentrant arrhythmias involving the Purkinje system can also occur, including VF and automatic rhythms [46,47,48]. PVCs initiating VF most often originate from the Purkinje system. Structural abnormalities in the vicinity of the Purkinje fibers are frequently present and may facilitate the anchoring of reentry [49].
Bundle branch reentry, which is a form of macroreentry utilizing the conduction system, usually occurs in patients with damaged His–Purkinje conduction system and is usually associated with left ventricular (LV) dysfunction due to dilated cardiomyopathy, valvular heart disease, and less often ischemic heart disease [50].
Left ventricular intrafascicular verapamil-sensitive VT usually occurs in patients with structurally normal heart. The mechanism is reentry that involves a portion of the LV Purkinje fibers, most often in the region of the left posterior or anterior fascicle, giving rise to a characteristic right bundle branch block (RBBB) superior axis or inferior QRS configuration and a QRS duration that is only modestly prolonged [51]. The response to verapamil suggests that there is a portion of the reentrant circuit in which the action potential is calcium dependent.
Indications for VT Ablation
Class 1 indication as per 2019 HRS expert consensus statement on catheter ablation of ventricular arrhythmias by Cronin et al. [1].
Patients with Structurally Normal Heart
-
1.
In patients with frequent and symptomatic PVCs originating from the RVOT, catheter ablation is recommended in preference to metoprolol or propafenone.
-
2.
In patients with symptomatic idiopathic sustained monomorphic VT, catheter ablation is useful.
-
3.
In patients with symptomatic VAs from the RV at sites other than the outflow tracts (tricuspid annulus, moderator band, or papillary muscles) in an otherwise normal heart for whom antiarrhythmic medications are ineffective, not tolerated, or not the patient’s preference, catheter ablation is useful.
-
4.
In patients with symptomatic VAs from the LV at sites other than the outflow tracts (mitral annulus, papillary muscles, or AMC) in an otherwise normal heart for whom antiarrhythmic medications are ineffective, not tolerated, or not the patient’s preference, catheter ablation is useful.
Patients with Ischemic Heart Disease
-
1.
In patients with ischemic heart disease (IHD) who experience recurrent monomorphic VT despite chronic amiodarone therapy, catheter ablation is recommended in preference to escalating antiarrhythmic drugs (AAD) therapy.
-
2.
In patients with IHD and recurrent symptomatic monomorphic VT despite AAD therapy, or when AAD therapy is contraindicated or not tolerated, catheter ablation is recommended to reduce recurrent VT.
-
3.
In patients with IHD and VT storm refractory to AAD therapy, catheter ablation is recommended.
-
4.
In patients with post-infarction reentrant Purkinje fiber-mediated VT, catheter ablation is useful.
Patients with Nonischemic Cardiomyopathy (NICM )
-
1.
In patients with nonischemic cardiomyopathy (NICM) and recurrent sustained monomorphic VT for whom antiarrhythmic medications are ineffective, contraindicated, or not tolerated, catheter ablation is useful for reducing recurrent VT and ICD shocks.
-
2.
In patients with NICM and electrical storm refractory to AAD therapy, catheter ablation is useful for reducing recurrent VT and ICD shocks.
-
3.
In patients with bundle branch reentrant VT, catheter ablation is useful for reducing the risk of recurrent VT.
Patients with Congenital Heart Disease and Inherited Arrhythmias
-
1.
In patients with repaired tetralogy of Fallot and sustained monomorphic VT or recurrent appropriate ICD therapy for VAs, catheter ablation is effective.
-
2.
In patients with ARVC who experience recurrent sustained VT or frequent appropriate ICD interventions for VT in whom AAD therapy is ineffective or not tolerated, catheter ablation, at a center with specific expertise, is recommended.
-
3.
In patients with ARVC who have failed one or more attempts of endocardial VT catheter ablation, an epicardial approach for VT ablation is recommended.
Procedural Planning for VT Ablation
Preprocedural Risk Assessment
Catheter ablation for ventricular tachycardia is an important therapeutic modality in patients with various forms of VA. Over the last decade, significant improvements in the techniques and technologies available for catheter ablation have been paralleled by an increasing number of procedures performed for high-risk and complex patient populations [52,53,54,55].
In these cases, the competing risks associated with the concomitant presence of advanced heart failure syndromes and high burden of associated comorbidities pose substantial periprocedural and postprocedural management challenges. A proper preprocedural risk stratification is crucial to minimize the risk of adverse periprocedural outcomes such as acute hemodynamic decompensation (AHD), which can have devastating consequences [56, 57].
A recent risk assessment score “PAAINESD risk score” (chronic obstructive Pulmonary disease [5 points], Age > 60 years [3 points], General anesthesia [4 points], Ischemic cardiomyopathy [6 points], New York Heart Association class III or IV [6 points], Ejection fraction, <25% [3 points], presentation with VT Storm [5 points], Diabetes mellitus [3 points]) has been demonstrated to be helpful to identify patients undergoing scar-related VT ablation, who are at increased risk of adverse periprocedural outcomes, and represents the most studied risk stratification tool in this context [53].
The cumulative evidence arising from available studies suggests that a PAAINESD score ≥ 15–17 (depending on whether the variable “general anesthesia” is included in the risk score calculation or not) identifies patients with SHD and VT at particularly high risk of adverse periprocedural and postprocedural outcomes. Careful consideration of the optimal sedation or anesthesia strategy as well as the use of mechanical assist devices periprocedural may further help to prevent AHD in patients with multiple other risk factors [53, 54, 58].
12-Lead Electrocardiogram and Body Surface Mapping Before Ventricular Tachycardia Ablation
The standard 12-lead ECG is of paramount importance in planning catheter ablation procedures for treatment of VAs, including VT and PVC, and in the case of hemodynamically tolerated VAs, every effort should be done to record the 12-lead ECG. In cases of focal VA in structurally normal heart, the 12-lead ECG is a relatively accurate indicator of the source location, whereas in the presence of myocardial scarring from whatever cause (in which reentry is the predominant mechanism of arrhythmia), the ECG reflects the exit site from the reentrant circuit, rather than the isthmus site, which typically represents the best ablation target.
Ventricular Tachycardia in the Absence of Structural Heart Disease
The 12-lead ECG features, during PVCs or VT, arising in structurally normal heart have been described in detail. Examples of such cases are VAs from RV or LV outflow tracts (OT) (including the aortic sinus of Valsalva), idiopathic fascicular (verapamil-sensitive) VT related to the LV Purkinje system, and other LV sources, including anterior and posterior papillary muscle VT, para-Hisian VT, mitral annular VT, LV summit VT and other RV sources, including tricuspid annular VT, moderator band VT, and RV papillary muscle VT.
Certain ECG patterns can indicate these regions of origin. An LBBB inferior axis morphology with late transition (> lead V3) indicates an origin in the RVOT. Lead I QRS vector indicates whether the origin is anterior and leftward (negative) or posterior and rightward (positive) located [59]. Notching and widening of the QRS in the inferior leads indicates a lateral (free wall) position in the RVOT (Fig. 19.2) [60].
RVOT PVC morphologies from the septal aspect and free wall. A number system is used from 1 to 9 to approximate distance from the pulmonic valve. Most idiopathic PVCs/VT originate from sites adjacent to or just above the pulmonic valve designated by site 1, most posterior and rightward; site 2, mid position; and site 3, most leftward position. PVCs from free wall aspect are smaller in amplitude, may have notching in inferior leads, are wider, and have a later precordial transition than PVCs from the septal aspect
An earlier transition of PVC from LBBB morphology compared to the sinus beat transition in the precordial leads or an early transition (≤ lead V3) indicates a left-sided origin, and a broader and taller R wave in V1 or V2 could indicate an origin from the sinus of Valsalva [61]. A hallmark feature of an RCC-LCC cusp junction origin is the presence of a QrS morphology or a QS morphology in lead V1 with notching on the downward deflection (Fig. 19.3) [62, 63].
VAs arising from the papillary muscles (PM) have distinguishing ECG features, typically RBBB morphology, and transition at leads V3–V5. Posteromedial PM origin is characterized by a superior axis, while an inferior axis or discordant axis is seen from the anterolateral papillary muscle. Characteristic of the papillary muscle origin is a qR pattern in lead V1. In contradistinction to papillary muscle origins, a fascicular origin has a much narrower QRS complex and an rsR pattern in V1 (Fig. 19.4) [64,65,66].
There are several ECG criteria that have been proposed to identify LV summit ventricular arrhythmias. These either have a right bundle branch block configuration in lead V1 or a left bundle branch block configuration with early precordial transition (V3 or earlier). The axis is always inferior. Importantly, there is generally a pattern break in lead V2, with more net negativity than leads V1 or V3. There is often a Q wave in lead I from these epicardial sites [67, 68].
Despite the range of heart positions in the chest and body habitus and variations of ECG lead positions, these ECG features can be remarkably accurate at indicating the SOO to within a 1- to 2-cm radius. Refinement of the exact site of impulse formation using activation or pace mapping is still needed prior to ablation [69,70,71,72].
Post-infarction Ventricular Tachycardia
The 12-lead ECG in post-infarct VAs indicates the exit site of the circuit. As noted above, the exit site location may not be in close proximity to the mid portion of the diastolic corridor (isthmus site of the VT). In general, correlation of exit sites with VT morphology follows these principles (Fig. 19.5):
-
1.
The majority of post-MI VT exit sites are LV endocardial.
-
2.
LBBB VTs tend to have exit sites from, or within 1 cm of, the intraventricular septum.
-
3.
Patients with inferior infarctions often have Q waves in the inferior leads, indicating inferior wall scarring and an inferior wall exit site, which tend to be located on the inferobasal septum or the inferolateral free wall, with a common diastolic corridor on the inferobasal free wall along the mitral annulus.
-
4.
VTs with a predominant inferior axis tend to have exit sites on the anterior wall.
-
5.
VTs with a predominant superior axis tend to have exit sites on the inferior aspect of the LV.
-
6.
VTs with a leftward axis tend to have exit sites from or within 1 cm of the septum.
-
7.
VTs with concordant positive precordial QRS complexes tend to have basal exit sites.
-
8.
VTs with concordant negative precordial QRS complexes tend to have apical exit sites.
A 65-year-old female with ischemic cardiomyopathy with LV ejection fraction of 20% and remote anterior myocardial infraction with recurrent ICD shocks secondary to VT. (a) Four monomorphic VTs induced during VT ablation. VT-2 represents the clinical VT with which the patient presented to the hospital. (b) Entrainment mapping of VT-2 demonstrating an isthmus site of the VT circuit with mid-diastolic potentials on ablation catheter. (c) Ablation at the site terminated the VT in 8 s. (d) Carto map (RAO view) of the LV unipolar and bipolar voltage map with final lesions. Programmed stimulation was not inducible using up to triple extrastimuli at the end of the procedure
Ventricular Tachycardia in Nonischemic Cardiomyopathy
The 12-lead ECG of the VT in NICM is less helpful in directing ablation in a similar fashion as seen in post-MI VT. It has been suggested that there are two distinct subtypes of scar-related VTs in these patients: basal lateral scar which often requires ablation on the epicardial surface to successfully eliminate VTs; the other subgroup is VTs originating from anteroseptal scar, which typically have an LBBB inferior axis morphology. These patients might either have AV conduction disturbances due to septal scarring of the conduction system or at high risk to develop heart block post-ablation. In this group, epicardial ablation does not work and at times, bipolar catheter ablation might be needed to eliminate VTs deep in the septum (Fig. 19.6) [73].
A 55-year-old male with nonischemic cardiomyopathy with EF 25% with recurrent VT, failed 2 VT ablation at basal septum. (a) Clinical VT induced in the EP lab with an LBLI axis QRS morphology. (b) Abnormal bipolar voltage map of LV septum and LV transseptal activation. (c) Bipolar ablation targeting the septum from the RV and LV simultaneously. (d) Final ablation lesion with the patient noninducible at the end of the procedure and at the time of repeat stimulation through ICD device 2 days later
Of note, we believe this dichotomous classification of VT in NICM is too simplistic and inaccurate. Many patients with basal septal involvement will have scar extension to the LV anterior free wall surrounding the mitral valve with endo- and/or epicardial circuits. Certainly, scar extension and VT circuits involving the LV summit region are very common in patients who demonstrate septal involvement (Fig. 19.7).
A 49-year-old male with nonischemic cardiomyopathy with left ventricular ejection fraction of 35% admitted with recurrent ICD shocks for VT ablation. (a) Cardiac MRI showing LV basal inferior lateral subepicardial scar. (b) Intracardiac ultrasound demonstrating the epicardial scar. CartoSound reconstruction of the LV with the epicardial scar denoted in brown. (c) Bipolar voltage map of LV with abnormal voltage at the inferior lateral LV with late potentials on the multipolar catheter. (d) Induction of clinical VT with program stimulation. (e) Entrainment from the LV epicardial scar demonstrating concealed fusion with PPI-TCL 19 ms, S-QRS/TCL of 53% consistent with an isthmus site of VT circuit. (f) Entrainment from the LV endocardial scar demonstrating concealed fusion with PPI-TCL 19 ms, S-QRS/TCL 12% consistent with an exit site of the VT circuit. (g) Ablation at the isthmus site on the epicardial aspect of the scar terminates clinical VT. (h) Mapping the epicardial scar with multipolar catheter demonstrates elimination of late potentials after the ablation procedure
Bundle Branch Reentrant Ventricular Tachycardia
As indicated, patients with bundle branch reentrant ventricular tachycardia (BBRVT) typically have evidence of advanced heart disease (nonischemic more than ICM), with an LBBB pattern in the baseline conducted rhythm, although RBBB or nonspecific interventricular conduction delay can also occur. During BBRVT, the QRS morphology may closely resemble the baseline QRS if LBBB is present, with characteristically rapid initial forces (in contrast to the delayed up-strokes in most other myocardial VTs). This is an important entity to recognize because of its prevalence as well as its curability with a rather simple ablation procedure, targeting typically the right bundle, or in the setting of LBBB in sinus rhythm, targeting the left bundle to eliminate persistent retrograde conduction (Fig. 19.8).
ICD Electrogram Information
Patients with structural heart disease and VAs present with recurrent ICD shocks that effectively terminated VTs, precluding recording of the 12-lead ECG of the clinical arrhythmia. The 12-lead ECG can be recorded if the VT is below the programmed therapy zone (slow VT) or when the device is disabled (during programmed stimulation at the beginning of VT ablation procedure). In patients with SHD, having the 12-lead ECG of all spontaneously occurring VTs is especially important during procedures in which multiple morphologies of VT are induced, and the operator must decide which morphology (or morphologies) is most important to target for ablation. In the absence of a 12-lead ECG, ICD electrograms of the recorded VT episodes have been particularly helpful to identify the “clinical VT” when multiple VTs are induced during an ablation procedure [74].
Epicardial Ventricular Tachycardia
The surface ECG during VT provides important clues to an epicardial origin, including the presence of a pseudo-delta wave (≥34 ms in duration) in the precordial leads, an intrinsicoid deflection to the peak of the R wave in lead V2 ≥85 ms, and an RS duration of >121 ms, although with limited specificity in patients with ICM [75].
An ECG algorithm to identify epicardial VT from the anterolateral LV in patients with NICM includes the absence of Q waves in the inferior leads with either pseudo-delta waves (≥75 ms), a maximum deflection index ≥0.59, and a Q wave in lead I. The reported sensitivity and specificity of these criteria to predict successful epicardial ablation in NICM can exceed 90% [76].
Most epicardial idiopathic VTs come from the region of the LV summit or the inferior LV crux and produce characteristic ECG patterns marked by initial slurring of the QRS, producing an increase in the maximum deflection index (MDI) to greater than 0.55. The MDI is measured from the onset of the QRS to the earliest peak of the R wave in any of the precordial leads. Also characteristic is the presence of a pattern break in V2 with more net negativity than leads V1 or V3 when from the summit and more net positivity in V2 than V1 and V3 from the crux. A QS pattern is frequently seen in lead 1 from the summit and in lead 3 and a VF from the crux, consistent with an epicardial to endocardial activation wave front.
Mapping and Imaging Techniques
Mapping Catheters
The use of multielectrode mapping catheters has increased over the past several years with the introduction of mapping systems capable of acquiring high-definition electro-anatomical data from multiple sites for each beat simultaneously. These catheters have significant advantages over the ablation catheter in terms of mapping, especially with respect to mapping density, resolution, and speed.
Multielectrode catheters have small electrodes with short interelectrode spacing, thereby increasing mapping resolution, which can be advantageous for detailed substrate mapping, and facilitate identification of surviving myocardial bundles within heterogeneous scar tissue that may escape detection when mapping with standard ablation catheters with larger electrodes and spacing is performed [77, 78]. These catheters are also useful for activation mapping during VT, as they allow rapid acquisition of multiple sites at high spatial resolution that can facilitate rapid identification of an isthmus site or VT focus of origin for idiopathic VTs [78].
There are several multielectrode catheters available. The Pentaray catheter (Biosense Webster, Diamond Bar, CA) is a flower-shaped catheter and has five flexible splines, each with four electrodes (a total of 20 electrodes), which can be used with the Carto electroanatomical mapping (CARTO EAM) system. The Livewire catheter (Abbott Laboratories, Abbott Park, IL) is a duodecapolar catheter with tight interelectrode spacing that has been used in mapping endocardial and epicardial ventricular surfaces. The Advisor HD Grid catheter (Abbott Laboratories, Abbott Park, IL) is a 4-by-4 unipolar electrode array with 1-mm (diameter) electrodes, equidistantly spaced 3 mm apart from each other, which can be used in conjunction with the EnSite Precision system. The fourth catheter is a small basket catheter with 64 very small electrodes arranged on 8 splines that can be used with the Rhythmia EAM system (Orion, Boston Scientific, Marlborough, MA) (Fig. 19.9).
The main limitations of these catheters include frequent ectopy during mapping, transient injury of the superficial conduction system, limited maneuverability, poor tissue contact and lack of tissue contact information, and increased potential for thrombus formation with the need for careful anticoagulation and the additional cost.
Activation Mapping
In patients with hemodynamically stable monomorphic tachycardia, activation and entrainment mapping are the gold standards for localization of the best site for catheter ablation [79, 80].
Activation mapping is performed by recording local electrograms from multiple sites during VT aided by 3D mapping systems, which display the position of the catheter and relative timing of activation on the EAM system. For focal VTs, the earliest presystolic site of activation identifies the SOO and is the target of ablation. At this site, the local bipolar electrogram precedes the surface QRS onset, and the unipolar signal exhibits a QS configuration, consistent with a spread of activation away from the SOO.
In patients with SHD, the most common VT mechanism is scar-related reentry with continuous excitation of the circuit throughout the tachycardia cycle length (CL). Electrograms at exit sites, when activation mapping is performed during VT, are identified during the latter half of electrical diastole, frequently just before the QRS complex. Ablation at exit sites from the isthmus can terminate the tachycardia; however, it can also result in a change of the tachycardia configuration and/or cycle length, in which case the diastolic pathway can exit at different locations from the scar [78].
Ablation of the diastolic pathway “isthmus” is therefore a more desirable target, given it can eliminate the machinery required for reentry. Electrograms at isthmus sites occur earlier during diastole, are typically of very low-voltage amplitude (<0.5 mV), and can have multiple potentials consistent with more marked delayed conduction.
In general, an activation map of a VT circuit should demonstrate entrance, isthmus, and exit sites that serve as essential parts of the circuit, such that it cannot continue without all of these elements. It is not uncommon in nonischemic substrate that part of the circuit has an intramural component that might not be recorded on the surface. These usually exhibit a “gap” in the activation sequence, such that part of the circuit is “concealed” from the surface map, residing deep in the myocardium (Fig. 19.7).
Entrainment Mapping
Entrainment is a pacing maneuver during VT that helps to distinguish reentrant from nonreentrant arrhythmias and can be used as a powerful mapping tool to target ablation at critical parts of the reentry circuit. Entrainment involves the continuous resetting of a reentry circuit during pacing at sites that are either within or outside the reentry circuit. This method is used to define critical sites of the arrhythmia circuit through the analysis of the QRS morphology, the measured intervals, and the recorded electrograms (Fig. 19.10) [81].
Algorithm identifying optimal site for scar-related VT ablation. (Reprinted from Haqqani and Callans [101], with permission from Elsevier)
The advantage of entrainment mapping over a substrate mapping approach is that VTs can be reliably and permanently eliminated with few RF ablation lesions when reentry circuit targets are identified (Table 19.1) [20, 82].
Entrainment mapping can potentially identify critical components of the reentry circuit other than the exit site that might be within the reach of the ablation catheter. Unfortunately, most patients with heart disease have multiple VTs that are not hemodynamically tolerated; hence, entrainment mapping is typically combined with substrate mapping in patients with VT associated with scars and SHD.
Pace Mapping
Pace mapping is a technique used to locate the origin of a PVC or VT by pacing the myocardium to reproduce the clinical 12-lead morphology in the absence of VT; it is mostly helpful if the targeted arrhythmia is difficult to induce or is hemodynamically unstable. The optimal site should exactly match the surface VT QRS, including individual notches as well as major deflections. Electroanatomical maps can provide a percentage template match electronically, but this needs to be reviewed.
Pace mapping indicates the location of the origin of focal VAs [83] and should provide confirmation of results from activation mapping when site produces an ideal QRS match [84].
In scar-related VT, pace mapping is used to identify the exit site of the reentrant VT circuit [43]. Critical sites of the reentrant VT circuit and regions of slow conduction can be suggested based on pace mapping with a long stimulus to QRS interval and electrogram characteristics showing late activation in sinus rhythm.
Within scar, the spatial resolution of pace mapping is quite variable and can indicate a region of interest that measures up to 18 cm2 [74]. Even in patients without SHD, a perfect pace map can be observed at sites up to 2 cm away from the VT isthmus [85].
Sinus Rhythm Substrate Mapping
Many of the patients with scar-related VAs presenting for VT ablation have hemodynamically unstable VTs that prevent accurate definition of the critical component of the reentrant circuit with activation or entrainment mapping [29]. Substrate mapping is an approach to characterize areas likely to support reentry based on electrophysiological characteristics that can be determined during stable sinus or paced rhythm. These features to define scar include bipolar electrogram amplitude using a 4-mm tip mapping catheter where bipolar voltage abnormality is defined as electrogram amplitude <1.5 mV and dense scar defined as an amplitude <0.5 mV [86].
Bipolar voltage mapping has a limited field of view to detect epicardial or intramurally located scar, typically seen in patients with NICM. In these cases, unipolar voltage mapping can be used to extend the field of view of endocardial mapping. Abnormal RV endocardial unipolar free wall voltage is <5.5 mV, RV septal voltage is 7.0 mV and the RV septal cut-off in the posterior septal RVOT in front of the aortic root is 6.0 mV. The LV endocardial unipolar voltage cut-off for defining abnormal mid or epicardial scar is <8.3 mV [88,89,90,91,91].
Other surrogates of scar are presence of sinus rhythm or pacing of fragmented electrograms, late potentials, low-amplitude ventricular activity or LAVA [92]. Targeting these abnormal electrograms in the region of low voltage allows elimination of VT irrespective of inducibility or hemodynamic tolerance. Even for hemodynamically stable VTs, substrate mapping is often used to help focus activation or entrainment mapping to a region of interest [19, 93, 94].
End Points of Catheter Ablation of Ventricular Tachycardia
For focal arrhythmias in patients without SHD, assessment of efficacy is termination of VT, or elimination of PVCs, and subsequent noninducibility by catecholamine infusion or electrical stimulation that had reliably provoked episodes prior to ablation.
For patients with SHD in whom reentry is the primary VT mechanism, the effectiveness of ablation is typically assessed by repeating PES after the ablation procedure (typically through triple ventricular extrastimuli at 2 RV sites and 1–2 drive cycle lengths). Noninducibility of VT by PES after ablation is a reasonable end point for VT recurrence post VT ablation in these patients [96,97,97].
Due to the limitations of PES, end points other than non-inducibility have been described, including elimination of local excitability at the site of each ablation lesion, elimination of LPs or LAVA, substrate homogenization, and core isolation in which encircling lesions result in a central core of inexcitability [98, 99].
It is recommended to consider using noninvasive program stimulation (NIPS) 1–3 days after ablation to identify patients who, though noninducible right after the procedure, demonstrate an increased risk for recurrence because of demonstration of VT inducibility at NIPs. If clinical VT can be initiated at that time, repeat ablation should be considered [100].
References
Cronin EM, Bogun FM, Maury P, Peichl P, Chen M, Namboodiri N, et al. 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias. Heart Rhythm. 2019;pii: S1547-5271(19)30210-3. https://doi.org/10.1016/j.hrthm.2019.03.002.
Connolly SJ, Dorian P, Roberts RS, Gent M, Bailin S, Fain ES, et al. Comparison of beta-blockers, amiodarone plus beta-blockers, or sotalol for prevention of shocks from implantable cardioverter defibrillators: the OPTIC Study: a randomized trial. JAMA. 2006;295:165–71.
Moss AJ, Greenberg H, Case RB, Zareba W, Hall WJ, Brown MW, et al. Long-term clinical course of patients after termination of ventricular tachyarrhythmia by an implanted defibrillator. Circulation. 2004;110:3760–5.
Buxton AE, Waxman HL, Marchlinski FE, Simson MB, Cassidy D, Josephson ME. Right ventricular tachycardia: clinical and electrophysiologic characteristics. Circulation. 1983;68:917–27.
Lerman BB. Response of nonreentrant catecholamine-mediated ventricular tachycardia to endogenous adenosine and acetylcholine: evidence for myocardial receptor-mediated effects. Circulation. 1993;87:382–90.
Lerman BB, Belardinelli L, West GA, Berne RM, DiMarco JP. Adenosine-sensitive ventricular tachycardia: evidence suggesting cyclic AMP- mediated triggered activity. Circulation. 1986;74:270–80.
Tada H, Tadokoro K, Ito S, et al. Idiopathic ventricular arrhythmias originating from the tricuspid annulus: prevalence, electrocardiographic characteristics, and results of radiofrequency catheter ablation. Heart Rhythm. 2007;4:7–16.
Tada H, Tadokoro K, Miyaji K, et al. Idiopathic ventricular arrhythmias arising from the pulmonary artery: prevalence, characteristics, and topography of the arrhythmia origin. Heart Rhythm. 2008;5:419–26.
Sorgente A, Epicoco G, Ali H, et al. Negative concordance pattern in bipolar and unipolar recordings: an additional mapping criterion to localize the site of origin of focal ventricular arrhythmias. Heart Rhythm. 2016;13:519–26.
Tedrow UB, Stevenson WG. Recording and interpreting unipolar electrograms to guide catheter ablation. Heart Rhythm. 2011;8:791–6.
Yamada T, Yoshida Y, Inden Y, Murohara T, Kay GN. Idiopathic premature ventricular contractions exhibiting preferential conduction within the aortic root. Pacing Clin Electrophysiol. 2010;33:e10–3.
Antzelevitch C, Shimizu W. Cellular mechanisms underlying the long QT syndrome. Curr Opin Cardiol. 2002;17:43–51.
Haissaguerre M, Extramiana F, Hocini M, et al. Mapping and ablation of ventricular fibrillation associated with long-QT and Brugada syndromes. Circulation. 2003;108:925–8.
Lerman BB. Mechanism of outflow tract tachycardia. Heart Rhythm. 2007;4:973–6.
Damle RS, Landers M, Kelly PA, Reiter MJ, Mann DE. Radiofrequency catheter ablation of idiopathic left ventricular tachycardia originating in the left anterior fascicle. Pacing Clin Electrophysiol. 1998;21:1155–8.
Lopera G, Stevenson WG, Soejima K, et al. Identification and ablation of three types of ventricular tachycardia involving the His-Purkinje system in patients with heart disease. J Cardiovasc Electrophysiol. 2004;15:52–8.
Bogun F, Good E, Reich S, et al. Role of Purkinje fibers in post-infarction ventricular tachycardia. J Am Coll Cardiol. 2006;48:2500–7.
Spitzer KW, Pollard AE, Yang L, Zaniboni M, Cordeiro JM, Huelsing DJ. Cell-to-cell electrical interactions during early and late repolarization. J Cardiovasc Electrophysiol. 2006;17(Suppl. 1):S8–14.
Kumar S, Romero J, Mehta NK, et al. Long-term outcomes after catheter ablation of ventricular tachycardia in patients with and without structural heart disease. Heart Rhythm. 2016;13:1957–63.
El-Shalakany A, Hadjis T, Papageorgiou P, Monahan K, Epstein L, Josephson ME. Entrainment/mapping criteria for the prediction of termination of ventricular tachycardia by single radiofrequency lesion in patients with coronary artery disease. Circulation. 1999;99:2283–9.
Josephson ME, Horowitz LN, Farshidi A, Kastor JA. Recurrent sustained ventricular tachycardia mechanisms. Circulation. 1978;57:431–40.
Vaseghi M, Hu TY, Tung R, et al. Outcomes of catheter ablation of ven- tricular tachycardia based on etiology in nonischemic heart disease: an international Ventricular Tachycardia Ablation Center collaborative study. JACC Clin Electrophysiol. 2018;4:1141–50.
Marchlinski FE, Zado E, Dixit S, et al. Electroanatomic substrate and outcome of catheter ablative therapy for ventricular tachycardia in setting of right ventricular cardiomyopathy. Circulation. 2004;110:2293–8. Kumar S, Androulakis AF, Sellal JM, et al. Multicenter experience with catheter ablation for ventricular tachycardia in Lamin A/C cardiomyopathy. Circ Arrhythm Electrophysiol. 2016;9:e004357.
Kumar S, Barbhaiya C, Nagashima K, et al. Ventricular tachycardia in cardiac sarcoidosis: characterization of ventricular substrate and outcomes of catheter ablation. Circ Arrhythm Electrophysiol. 2015;8:87–93.
Sosa E, Scanavacca M, d’Avila A, Bellotti G, Pilleggi F. Radiofrequency catheter ablation of ventricular tachycardia guided by nonsurgical epicar- dial mapping in chronic Chagasic heart disease. Pacing Clin Electrophysiol. 1999;22:128–30.
Kapel GF, Sacher F, Dekkers OM, et al. Arrhythmogenic anatomical isthmuses identified by electroanatomical mapping are the substrate for ventricular tachycardia in repaired tetralogy of Fallot. Eur Heart J. 2017;38:268–76.
Eckart RE, Hruczkowski TW, Tedrow UB, Koplan BA, Epstein LM, Stevenson WG. Sustained ventricular tachycardia associated with correc- tive valve surgery. Circulation. 2007;116:2005–11.
Stevenson WG, Khan H, Sager P, et al. Identification of reentry circuit sites during catheter mapping and radiofrequency ablation of ventricular tachycardia late after myocardial infarction. Circulation. 1993;88:1647–70.
Stevenson WG, Friedman PL, Kocovic D, Sager PT, Saxon LA, Pavri B. Radiofrequency catheter ablation of ventricular tachycardia after myocardial infarction. Circulation. 1998;98:308–14.
Wilber DJ, Kopp DE, Glascock DN, Kinder CA, Kall JG. Catheter ablation of the mitral isthmus for ventricular tachycardia associated with infe- rior infarction. Circulation. 1995;92:3481–9.
Nagashima K, Tedrow UB, Koplan BA, et al. Reentrant ventricular tachy- cardia originating from the periaortic region in the absence of overt structural heart disease. Circ Arrhythm Electrophysiol. 2014;7:99–106.
Glashan CA, Androulakis AFA, Tao Q, et al. Whole human heart histology to validate electroanatomical voltage mapping in patients with non- ischaemic cardiomyopathy and ventricular tachycardia. Eur Heart J. 2018;39:2867–75.
Pogwizd SM, Hoyt RH, Saffitz JE, Corr PB, Cox JL, Cain ME. Reentrant and focal mechanisms underlying ventricular tachycardia in the human heart. Circulation. 1992;86:1872–87.
Anter E, Kleber AG, Rottmann M, et al. Infarct-related ventricular tachycardia: redefining the electrophysiological substrate of the isthmus during sinus rhythm. JACC Clin Electrophysiol. 2018;4:1033–48.
Dillon SM, Allessie MA, Ursell PC, Wit AL. Influences of anisotropic tissue structure on reentrant circuits in the epicardial border zone of subacute canine infarcts. Circ Res. 1988;63:182–206.
Downar E, Kimber S, Harris L, et al. Endocardial mapping of ventricular tachycardia in the intact human heart. II. Evidence for multiuse reentry in a functional sheet of surviving myocardium. J Am Coll Cardiol. 1992;20:869–78.
de Bakker JM, van Capelle FJ, Janse MJ, et al. Reentry as a cause of ventricular tachycardia in patients with chronic ischemic heart disease: electrophysiologic and anatomic correlation. Circulation. 1988;77:589–606.
Baba S, Dun W, Cabo C, Boyden PA. Remodeling in cells from different regions of the reentrant circuit during ventricular tachycardia. Circulation. 2005;112:2386–96.
Peters NS, Wit AL. Myocardial architecture and ventricular arrhythmogenesis. Circulation. 1998;97:1746–54.
de Bakker JM, van Capelle FJ, Janse MJ, et al. Slow conduction in the infarcted human heart. ‘Zigzag’ course of activation. Circulation. 1993;88:915–26.
de Bakker JM, Stein M, van Rijen HV. Three-dimensional anatomic structure as substrate for ventricular tachycardia/ventricular fibrillation. Heart Rhythm. 2005;2:777–9.
Raiman M, Tung R. Automated isochronal late activation mapping to identify deceleration zones: rationale and methodology of a practical electroanatomic mapping approach for ventricular tachycardia ablation. Comput Biol Med. 2018;102:336–40.
Bogun F, Good E, Reich S, et al. Isolated potentials during sinus rhythm and pace-mapping within scars as guides for ablation of post-infarction ventricular tachycardia. J Am Coll Cardiol. 2006;47:2013–9.
Cheniti G, Vlachos K, Meo M, et al. Mapping and ablation of idiopathic ventricular fibrillation. Front Cardiovasc Med. 2018;5:123.
Kleber AG, Rudy Y. Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol Rev. 2004;84:431–88.
Caceres J, Jazayeri M, McKinnie J, et al. Sustained bundle branch reentry as a mechanism of clinical tachycardia. Circulation. 1989;79:256–70.
Crijns HJ, Smeets JL, Rodriguez LM, Meijer A, Wellens HJ. Cure of in- terfascicular reentrant ventricular tachycardia by ablation of the anterior fascicle of the left bundle branch. J Cardiovasc Electrophysiol. 1995;6:486–92.
Huang J, Dosdall DJ, Cheng KA, Li L, Rogers JM, Ideker RE. The importance of Purkinje activation in long duration ventricular fibrillation. J Am Heart Assoc. 2014;3:e000495.
Jeyaratnam J, Umapathy K, Masse S, et al. Relating spatial heterogeneities to rotor formation in studying human ventricular fibrillation. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:231–4.
Caceres J, Jazayeri M, McKinnie J, Avitall B, Denker ST, Tchou P, et al. Sustained bundle branch reentry as a mechanism of clinical tachycardia. Circulation. 1989;79:256–70.
Nakagawa H, Beckman KJ, McClelland JH, Wang X, Arruda M, Santoro I, et al. Radiofrequency catheter abla- tion of idiopathic left ventricular tachycardia guided by a Purkinje potential. Circulation. 88:2607–17.
Della Bella P, Baratto F, Tsiachris D, et al. Management of ventricular tachycardia in the setting of a dedicated unit for the treatment of complex ventricular arrhythmias: long-term outcome after ablation. Circulation. 2013;127:1359–68.
Santangeli P, Muser D, Zado ES, et al. Acute hemodynamic decompensation during catheter ablation of scar-related ventricular tachycardia: inci- dence, predictors, and impact on mortality. Circ Arrhythm Electrophysiol. 2015;8:68–75.
Santangeli P, Frankel DS, Tung R, et al. Early mortality after catheter ablation of ventricular tachycardia in patients with structural heart disease. J Am Coll Cardiol. 2017;69:2105–15.
Tzou WS, Tung R, Frankel DS, et al. Ventricular tachycardia ablation in severe heart failure: an international ventricular tachycardia ablation center collaboration analysis. Circ Arrhythm Electrophysiol. 2017;10:e004494.
Enriquez A, Liang J, Gentile J, et al. Outcomes of rescue cardiopulmonary support for periprocedural acute hemodynamic decompensation in patients undergoing catheter ablation of electrical storm. Heart Rhythm. 2018;15:75–80.
Mathuria N, Wu G, Rojas-Delgado F, et al. Outcomes of pre-emptive and rescue use of percutaneous left ventricular assist device in patients with structural heart disease undergoing catheter ablation of ventricular tachycardia. J Interv Card Electrophysiol. 2017;48:27–34.
Muser D, Liang JJ, Castro SA, et al. Outcomes with prophylactic use of percutaneous left ventricular assist devices in high-risk patients undergoing catheter ablation of scar-related ventricular tachycardia: a propensity-score matched analysis. Heart Rhythm. 2018;15:1500–6.
Dixit S, Gerstenfeld EP, Callans DJ, Marchlinski FE. Electrocardiographic patterns of superior right ventricular outflow tract tachycardias: distinguishing septal and free-wall sites of origin. J Cardiovasc Electrophysiol. 2003;14:1–7.
Tanner H, Hindricks G, Schirdewahn P, et al. Outflow tract tachycardia with R/S transition in lead V3: six different anatomic approaches for successful ablation. J Am Coll Cardiol. 2005;45:418–23.
Ouyang F, Fotuhi P, Ho SY, et al. Repetitive monomorphic ventricular tachycardia originating from the aortic sinus cusp: electrocardiographic characterization for guiding catheter ablation. J Am Coll Cardiol. 2002;39:500–8.
Yamada T, Yoshida N, Murakami Y, et al. Electrocardiographic characteristics of ventricular arrhythmias originating from the junction of the left and right coronary sinuses of Valsalva in the aorta: the activation pattern as a rationale for the electrocardiographic characteristics. Heart Rhythm. 2008;5:184–92.
Bala R, Garcia FC, Hutchinson MD, Gerstenfeld EP, et al. Electrocardiographic and electrophysiologic features of ventricular arrhythmias originating from the right/left coronary cusp commissure. Heart Rhythm. 2010;7(3):312–22.
Good E, Desjardins B, Jongnarangsin K, et al. Ventricular arrhythmias originating from a papillary muscle in patients without prior infarction: a comparison with fascicular arrhythmias. Heart Rhythm. 2008;5:1530–7.
Enriquez A, Supple GE, Marchlinski FE, Garcia FC. How to map and ablate papillary muscle ventricular arrhythmias. Heart Rhythm. 2017;14:1721–8.
Enriquez A, Pathak RK, Santangeli P, et al. Inferior lead discordance in ventricular arrhythmias: a specific marker for certain arrhythmia locations. J Cardiovasc Electrophysiol. 2017;28:1179–86.
Yamada T, McElderry HT, Doppalapudi H, et al. Idiopathic ventricular arrhythmias originating from the left ventricular summit: anatomic concepts relevant to ablation. Circ Arrhythm Electrophysiol. 2010;3:616–23.
Enriquez A, Malavassi F, Saenz LC, et al. How to map and ablate left ventricular summit arrhythmias. Heart Rhythm. 2017;14:141–8.
Miller JM, Marchlinski FE, Buxton AE, Josephson ME. Relationship between the 12-lead electrocardiogram during ventricular tachycardia and endocardial site of origin in patients with coronary artery disease. Circulation. 1988;77:759–66.
Kamakura S, Shimizu W, Matsuo K, et al. Localization of optimal ablation site of idiopathic ventricular tachycardia from right and left ventricular outflow tract by body surface ECG. Circulation. 1998;98:1525–33.
Betensky BP, Park RE, Marchlinski FE, et al. The V(2) transition ratio: a new electrocardiographic criterion for distinguishing left from right ven- tricular outflow tract tachycardia origin. J Am Coll Cardiol. 2011;57:2255–62.
Kumagai K. Idiopathic ventricular arrhythmias arising from the left ven- tricular outflow tract: tips and tricks. J Arrhythmia. 2014;30:211–21. Larroussi L, Badhwar N. Ventricular tachycardia arising from cardiac crux: electrocardiogram recognition and site of ablation. Card Electrophysiol Clin. 2016;8:109–13.
Oloriz T, Silberbauer J, Maccabelli G, et al. Catheter ablation of ventricular arrhythmia in nonischemic cardiomyopathy: anteroseptal versus infero- lateral scar sub-types. Circ Arrhythm Electrophysiol. 2014;7:414–23.
Yoshida K, Liu TY, Scott C, et al. The value of defibrillator electrograms for recognition of clinical ventricular tachycardias and for pace mapping of post-infarction ventricular tachycardia. J Am Coll Cardiol. 2010;56:969–79.
Berruezo A, Mont L, Nava S, Chueca E, Bartholomay E, Brugada J. Electrocardiographic recognition of the epicardial origin of ventricular tachycardias. Circulation. 2004;109:1842–7.
Valles E, Bazan V, Marchlinski FE. ECG criteria to identify epicardial ventricular tachycardia in nonischemic cardiomyopathy. Circ Arrhythm Electrophysiol. 2010;3:63–71.
Berte B, Relan J, Sacher F, et al. Impact of electrode type on mapping of scar-related VT. J Cardiovasc Electrophysiol. 2015;26:1213–23.
Anter E, Tschabrunn CM, Buxton AE, Josephson ME. High-resolution mapping of postinfarction reentrant ventricular tachycardia: electrophysi- ological characterization of the circuit. Circulation. 2016;134:314–27.
Josephson ME. Recurrent ventricular tachycardia, clinical cardiac electrophysiology: techniques and interpretations. 5th ed. Philadelphia: Wolters Kluwer; 2015.
Josephson ME, Anter E. Substrate mapping for ventricular tachycardia: assumptions and misconceptions. JACC Clin Electrophysiol. 2015;1:341–52.
Stevenson WG, Friedman PL, Sager PT, et al. Exploring postinfarction reentrant ventricular tachycardia with entrainment mapping. J Am Coll Cardiol. 1997;29:1180–9.
Bogun F, Kim HM, Han J, et al. Comparison of mapping criteria for hemodynamically tolerated, postinfarction ventricular tachycardia. Heart Rhythm. 2006;3:20–6.
Calkins H, Kalbfleisch SJ, El-atassi R, Langberg JJ, Morady F. Relation between efficacy of radiofrequency catheter ablation and site of origin of idiopathic ventricular tachycardia. Am J Cardiol. 1993;71:827–33.
Bogun F, Taj M, Ting M, et al. Spatial resolution of pace mapping of idiopathic ventricular tachycardia/ectopy originating in the right ventricular outflow tract. Heart Rhythm. 2008;5:339–44.
Azegami K, Wilber DJ, Arruda M, Lin AC, Denman RA. Spatial resolution of pacemapping and activation mapping in patients with idiopathic right ventricular outflow tract tachycardia. J Cardiovasc Electrophysiol. 2005;16:823–9.
Soejima K, Stevenson WG, Maisel WH, Sapp JL, Epstein LM. Electrically unexcitable scar mapping based on pacing threshold for identification of the reentry circuit isthmus: feasibility for guiding ventricular tachycardia ablation. Circulation. 2002;106:1678–83.
Hutchinson MD, Gerstenfeld EP, Desjardins B, et al. Endocardial unipolar voltage mapping to detect epicardial ventricular tachycardia substrate in patients with nonischemic left ventricular cardiomyopathy. Circ Arrhythm Electrophysiol. 2011;4:49–55.
Polin GM, Haqqani H, Tzou W, et al. Endocardial unipolar voltage mapping to identify epicardial substrate in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Heart Rhythm. 2011;8:76–83.
Chopra N, Tokuda M, Ng J, et al. Relation of the unipolar low-voltage penumbra surrounding the endocardial low-voltage scar to ventricular tachy- cardia circuit sites and ablation outcomes in ischemic cardiomyopathy. J Cardiovasc Electrophysiol. 2014;25:602–8.
Soto-Becerra R, Bazan V, Bautista W, et al. Ventricular tachycardia in the setting of Chagasic cardiomyopathy. Circ Arrhythm Electrophysiol. 2017;10:e004950.
Kelesidis I, Desjardins B, Muser D, Santangeli P, Zado ES, Marchlinski FE. Unipolar voltage mapping criteria for right ventricular septum: influence of the aortic root. J Cardiovasc Electrophysiol. 2018;29(8):1113–8.
Sacher F, Lim HS, Derval N, Denis A, Berte B, Yamashita S, et al. Substrate mapping and ablation for ventricular tachycardia: the LAVA approach. J Cardiovasc Electrophysiol. 2015;26(4):464–71.
Callans DJ, Ren JF, Michele J, Marchlinski FE, Dillon SM. Electroanatomic left ventricular mapping in the porcine model of healed anterior myocardial infarction: correlation with intracardiac echocardiography and pathological analysis. Circulation. 1999;100:1744–50.
Volkmer M, Ouyang F, Deger F, et al. Substrate mapping vs. tachycardia mapping using CARTO in patients with coronary artery disease and ventricular tachycardia: impact on outcome of catheter ablation. Europace. 2006;8:968–76.
Horowitz LN, Josephson ME, Farshidi A, Spielman SR, Michelson EL, Greenspan AM. Recurrent sustained ventricular tachycardia 3. Role of the electrophysiologic study in selection of antiarrhythmic regimens. Circulation. 1978;58:986–97.
Ghanbar H, Baser K, Yokokawa M, et al. Noninducibility in postinfarction ventricular tachycardia as an end point for ventricular tachycardia ablation and its effects on outcomes: a meta-analysis. Circ Arrhythm Electrophysiol. 2014;7:677–83.
Essebag V, Joza J, Nery PB, et al. Prognostic value of noninducibility on outcomes of ventricular tachycardia ablation: a VANISH substudy. JACC Clin Electrophysiol. 2018;4:911–9.
Santangeli P, Frankel DS, Marchlinski FE. End points for ablation of scar-related ventricular tachycardia. Circ Arrhythm Electrophysiol. 2014;7:949–60.
Santangeli P, Marchlinski FE. Substrate mapping for unstable ventricular tachycardia. Heart Rhythm. 2016;13:569–83.
Frankel DS, Mountantonakis SE, Zado ES, et al. Noninvasive programmed ventricular stimulation early after ventricular tachycardia ablation to predict risk of late recurrence. J Am Coll Cardiol. 2012;59:1529–35.
Haqqani HM, Callans DJ. Ablation of ventricular tachycardia in coronary artery disease. In: Huang SKS, Wood MA, editors. Catheter ablation of cardiac arrthythmias. 2nd ed. Philadelphia: Elsevier Saunders; 2011.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Al-Rawahi, M., Marchlinski, F.E. (2020). Ventricular Tachycardia: Catheter Ablation. In: Yan, GX., Kowey, P., Antzelevitch, C. (eds) Management of Cardiac Arrhythmias. Contemporary Cardiology. Humana, Cham. https://doi.org/10.1007/978-3-030-41967-7_19
Download citation
DOI: https://doi.org/10.1007/978-3-030-41967-7_19
Published:
Publisher Name: Humana, Cham
Print ISBN: 978-3-030-41966-0
Online ISBN: 978-3-030-41967-7
eBook Packages: MedicineMedicine (R0)