Abstract
Background
The prevalence of chromosomal translocations is 1/500 in the general population. While in the vast majority of cases, carriers have a normal phenotype; they can present with difficulty conceiving due to the presence of a proportion of unbalanced gametes as a consequence of abnormal chromosomal segregation during meiosis. Since complex translocations involve three or more chromosomes, meiotic segregation leads to a greater number of possible combinations which effectively complicate both their study and therapeutic care.
Case presentation
We report on the case of a male carrier of a complex homogeneous double Robertsonian translocation: 44, XY, der(13;14)(q10;q10),der(21;22)(q10;q10). We studied his meiotic segregation by FISH on spermatozoa from the initial sample, as well as following discontinuous gradient centrifugation and after incubation in an hypo-osmotic solution.
Conclusion
We report a method to study in a simple single-step manner the meiotic segregation of double Robertsonian translocations in spermatozoa. Further, our results suggest that reproductive prognosis of affected individuals may be markedly improved by HOST-based sperm selection (HBSS).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Balanced chromosomal rearrangements have a prevalence of about 1/500 in the general population and are the most common form of chromosomal rearrangement in humans. Two types are observed: Robertsonian translocations which correspond to the centromeric fusion of two acrocentric chromosomes (chromosomes 13, 14, 15, 21, 22) and reciprocal translocations which correspond to an exchange of chromosomal segments between two non-homologous chromosomes. They are usually associated with a normal phenotype, except for the existence of chromosomally unbalanced gametes which are related to abnormal meiotic events. Indeed, meiosis can be achieved only after homologous chromosome pairing, an obligatory prerequisite to chromosome segregation and to the formation of chromosomally balanced gametes. Therefore, in the case of translocated chromosomes, meiotic pairing of homologous chromosomal segments leads to the formation of particular chromosome associations, named trivalents in Robersonian translocations and quadrivalents in reciprocal ones, whose segregation can adopt different modes. One of these modes, termed the “alternate” mode, leads to the formation of balanced gametes and is associated with a normal fetal development. All the other modes (adjacent 1, adjacent 2, 3:0, and 3:1) lead to the formation of chromosomally unbalanced gametes, which in turn may lead to spontaneous abortions or fetal malformations [1].
Evaluating the proportion of chromosomally unbalanced spermatozoa is an important element of genetic counseling in translocation carriers since it allows affected couples to choose to have a child either by natural pregnancy with a prenatal diagnosis or by in vitro fertilization with a pre-implantation genetic diagnosis (PGD). Generally, the proportion of unbalanced gametes in Robertsonian translocations is 10 to 49% according to a recent review of the literature by Lemotte et al. [2]. Such an evaluation is particularly important in rare individuals who carry two different translocations. We present here a sperm segregation study performed in a man with a complex double Robertsonian translocation and discuss the interest of two different techniques that we described previously in improving the proportion of chromosomally balanced gametes [3, 4].
Case presentation
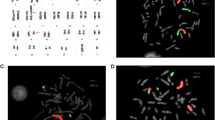
The patient, a 38-year-old male, presented with a primary infertility of over 1 year. No spontaneous abortion was reported in his 38-year-old partner. Semen analysis revealed oligo-astheno-teratozoospermia (sperm concentration 2.9 million/ml, vitality 34%, progressive motility 30%, normal morphology 3%.). Infertility work-up (FSH, LH, testosterone, prolactin, scrotal sonogram) was within normal limits. Blood lymphocytes karyotype revealed a complex homogenous double Robertsonian translocation: 44,XY,der(13;14)(q10;q10),der(21;22)(q10;q10) (Fig. 1).
After informed consent for genetic studies was signed, the patient’s sperm sample was obtained by masturbation after a 1–5-day abstinence period and split into three fractions. The first fraction was washed in phosphate-buffered saline (PBS) and fixed on microscope slides in methanol and acetic acid (3:1). The second fraction underwent discontinuous gradient centrifugation (DGC) as previously described [3]. Spermatozoa from the pellet were spread on a slide and fixed. Finally, the third fraction underwent DGC before being incubated in an hypo-osmotic citrate sodium and fructose solution (450 mOsm, 75 mM fructose, 25 mM sodium citrate), a process called HOST (hypo-osmotic swelling test), in order to look for B+ spermatozoa, which have been shown to comprise significantly less chromosomal imbalances in chromosomal rearrangement carriers [4] (Fig. 2).
Fluorescence in situ hybridization (FISH) sperm studies were then conducted with a set of contiguous telomeric probes that allowed for the joint analysis of both translocations. We used the following: a green 14q telomeric probe, a green and a red 13q telomeric probe (resulting in a yellow signal), and red 21q and 22q telomeric probes for chromosomes 21 and 22. The specificity of the probes was first confirmed by conducting FISH on the patient’s blood chromosomes (Fig. 1). Each signal combination was specific for one or several chromosomal combination patterns. Importantly, balanced spermatozoa carried a specific signal combination (green/red/red/yellow) so as to prevent confusion with their unbalanced counterparts (Fig. 3). Two hundred spermatozoa were counted for the first and second fractions, and 42 HOST B+ spermatozoa were analyzed.
a A set of telomeric probes were used to identify chromosomal patterns in the patient’s spermatozoa. We used the following: green and red (resulting in a yellow fluorescence) telomeric probes for chromosome 13, green telomeric probes for chromosome 14, and red telomeric probes for chromosomes 21 and 22. b The spermatozoa were analyzed on a fluorescent microscope, with each signal combination being associated with one or several chromosomal combinations. Note that the spermatozoon in the lower right corner is balanced (green/red/red/yellow)
The different combinations of fluorescent signals are given in Table 1. The only combination corresponding to a balanced state was the association of a green signal with two red ones and a yellow one. All the other patterns were associated with an unbalanced chromosomal content although some chromosome combinations could not be distinguished unequivocally.
For the fraction washed only in PBS, we found 60% unbalanced spermatozoa. After DGC, a slight decrease to 55% was observed, although statistically not significant with the number of analyzed spermatozoa. After the HOST procedure, we found a significant decrease of the proportion of unbalanced gametes to 24% (p < 0.05) (Fig. 4).
Discussion
Sperm FISH is a reliable method to study the segregation of chromosomal translocations in spermatozoa and to provide accurate genetic counseling to couples, since the existence of a balanced chromosomal translocation leads to the formation of a variable percentage of chromosomally unbalanced gametes. This has been performed extensively for many years now, both in Robertsonian and reciprocal translocation carriers [2]. Typically, Robertsonian translocations are associated with a lower proportion of unbalanced spermatozoa as compared with reciprocal translocations (3–40% vs. 20–75% for reciprocal translocations; [2, 5, 6]). We will discuss both types in this short review, since they are both associated with the same segregation analysis issues.
Sperm FISH has also been used in rare cases of complex chromosomal rearrangements involving three or more different chromosomes even if the increasing number of fluorescent signals makes this technique difficult to applicate. The case of complex translocations involving three chromosomes, such as the t(1;19;13)(p31;q13.2;q31) and t(5;13;14)(q23;q21;q31) reported respectively by Loup et al. [7] and Pellestor et al. [8] in 2010 is different from the present case, with only three chromosomes to be studied versus four here. Both authors describe the use of FISH combined to primed in situ labeling (PRINS) in such a way as to simultaneously analyze five fluorochromes together. Godo et al. in 2013 [9] showed that it was possible to study the segregation of a complex three-chromosome rearrangement, in the case of a t(1;8;2)(q42;p21;p15) translocation by using a sequential strategy: two consecutive rounds of FISH were conducted with the location of each cell on the slide being noted between the two rounds. While this is an accurate and effective strategy, it is time-consuming and therefore difficult to implement in a routine clinical setting. The chromosomal segregation of a double Robertsonian translocation has not been studied so far although Juchniuk de Vozzi et al. in 2009 [10] have reported the case of a male patient carrying two Robertsonian translocations rob(13;13) and rob(13;14) in a mosaic state. Since no cell, and particularly germ cells, could carry both translocations simultaneously, segregation analysis did not cause any particular issues and was limited to the simple classical study of two chromosomes.
Technically, our results emphasize the need to study both rearrangements in a simultaneous manner, as opposed to independently. Ferfouri et al. in 2012 [11] reported on the case of a double chromosomal rearrangement, specifically a reciprocal translocation associated with a pericentric inversion (46,XY,t(3;6)(p24;p21.2),inv(8)(p11;2q21.2)). However, in this case, the two rearrangements were studied in the patient’s spermatozoa independently from each other (61.2% of unbalanced with regard to the translocation and 1.7% with regard to the inversion), which provided no information regarding the actual proportion of chromosomally balanced and unbalanced spermatozoa, since the overlap between these two populations could not be quantified with their procedure.
The findings by Burns et al. [12] supported the assumption that both translocations have to be studied simultaneously in double carrier males. They reported on the case of a male complex rearrangement carrier: t(5;11)(p13;q23.2),t(7;14)(ql1;q24.1) whose segregation was adequately studied by using the hamster penetration test which allows the analysis of the whole karyotype but at the expense of analyzing far fewer cells.
The strategy we describe here allowed us to study the segregation of this double Robertsonian translocation in one step, with only three different signals. While not all signal combinations were specific for a particular segregation mode, the alternate one (balanced one) was associated with a specific combination. We were therefore able to establish the proportion of unbalanced and balanced spermatozoa for this patient. We evidenced a higher proportion of unbalanced spermatozoa (60%) compared with that typically found among Robertsonian translocation carriers. This is explained by a higher number of chromosomes involved in the rearrangement.
Our team has shown that discontinuous gradient centrifugation (DGC), a procedure usually performed prior to intrauterine inseminations and in vitro fertilization, allowed for a mean 30% decrease in the proportion of unbalanced spermatozoa in chromosomal rearrangement carriers [3]. In the case of the present patient, we found a decrease in the proportion of unbalanced spermatozoa from 60 to 55% (decrease 8%). Considering the number of analyzed spermatozoa, this decrease was however not statistically significant.
Additionally, we showed that the selection of a certain type of spermatozoa (termed “B+ spermatozoa”) after incubation in an hypo-osmotic solution, as previously described, allowed for a mean 84% decrease in the proportion of unbalanced spermatozoa [4]. Those spermatozoa can be identified by the presence of a small loop at the tip of the flagellum, with the diameter of the loop being 20 times shorter than the length of the flagellum (Fig. 5). Here, the proportion of unbalanced spermatozoa among B+ spermatozoa was 5% (decrease: 92%). This shows the remarkable potential of selecting spermatozoa from this patient with this procedure prior to ICSI in a way that optimizes the chances of fertilization and normal pregnancy. The patient is currently scheduled to undergo PGD (pre-implantation genetic diagnosis), with HOST-based sperm selection (HBSS).
In the present case, we show that it is possible to analyze the meiotic segregation of a complex double Robertsonian translocation using FISH in one single step. We emphasize the importance of studying both rearrangements simultaneously rather than independently in a manner that evaluates the proportion of unbalanced spermatozoa with regard to all possible rearrangements at once.
References
McKinslay G, Sutherland C. Abnormalities and genetic counseling. 4th ed. USA, chapters 4 & 6: Oxford University Press; 2011.
Lamotte A, Martinez G, Devillard F, Hograindleur J-P, Satre V, Coutton C, et al. Is sperm FISH analysis still useful for Robertsonian translocations? Meiotic analysis for 23 patients and review of the literature. Basic Clin Androl. 2018;28:5.
Rouen A, Balet R, Dorna M, Hyon C, Pollet-Villard X, Chantot-Bastaraud S, et al. Discontinuous gradient centrifugation (DGC) decreases the proportion of chromosomally unbalanced spermatozoa in chromosomal rearrangement carriers. Hum Reprod. 2013;28:2003–9.
Rouen A, Carlier L, Heide S, Egloff M, Marzin P, Ader F, et al. Potential selection of genetically balanced spermatozoa based on the hypo-osmotic swelling test in chromosomal rearrangement carriers. Reprod BioMed Online. 2017;35:372–8.
Roux C, Tripogney C, Morel F, Joanne C, Fellmann F, Clavequin MC, et al. Segregation of chromosomes in sperm of Robertsonian translocation carriers. Cytogenet Genome Res. 2005;111:291–6.
Moradkhani K, Puechberty J, Bhatt S, Lespinasse J, Vago P, Lefort G, et al. Rare Robertsonian translocations and meiotic behaviour: sperm FISH analysis of t(13;15) and t(14;15) translocations: a case report. Hum Reprod. 2006;21:3193–8.
Loup V, Bernicot I, Janssens P, Hedon B, Hamamah S, Pellestor F, et al. Combined FISH and PRINS sperm analysis of complex chromosome rearrangement t(1;19;13): an approach facilitating PGD. Mol Hum Reprod. 2010;16:111–6.
Pellestor F, Puechberty J, Weise A, Lefort G, Anahory T, Liehr T, et al. Meiotic segregation of complex reciprocal translocations: direct analysis of the spermatozoa of a t(5;13;14) carrier. Fertil Steril. 2011;95:2433.e17–22.
Godo A, Blanco J, Vidal F, Parriego M, Boada M, Anton E. Sequential FISH allows the determination of the segregation outcome and the presence of numerical anomalies in spermatozoa from a t(1;8;2)(q42;p21;p15) carrier. J Assist Reprod Genet. 2013;30:1115–23.
Juchniuk de Vozzi MS, Santos SA, Pereira CS, Cuzzi JF, Laureano LA, Franco JG, et al. Meiotic segregation and interchromosomal effect in the sperm of a double translocation carrier: a case report. Mol Cytogenet. 2009;2:24.
Ferfouri F, Boitrelle F, Clement P, Molina Gomes D, Selva J, Vialard F. Sperm FISH analysis of a 44,X,der(Y),t(Y;15)(q12;q10)pat,rob(13;14)(q10;q10)mat complex chromosome rearrangement. Andrologia. 2014;46:576–82.
Burns JP, Koduru PR, Alonso ML, Chaganti RS. Analysis of meiotic segregation in a man heterozygous for two reciprocal translocations using the hamster in vitro penetration system. Am J Hum Genet. 1986;38:954–64.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pierron, L., Irrmann, A., de Chalus, A. et al. Double chromosomal translocation in an infertile man: one-step FISH meiotic segregation analysis and reproductive prognosis. J Assist Reprod Genet 36, 973–978 (2019). https://doi.org/10.1007/s10815-019-01430-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-019-01430-z