Abstract
Introduction
Complex chromosomal rearrangements (CCRs) involve two or more chromosomes and at least three breakpoints. Due to their complexity, they are associated with a high number of unbalanced gametes, whose fertilization is often incompatible with viable fetal development. Preimplantation genetic diagnosis (PGD) is usually offered to those patients and typically shows modest results considering the high number of unbalanced embryos. We previously showed that a sperm selection process using the hypo-osmotic swelling test (HOST) allows for an 83% reduction in the proportion of unbalanced spermatozoa (US) in male rearrangements carriers. This is the first report of the use of this procedure in a CCR carrier.
Case description
We report on the case of a 36-year-old male t(4;7;14)(q12;p21;q11.2) carrier who presented to our center for infertility. Sperm fluorescent in situ hybridization showed an 88% proportion of unbalanced spermatozoa. After hypo-osmotic incubation and selection of spermatozoa with a specific flagellar conformation, the proportion of unbalanced spermatozoa dropped to 15%.
Discussion
In the present case, we show that it is possible to select chromosomally balanced prior to in vitro fertilization in male CCR carriers. This technique has the potential of increasing the proportion of euploid embryos and therefore the chances of healthy pregnancy and birth.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The most common form of chromosomal rearrangement in humans, with a prevalence of approximately 1/500 in the general population, is balanced chromosomal rearrangements [1]. Two types are observed: reciprocal translocations and Robertsonian translocations.
Complex chromosomal rearrangements (CCRs) are balanced chromosomal structural aberrations that involve two or more chromosomes and at least three breakpoints [2]. This abnormality generally has no influence on the patient’s phenotype when it is balanced [3]. However, they exhibit a higher risk of reproductive failure, with an increased risk of spontaneous abortions, fetal malformations, and infertility. Indeed, the proportion of unbalanced gametes is typically high in complex translocation carriers. It has been reported that the chances of balanced or normal embryos in couples with a CCR carrier are < 6% [4].
CCRs are classified according to the total number of breakpoints and their locations on the chromosomes [5]. Indeed, they can be divided into three distinct categories. The first is called “three-way exchange” and involves three chromosomes with each undergoing a break and an exchange of the distal segments. The second is called “exceptional CCR” and involves more than one breakpoints per chromosome. Finally, “double bidirectional translocations” involve two or three independent translocations in the same carrier.
As in more common chromosomal rearrangements, the only mode of segregation leading to the formation of balanced gametes is the alternate mode and is associated with normal fetal development. All other modes can lead to spontaneous abortions or fetal malformations. The segregation modes can theoretically be of type 3:3, 4:2, 5:1, and 6:0 leading to 64 possibilities. The 3:3 mode includes 20 different possibilities, but only two of them lead to the formation of balanced gametes [1].
An assessment of the proportion of chromosomally balanced and unbalanced spermatozoa is an indispensable part of genetic counseling. It allows the couples to choose to have a child either by natural pregnancy with prenatal diagnosis or by in vitro fertilization with preimplantation genetic diagnosis. However, the chances of producing a normal or balanced embryo for CCR carriers are low.
Our team has recently shown that it was possible to select for balanced spermatozoa in balanced chromosomal rearrangement carriers [6], as well as in double Robertsonian translocation carriers [7]. A study of sperm morphology after incubation in a hypo-osmotic medium (HOST) allows an average reduction in the proportion of sperm carrying unbalanced chromosomal content of 83%.
Here, we present a sperm segregation study performed in a male carrier of a 46,XY,t(4;7;14)(q12;p21;q11.2) balanced complex translocation and discuss the value of HOST-based sperm selection in increasing the proportion of chromosomally balanced gametes (and therefore decrease the proportion of aneuploid spermatozoa).
Case presentation
We present the case of a 36-year-old male patient who initially presented with primary infertility and type 2 narcolepsy (without cataplexy). Routine sperm analysis did not show any abnormalities.
His 38-year-old partner had a normal infertility work-up. Routine semen analysis showed teratozoospermia (concentration: 40 million/ml, volume: 1.9 ml, vitality: 66%, progressive motility 35%, sperm morphology: 1% normal). One spontaneous miscarriage occurred in 2005. The couple elected for intra-uterine insemination twice in 2012 and 2013. The first one performed with the partner’s sperm was unsuccessful, while the second performed with donor sperm led to the birth of twin girls.
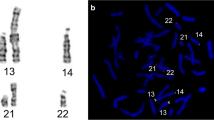
Informed consent was obtained from the patient. Karyotype on blood lymphocytes revealed a balanced complex translocation of the “three-way” type: 46,XY,t(4;7;14)(q12;p21;q11.2) (Fig. 1). CGH-array confirmed that the rearrangement was balanced (Fig. 2).
The patient’s semen sample was obtained by masturbation after 2 to 7 days of abstinence. The semen sample was divided into three parts: native, discontinuous gradient centrifugation (DGC), and DGC + HOST (Fig. 3a). The native sample was washed in phosphate buffered saline (PBS) and then fixed on slides. The other two fractions underwent discontinuous gradient centrifugation (DGC) as described previously (Rouen A, et al. 2019). The DGC-only fraction part was fixed. The DGC + HOST sample was incubated in a hypo-osmotic solution of sodium citrate and fructose (450 mOsm, 75 mM fructose, 25 mM sodium citrate). This process, called HOST (hypo-osmotic swelling test), makes it possible to distinguish category B + spermatozoa which have been shown to contain significantly fewer chromosomal imbalances in carriers of chromosomal rearrangements (Fig. 3b) [6].
A The sperm sample is divided into 3 parts. The first part (native) was washed with PBS before being fixed. The second (DGC) underwent discontinuous gradient centrifugation before being fixed. The third part (DGC + HOST) also underwent discontinuous gradient centrifugation then was incubated in a hypo-osmotic solution (HOST) for 10 min before being fixed. B After incubation in the HOST solution, the flagella adopt different conformations allowing them to be classified into 7 categories. C B + spermatozoa can be identified by the presence of a small loop at the tip of the flagellum with an L/D ratio > 20, with L being the length of the flagellum and D the diameter of the loop [6]
FISH was performed as previously described [7]. In-house contiguous probes and commercial probes were selected to allow for a non-ambiguous analysis of chromosomal content in sperm nuclei (Fig. 4).
A A set of probes was used to study chromosomal segregation in the patient’s spermatozoa. In order to obtain a non-ambiguous set of signal combinations, allowing for the identification of balanced and unbalanced modes, we used the following probes: 4q red and 4q green (resulting in a yellow-colored signal), 4 centromeric blue, 7p22 red, 7q red, and 14q 32 green. B FISH on blood lymphocyte chromosomes. From this, we assessed the specificity of the probes that were used on spermatozoa
We used the following probes (Fig. 4A): Vysis 4q red and contiguous 4q green (this combination gives a yellow signal), Rainbow 7p22 red, Vysis 7q red, rainbow 14q 32 green, vysis 4 centromeric blue. The specificity of the probes was first confirmed by performing FISH on the patient’s blood chromosomes (Fig. 4B).
This type of complex translocation can potentially lead to 64 different chromosomal combinations. Each combination of signals was specific for one or more chromosome segregation modes. However, probe locations on the chromosomes allowed for balanced spermatozoa to be associated with a specific signal combination (blue, green, red, red, yellow) (Table 1). Three hundred spermatozoa were analyzed for the first and second fractions, and 20 HOST B + sperm were analyzed.
In the native sample, 88% of spermatozoa were unbalanced. After DGC, 85% of spermatozoa were unbalanced (p = 0.77). After HOST-based sperm selection, 15% of spermatozoa were unbalanced (p = 0.002).
Discussion
Advances in cytogenetics as well as in reproductive medicine have improved the detection of CCR. Over 160 cases of men with CCR have been reported in scientific publications [8]. The majority of these men exhibit an abnormal spermatogenesis resulting in infertility [9]. About 75% of these anomalies appear de novo or are inherited from the mother [5, 8]. CCR arise as a result of replication or recombination errors that lead to the accumulation of DNA defects. Additionally, some germline CCR can be caused by a chromothripsis event during spermatogenesis [10, 11].
Sperm FISH techniques make it possible to directly study the meiotic chromosomal segregation of those complex rearrangement. Several reports of sperm FISH studies on complex chromosomal rearrangements involving three different chromosomes have been published. The first to our knowledge was performed on the carrier of a t(2;11;22) complex translocation [12]. Of the 208 spermatozoa analyzed, the proportion of chromosomally balanced spermatozoa was 13.5%. Another meiotic segregation analysis of a balanced t(5;13;14)(q23;q21;q31) CCR showed a proportion of 27% balanced spermatozoa [5]. Loup et al. performed an original analysis of a CCR t(1;19;13) carrier, using a combination of combining FISH and PRINS techniques, allowing for a larger number of fluorochromes to be used simultaneously on the same sperm preparation [13]. Overall, those reports highlight the paucity of balanced spermatozoa in CCR carriers. In addition, the proportion of those balanced gametes seems to differ from one CCR carrier to another, as is also the case in reciprocal and Robertsonian translocations [6]. Segregation analysis, on a given chromosomal rearrangement carrier, is indispensable to provide appropriate genetic counseling.
The strategy we describe here allowed us to establish the proportion of unbalanced and balanced spermatozoa for this patient (Tables 1, 2, and 3). We were able to study the segregation of this complex translocation involving three chromosomes in a single hybridization step, with four different signals (Fig. 4). The balanced segregation mode was associated with a specific combination (blue, green, red, red, yellow), while it was not possible to distinguish between balanced carrier and balanced non-carrier spermatozoa.
In the native sample, we observed a proportion of 88% unbalanced spermatozoa. After DGC, we observed a proportion of 85% unbalanced spermatozoa. DGC is a technique routinely used in assisted reproduction laboratories for sperm preparation. We showed in a previous study that it allowed for a modest but significant decrease in the proportion of unbalanced spermatozoa [14]. In this study, we observed a non-significant decrease of unbalanced spermatozoa after DGC.
HOST has been used in assisted reproduction laboratories to select viable spermatozoa [15, 16]. Spermatozoa react to incubation in a hypo-osmotic solution, causing the flagella to adopt different shapes. Spermatozoa are categorized based on their flagellar conformation.
According to our previous study, the selection of spermatozoa called “B + ” would allow for a mean 84% decrease of unbalanced spermatozoa [6]. The L/D (length of the flagellum over the diameter of the loop) ratio was evaluated over several thousand spermatozoa in that study and was empirically found to be over 20 in B + spermatozoa. HOST-based sperm selection has previously been demonstrated by our team to be of interest on patients with different cases of structural abnormalities [7]. In the present case, we observed a proportion of unbalanced spermatozoa of 15% after selection of B + . With HOST-based sperm selection, we observed a mean 83% decrease of unbalanced spermatozoa.
The mechanism through which HOST allows for the selection of balanced spermatozoa remains unclear. It has been shown that there is an association between HOST morphology and different sperm quality parameters [17,18,19,20,21]. We showed that in chromosomal rearrangement carriers, spermatic nuclear architecture was altered [22]. It is likely that this hinder normal spermatic nuclear condensation, which leads to larger spermatic nuclei among unbalanced spermatozoa [23]. This could act as a trigger for an apoptosis process, leading to an alteration of the membrane, and in turn to changes in the reaction to changes of osmolarity.
Preimplantation genetic diagnosis (PGD) is routinely offered to couples with chromosomal rearrangements in order discard unbalanced embryos. Only a few cases of PGD with a CCR carrier are reported. Pellestor et al. reviewed the PGD cycles in 6 patients with CCR. Among the 129 genetically analyzed embryos, only 9 were balanced [5]. Frumkin et al. estimated that the probability of balanced embryos for carriers of CCR is about 9.25%, compared to 20–30% chance of balanced embryos in case of translocation reciprocal between two chromosomes [24]. One of the limiting factors is therefore the number of retrieved oocytes, since most of them will be fertilized with an unbalanced spermatozoon.
Along with previous publications on the subject, this case reports suggests that HOST-based sperm selection can be offered to patients with rare and complex chromosomal rearrangements, associated with a high proportion of unbalanced gametes, to potentially improve reproductive outcome.
Data availability
The data that support the findings of this study are available on request from the corresponding author, Alexandre ROUEN.
References
Gardner RJMK, Sutherland GR, Shaffer LG. Chromosome abnormalities and genetic counseling. Oxford University Press, USA; 2011.
Patsalis PC. Complex chromosomal rearrangements. Genet Couns. 2007;18:57–69.
Madan K. Balanced complex chromosome rearrangements: reproductive aspects. A review. Am J Med Genet A. 2012;158A:947–63.
Hu L, Wei Y, Luo K, Xie P, Gong F, Xiong B, et al. Clinical outcomes in carriers of complex chromosomal rearrangements: a retrospective analysis of comprehensive chromosome screening results in seven cases. Fertil Steril. 2018;109:486–92.
Pellestor F, Anahory T, Lefort G, Puechberty J, Liehr T, Hédon B, et al. Complex chromosomal rearrangements: origin and meiotic behavior. Hum Reprod Update. 2011;17:476–94.
Rouen A, Carlier L, Heide S, Egloff M, Marzin P, Ader F, et al. Potential selection of genetically balanced spermatozoa based on the hypo-osmotic swelling test in chromosomal rearrangement carriers. Reprod Biomed Online. 2017;35:372–8.
Pierron L, Irrmann A, de Chalus A, Bloch A, Heide S, Rogers E, et al. Double chromosomal translocation in an infertile man: one-step FISH meiotic segregation analysis and reproductive prognosis. J Assist Reprod Genet. 2019;36:973–8.
Olszewska M, Stokowy T, Pollock N, Huleyuk N, Georgiadis A, Yatsenko S, et al. Familial infertility (azoospermia and cryptozoospermia) in two brothers-carriers of t(1;7) complex chromosomal rearrangement (CCR): molecular cytogenetic analysis. Int J Mol Sci. 2020;21:E4559.
Salahshourifar I, Shahrokhshahi N, Tavakolzadeh T, Beheshti Z, Gourabi H. Complex chromosomal rearrangement involving chromosomes 1, 4 and 22 in an infertile male: case report and literature review. J Appl Genet. 2009;50:69–72.
Cretu Stancu M, van Roosmalen MJ, Renkens I, Nieboer MM, Middelkamp S, de Ligt J, et al. Mapping and phasing of structural variation in patient genomes using nanopore sequencing. Nat Commun. 2017;8:1326.
Fukami M, Shima H, Suzuki E, Ogata T, Matsubara K, Kamimaki T. Catastrophic cellular events leading to complex chromosomal rearrangements in the germline. Clin Genet. 2017;91:653–60.
Cifuentes P, Navarro J, Míguez L, Egozcue J, Benet J. Sperm segregation analysis of a complex chromosome rearrangement, 2;22;11, by whole chromosome painting. Cytogenet Cell Genet. 1998;82:204–9.
Loup V, Bernicot I, Janssens P, Hedon B, Hamamah S, Pellestor F, et al. Combined FISH and PRINS sperm analysis of complex chromosome rearrangement t(1;19;13): an approach facilitating PGD. Mol Hum Reprod. 2010;16:111–6.
Rouen A, Balet R, Dorna M, Hyon C, Pollet-Villard X, Chantot-Bastaraud S, et al. Discontinuous gradient centrifugation (DGC) decreases the proportion of chromosomally unbalanced spermatozoa in chromosomal rearrangement carriers. Hum Reprod. 2013;28:2003–9.
Jeyendran RS, Van der Ven HH, Perez-Pelaez M, Crabo BG, Zaneveld LJ. Development of an assay to assess the functional integrity of the human sperm membrane and its relationship to other semen characteristics. J Reprod Fertil. 1984;70:219–28.
Boitrelle F, Shah R, Saleh R, Henkel R, Kandil H, Chung E, et al. The sixth edition of the WHO manual for human semen analysis: a critical review and SWOT analysis. Life (Basel). 2021;11:1368.
F B, M T, Ah S, S M, Mh N-E. Is there an association between HOST grades and sperm quality? Human reproduction (Oxford, England) [Internet]. Hum Reprod. 2012 [cited 2022 Jul 6];27. Available from: https://pubmed.ncbi.nlm.nih.gov/22595979/
Stanger JD, Vo L, Yovich JL, Almahbobi G. Hypo-osmotic swelling test identifies individual spermatozoa with minimal DNA fragmentation. Reprod Biomed Online. 2010;21:474–84.
Bloch A, Rogers EJ, Nicolas C, Martin-Denavit T, Monteiro M, Thomas D, et al. Detailed cell-level analysis of sperm nuclear quality among the different hypo-osmotic swelling test (HOST) classes. J Assist Reprod Genet. 2021;38:2491–9.
Charehjooy N, Najafi MH, Tavalaee M, Deemeh MR, Azadi L, Shiravi AH, et al. Selection of sperm based on hypo-osmotic swelling may improve ICSI outcome: a preliminary prospective clinical trial. Int J Fertil Steril. 2014;8:21–8.
Pang M-G, You Y-A, Park Y-J, Oh S-A, Kim D-S, Kim Y-J. Numerical chromosome abnormalities are associated with sperm tail swelling patterns. Fertil Steril. 2010;94:1012–20.
Mebrek ML, Clède S, de Chalus A, Heide S, Ruoso L, Rogers E, et al. Simple FISH-based evaluation of spermatic nuclear architecture shows an abnormal chromosomal organization in balanced chromosomal rearrangement carriers. J Assist Reprod Genet. 2020;37:803–9.
Rouen A, Lavillaureix A, Hyon C, Heide S, Clède S, Balet R, et al. Nuclear volume differences between balanced and unbalanced spermatozoa in chromosomal translocation carriers. Reprod Biomed Online. 2015;30:290–5.
Frumkin T, Peleg S, Gold V, Reches A, Asaf S, Azem F, et al. Complex chromosomal rearrangement-a lesson learned from PGS. J Assist Reprod Genet. 2017;34:1095–100.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rossi, C., Siffroi, JP., Ruosso, L. et al. Chromosomal segregation analysis and HOST-based sperm selection in a complex reciprocal translocation carrier. J Assist Reprod Genet 40, 33–40 (2023). https://doi.org/10.1007/s10815-022-02665-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-022-02665-z