Abstract
Purpose
To assess the variability of meiotic segregation patterns in sperm of Robertsonian translocation (RobT) carrier t(21;22) and present effect on reproductive outcome.
Methods
Infertile couple enrolled in IVF/ICSI program. Sperm chromosomal segregation analysis was done using FISH; preimplantation genetic testing for aneuploids (PGT-A) was performed by NGS.
Results
Patients had a low fertilization rate and a negative outcome after the first IVF/ICSI cycle, so they were advised to do chromosomal aberration analysis before their next attempt. The second IVF/ICSI procedure resulted in pregnancy, and two blastocysts were cryopreserved. The NIFTY test has shown low risk for all tested trisomies, sex chromosomes aneuploidis, and deletion syndromes, so a healthy female child was born. During pregnancy, karyotypisation results revealed that the male partner is a RobT carrier t(21;22). Sperm segregation analysis of chromosomes 21 and 22 has shown six types of sperm chromosome sets. The majority of sperm cells had a normal/balanced RobT form of a haploid set of chromosomes (68.5–76%) called an “alternate.” Sperm cells that had additional chromosome 21 or 22, or lack of chromosome 21 or 22, were present in 4–12%. PGT-A performed on two cryopreserved blastocysts revealed one embryo euploid and the other with the mosaic aneuploidy of chromosome 7 present in 50% of the cells.
Conclusion
Infertile couples with a RobT male carrier who have semen comprising of normal/alternate form in the majority have a good prognosis of IVF/ICSI outcome. PGT is recommended because of the possible occurrence of viable trisomic embryos and potential interchromosomal effect.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Robertsonian translocation (RobT) is the most common type of translocation in humans that affects 1:1000 newborns [1]. Zhao et al. [2] reported 872 cases of RobTs from 205 001 blood samples karyotyped postnatally, including 583 balanced RobTs, 264 unbalanced RobTs, 9 mosaic RobTs, and 18 complex RobTs.
This translocation is most often the consequence of the centromeric fusion of two acrocentric chromosomes (13, 14, 15, 21, and 22) which results in one chromosome with two long arms and subsequent loss of short arms. The most frequent forms of RobTs are between chromosomes 13 and 14, 14 and 21, and 14 and 15; while between 21 and 22 is rare, it is present with the incidence of 1:200 000 cases in the population [3].
A person with a balanced translocation is called a RobT carrier. The phenotype expression of these individuals is normal, but their fertility is affected because of impaired gametogenesis and/or production of gametes with unbalanced combination due to meiotic process disturbance. That is the reason why RobT carriers are present in 0.8% of the population of infertile males [4], which is nine times higher than in the general population [5]. Additionally, RobT carriers represent 1.6% of oligozoospermic males [6].
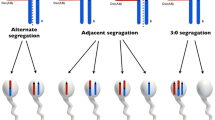
Lamotte et al. [7] have presented a scheme of chromosome segregation during spermatogenesis in the carriers of RobT. During spermatogenesis in prophase I, the meiotic pairing of the involved chromosomes gives a trivalent structure. The trivalent is formed by the association of the translocated chromosome der(A;B) and two corresponding acrocentric chromosomes A and B. There are four theoretical possibilities of sperm formation.
The first one results with “alternate” sperms, either with two acrocentric chromosomes or with one translocated chromosome. After oocyte fertilization with one of those sperms, possible outcomes are a normal embryo and a RobT embryo carrier respectively.
The second and third possibility of sperm formation gives “adjacent” sperms, which involves disomic sperms with chromosome A or B and a translocated der(A;B) chromosome, and nullisomic sperms with only one chromosome, A or B. The result of oocyte fertilization with nullisomic sperm is an embryo with monosomy, which is not viable, while fertilization with disomic sperm results in a potentially viable trisomic embryo.
The fourth possibility in gamete formation is the segregation of all three trivalent forming chromosomes into one sperm, which leaves the other sperm with a double monosomy. In this case, fertilization results with spontaneous abortion exclusively [4, 7].
Studies in which sperm segregation from a RobT male carrier (t(13;14), t(13;15), t(13;22), t(14;15), t(14;21)) was analyzed have shown that the majority of spermatozoa had alternate segregation (mean 85,42; SD 7,8). It seems that the meiotic segregation behavior in the RobT carriers is similar and alternate segregation is predominant [8,9,10].
The quality of semen of RobT carriers varies significantly from patient to patient. Earlier theory suggested that there was some type of mechanism through which aberrant germ cells were eliminated by apoptosis, and the consequence of this process was a reduction of spermatogenesis and sperm count [4, 11]. Recent studies have shown that carriers with normal sperm parameters and oligoasthenoteratozoospermia (OAT) carriers do not feature different frequency of imbalances, suggesting that the segregation pattern and impairment of spermatogenesis are probably independent processes [6, 12].
When speaking of RobT, it is important to take into consideration interchromosomal effect (ICE). Literature data are inconclusive, but it is frequently mentioned that the presence of RobT has an influence on the elevated level of disomy occurrence of chromosomes which are not included in RobT directly [6, 8, 10].
So far, there were five cases of male RobT carriers t(21;22) described in the literature [6, 10, 13,14,15].
The objective of this study was to assess the variability of meiotic segregation in the sperm of a RobT carrier t(21;22) and present an impact on the reproduction outcome. Since the male patient has been diagnosed with a rare type of RobT, we tested if sperm chromosomal segregation would be different after sperm preparation with the sperm wash method or the gradient density method in order to get a better quality of sperm population for the intracytoplasmic sperm injection (ICSI) procedure.
Patients and methods
The couple with 4 years of primary infertility was included in the IVF program because of male factor infertility. The ages of the patients were 38 (female) and 43 (male). The female partner had no pregnancies previously. At the age of 23, she had a cervical conization due to cervical intraepithelial neoplasia (CIN II). During work-up, a mock embryo transfer was performed, but no obstacles were observed and the uterine factor was therefore excluded. The patient also has a low ovarian reserve with an anti-Müllerian hormone (AMH) of 8 pmol/L, but her follicle-stimulating hormone (FSH) was adequate at 5 IU/L.
According to WHO criteria [16], semen analyses have shown Oligoasthenozoospermia with a concentration ranging from 3.6 × 106/mL to 14.5 × 106/mL, total motility between 5.8 and 15.7%, progressive motility between 1.7 and 2.9%, non-progressive motility between 2.9 and 14%, morphology between 5 and 9%, and vitality between 61 and 75%.
In our center, all couples routinely give written consent for all procedures performed in our IVF/ICSI program. The couple also gave written informed consent for their anonymized medical records to be used for the purpose of this research and written informed consent in which the patient allowed his semen to be used for sperm chromosomal segregation analysis.
IVF/ICSI procedure
The first attempt of ICSI/ET procedure was done using antagonist stimulation protocol with rFSH/HMG/GnRH antagonist/rHCG (Gonal F, Merck, London, UK/Menopur, Ferring Pharmaceuticals CZ s.r.o., Prague, CZ/Cetrotide, Merk Serono, Amsterdam, Netherland/Ovitrelle Merck Europe B.V., London, UK), while the second one was done with short agonist protocol GnRH agonist/rFSH/HMG/rHCG (Decapeptyl, Ferring Pharmaceuticals CZ s.r.o., Prague, CZ/Merck, London, UK/Menopur, Ferring Pharmaceuticals CZ s.r.o., Prague, CZ/Ovitrelle, Merck Europe B.V., London, UK).
Laboratory procedures were as follows
Gametes and embryos were cultivated in incubators adjusted to 6% CO2 and 5% O2. All culture media were prepared a day earlier; the culture was performed in drops of an oil-covered medium. The equipment used in the procedures was as follows: disposable plastic material for IVF procedures (Nunc Art IVF Product Line, Thermo Scientific™, USA), 17G needles (Ovum aspiration Needle, Cook Medical, William A. Cook Australia Pity.Ltd., Australia), micromanipulation pipettes (Research Instruments Ltd, UK), and Sydney IVF catheters for embryo transfer (Guardian Access, Cook Medical Inc., USA).
Semen preparation with the gradient density method
The ejaculate was centrifuged at 80%/40% of density gradient (PureCeption 80% Lower Phase Gradient/PureCeption 40% Upper Phase Gradient, SAGE In-vitro Fertilization, Inc., CooperSurgical, USA) for 15 min at 300 g, and then washed out twice with a washing medium (Quinns Advantage® Sperm Washing Medium, SAGE In-vitro Fertilization, Inc., CooperSurgical, USA) for 7 min at 600 g. The obtained sperm pellet was layered with a fertilization medium (Quinns Advantage® Fertilization Medium, SAGE In-vitro Fertilization, Inc., CooperSurgical, USA) and placed into the incubator until oocytes insemination.
After retrieval from a follicular fluid, the oocytes were washed in the fertilization medium, granulosa cells were mechanically removed up to the corona radiata, after which the oocytes were placed into a Petri dish with 40 μL culture medium droplets covered with oil (Oil for Tissue Culture, SAGE In-vitro Fertilization, Inc., CooperSurgical, USA). The ICSI procedure was performed after a 3-h period of preincubation. In another Petri dish with an oil-covered stabilized fertilization medium, the oocytes were first enzymatically denuded (ICSI Cumulase, Origio, Denmark), washed through a series of 40 μL drops, and placed into 10 μL drops for the ICSI procedure. The processed semen was placed into polyvinylpyrrolidone (PVP 7%, SAGE In-vitro Fertilization, Inc., CooperSurgical, USA), the sperm with the best morphologic features was chosen, and their tail was mechanically drilled prior to the ICSI procedure itself.
After 20 h, fertilization was checked, the culture medium was changed, and the culture was continued in a sequential medium (Quinns Advantage® Cleavage Medium, SAGE In-vitro Fertilization, Inc., CooperSurgical, USA). After 48 h, another quality grading of embryonic development was done. For extended cultivation, the medium (Quinns Advantage® Blastocyst Medium, SAGE In-vitro Fertilization, Inc., CooperSurgical, USA) was changed on day three. The embryonic development was checked and assessed on days four and five.
The embryos were put in one well dish with the preincubated medium without oil and transferred using the embryo transfer catheter.
Vitrification/thawing
On day five, the blastocysts of sufficient grading quality were vitrified (Vitrification Media, Kitazato Corp., Japan), according to the Istanbul Consensus (2011), using an open system carrier (Cryotop, Kitazato Corp., Japan) and cryopreserved in liquid nitrogen, as per manufacturer’s instructions. The embryo thawing was performed using thawing solutions (Vitrification Media, Kitazato Corp., Japan), as per manufacturer’s instructions. After thawing, the embryos were cultured in the droplets of the blastocyst medium covered with oil.
Somatic cell karyotyping and fish analysis
After the first unsuccessful IVF attempt, the couple had been recommended to do an analysis for potential chromosomal aberrations. Peripheral blood lymphocyte karyotypisation by standard G banding was done as a routine procedure in the laboratory of an authorized hospital. Due to the male patient karyotype findings, additional fluorescent in situ hybridization (FISH) analysis using centromere probes 13/21(D13Z1/21Z1) (Kreatech) and 14/21(D14Z1/22Z1) (Kreatech) was performed.
PRENATAL DIAGNOSTICS
The mother’s blood sample was taken in the 12th week of gestation. The non-invasive prenatal test NIFTY was performed in the genetic testing service facility (BGI Clinical Laboratories). From the blood sample, cell-free DNA was isolated, and the potential occurrence of fetal chromosomal aneuploidies and deletions were analyzed by doing low coverage whole genome sequencing by Next Generation Sequencing (NGS).
Sperm cell FISH analysis
Semen samples were prepared by both, the gradient density method and the washing method. After antegrade ejaculation, half (1.2 mL) of the semen sample was put onto a gradient and prepared as described above. The supernatant was removed and the pellet was resuspended in 0.2 mL of sperm wash medium, and smeared on adhesive microscope slides were made and air-dried. Another half of the same semen sample was washed twice with a sperm wash medium for 10 min at 600 g. The semen smear samples were made the same way as described above.
FISH probes specific for chromosomal regions 21q22.13 (SureFISH 21q22.13 DSCR8 494 kb RD, Agilent Technologies) and 22q11.23 (SureFISH 22q11.23 BCR DF 1388 kb GR, Agilent Technologies) were prepared according to the manufacturer’s instructions using the IQFISH Fast Hybridization Buffer (Agilent Technologies). An aliquot was applied to both types of semen smear samples and immediately covered with a cover slip. The slides were placed in a hybridizer (Dako) and denatured at 90 °C for 5 min, followed by 2-h hybridization at 45 °C. After hybridization, the slides were washed in a preheated saline-sodium citrate buffer with detergent Tween-20 at 65 °C for 2 min and cooled to room temperature by washing in Tris/HCl buffer for 2 min. The washed slides were mounted using Vectashield antifade mounting medium with 1.5 μg/mL DAPI (Vector Laboratories Inc., USA) and viewed using a fluorescence microscope (Olympus BX51).
Approximately 100 sperm cells were analyzed per sample type. The number of signals specific for 21q22 and/or 22q11.1 was noted for each cell and the final interpretation was done by two independent observers.
Preimplantation genetic testing for aneuploidies
Blastocysts were thawed for trophectoderm biopsy and cryopreserved after the procedure. The trophectoderm was analyzed using preimplantation genetic testing for aneuploidies (PGT-A) according to the manufacturer’s protocol (Illumina, San Diego, CA, USA) in a genetic testing service facility. The VeriSeq kit was used for preimplantation genetic screening utilizing NGS technology to provide screening of all 24 chromosomes for selection of the embryos most likely to be euploid. VeriSeq technology uses whole genome amplification SurePlex to increase the quantity of DNA from a single cell or a few cells. This amplification was done to ensure a sufficient amount of DNA for NGS. The output data was analyzed by SW BlueFuse MultiTM software.
Results
IVF attempts and outcome
The first attempt of the ICSI/ET procedure with an antagonist stimulation protocol resulted in six oocytes, one embryo, and with no pregnancy. In the second ICSI/ET attempt with a short agonist protocol, five oocytes were obtained and all of them were fertilized successfully. The procedure resulted in a positive pregnancy outcome after day three ET of two embryos (Fig. 1a). The other three embryos were left in culture until day five, two blastocysts were developed and cryopreserved (Fig. 1b).
The prenatal NIFTY test has shown low risk for trisomy 13, 18, and 21 (probability 1/138.919.693; 1/233.464.030; 1/1.002.588), and a low risk for trisomy 9, 16, and 22. Sex chromosome aneuploidies (X0, XXY, XXX, XYY) were not detected as well as deletion syndromes (Cri du Chat (5p); 1p36; 2q33.1; DiGeorge 2(10p14), 16p12.2, Jacobsen (11q23), Van der Woude (1q23), Prader-Willi/Angelman (15q11.2)). A healthy baby girl was spontaneously vaginally born in the 40th week of pregnancy weighing 3500 g, with a length of 50 cm, and an APGAR score of 10/10.
Somatic karyotyping and FISH results
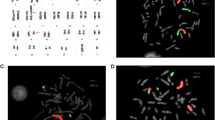
After the first unsuccessful IVF/ICSI attempt, karyotypisation was recommended. The patients insisted on the next IVF procedure before they have received the karyotypisation results, and this attempt resulted with a clinical pregnancy as described above. During the pregnancy, the patients received their karyotypisation results. Analysis of peripheral blood lymphocytes showed that the male karyotype was 45, XY, der(21;22) (p11.2;p11.2) (Fig. 2), while the karyotype of female partner was a normal 46, XX.
Additional fluorescence in situ hybridization (FISH) analysis of the male partner identified that this type of RobT is 45, XY, der(21;22) (p11.2;p11.2) ishdic (21;22) (D13Z1/21Z1+;D14Z1/D22Z1+), and that this derivative chromosome is dicentric, but the test results also stated that only one centromere is active.
Sperm cell segregation of chromosomes 21 and 22
The sperm cell samples were analyzed by FISH, and chromosomal meiotic segregation revealed cells with various chromosomal variations shown in Fig. 3.
FISH analysis of sperm cell samples using probes specific for regions 21q22 and 22q11.1 showed sperm cells. a Alternate forms (red and green signal). b Harboring additional chromosome 22 (green signal). c Harboring additional chromosome 21 (red signal). d Lacking chromosome 22. e Lacking chromosome 21 (original magnification × 1000)
Sperm segregation patterns showed similar results in semen preparation with gradient density and sperm washing method. The results showed that six types of chromosome sets are possible. The majority of sperm cells harbored a normal or balanced form of haploid set of chromosomes (68.5–76%). Because it was hard to distinguish these two types using these probes, they were put in the same group called “alternate.” Sperm cells harboring additional chromosome 21 or 22, or lacking chromosome 21 or 22, were present in 4–12%.
Presence of specific chromosome segregation patterns in the samples after different methods of semen preparation are listed in Table 1.
PGT-A of cryopreserved blastocysts
The indication for PGT-A of the cryopreserved blastocysts was the paternal balanced RobT. Trophectoderm analysis showed that one embryo was a balanced euploid, and in the other one, a 50% mosaic monosomy of chromosome 7 was detected (Fig. 4).
Discussion
To our knowledge, there were five cases of RobT carriers t(21;22) described in the literature so far, and the data has shown that semen quality varies from oligoasthenoterathozoospermia to normozoospermia in the male carriers of this specific RobT. Based on this, it cannot be concluded that a specific semen diagnosis is connected with RobT. This is in accordance with the results of the semen quality findings in other RobT cases [6, 12].
Data regarding sperm chromosome meiotic segregation in carriers of the RobT der (21;22) are presented in Table 2. The first analysis of this kind was done by heterospecific oocyte fertilization followed by sperm karyotyping [13] while the other four and our own study were performed using FISH. Sperm FISH is the most commonly used method to determine the proportion of aneuploidy present in sex chromosomes or autosomes of infertile men. Furthermore, it can also quantify the probability of transmitting aneuploidies and complex chromosomal rearrangement, which is the reason why sperm FISH analysis is increasingly included in infertility diagnostic protocols [10].
The results of studies from other authors of RobT carriers t(21;22) sperm chromosomes meiotic segregation (total percentage from 40 to 85.6%) are in accordance with our results. The discrepancy between the results of sperm alternate proportion (total percentage 96.6%) presented in the first study and the results obtained later was most likely due to the usage of different methodologies. Former FISH analyses mentioned in the literature, as well as in our study, did not allow for the differentiation between normal chromosomes 21 or 22 and chromosome der(21;22). Our results are comparable with the segregation pattern of other types of RobT mentioned in the “Introduction” section. This further confirms that alternate segregation is the most common result of meiosis in sperm cells of all RobT carriers.
Pierron et al. [15] and our results have shown that both sperm preparation methods show a similar distribution of alternate and adjacent sperm.
Concerning chromosomal segregation in female and male gametes, the literature data shows a difference. Ko et al. [17] examined 62 couples in which one of the partners was a RobT carrier. They concluded that alternate segregation was significantly higher in male carriers than in female carriers (43.9% vs. 29.9%, respectively), and adjacent segregation was higher in female carriers than in male carriers (44.7% vs. 38.7%, respectively).
Escudero et al. [18] have established a pregnancy predictive value based on the chromosomal sperm segregation analysis. According to the PGT selection before ET, it seems that patients with less than 65% chromosomally abnormal sperm have a good chance of conceiving, while the patients with higher rates of abnormal sperm have perceivably decreasing chances of pregnancy. Indeed, in our case after two attempts of fresh ICSI/ET procedures, the couple had one successful outcome with a healthy baby born without previous PGT selection.
However, PGT-A of two, a cryopreserved blastocyst has shown that one of them had a 50% mosaic monosomy of chromosome 7.
Some authors [19, 20] suggested that although the embryos of RobT carriers generate clinical pregnancies, they had an increased rate of aneuploidies of chromosomes not involved in the translocation. The interchromosomal effect (ICE) can significantly contribute to the genetic status of these embryos. A highly significant increase in the rate of malsegregation affects structurally normal chromosomes in association with RobT. This might be a consequence of disrupted chromosomal alignment on the spindle, or due to interference with other key aspects of the chromosome segregation process, leading to a generalized increase in the risk of producing aneuploid gametes and/or embryos. This observation has implications for understanding the genetic stability during preimplantation development and the clinical relevance for patients carrying a RobT.
In order to investigate whether ICE truly exists, Alfarawati et al. [19] did chromosome screening of 283 oocytes and early embryos derived from 44 patient carrying chromosomal RobT or reciprocal translocations. The authors have found a statistically significant increase in the rate of the malsegregation of chromosomes not directly involved in RobT. This effect was not found in other types of rearrangements such as reciprocal translocations. In the case of RobT, the authors found ICE only at the cleavage stage, but not in polar bodies or blastocysts. This finding suggests that ICE occurs during the first cell divisions after fertilization, which is known to be the most sensitive time of embryo development. This also indicates that these types of embryos have a low potential for developing to the blastocyst stage. Regarding the frequency of ICE in embryos produced by a RobT carrier, the authors concluded that out of eleven euploid zygotes, one is expected to become abnormal because of ICE.
A recent study from Findikli et al. [20] in which PGT-A was performed on 135 blastocysts generated from 41 couples, in which one partner is a RobT carrier, has shown an increased likelihood of aneuploidy occurrence of chromosomes not directly involved in translocation. The authors did not make the distinction between female or male carriers.
On the other hand, Tulay et al. [21] found no statistically significant difference in the malsegregation rate between 495 embryos obtained from the carriers of translocations (RobT and reciprocal) and from the 1284 embryos from the control group; therefore, there is no evidence for ICE. Xie et al. [22] also did not found evidence of ICE in 1214 cleavage and blastocyst stage embryos obtained from reciprocal and RobT carriers.
Taking into account these inconsistent findings of ICE, it is difficult to say whether the mosaic monosomy of chromosome 7 presented in this study is a result of this effect or not, especially considering that we had the results of PGT-A for only two blastocysts. However, this information should be taken into consideration when planning a pregnancy in patients where one of the partners is a RobT carrier.
Based on the discussion about RobT male carriers and the risk for their offspring, data regarding the rates of balanced and unbalanced chromosomal segregation, including the different types of unbalanced modes, is of great importance for genetic counseling.
Additionally, preimplantation genetic testing should be recommended for couples with this condition because of the possible occurrence of viable embryos with trisomy and potential interchromosomal effect.
References
Therman E, Susman B, Denniston C. The nonrandom participation of human acrocentric chromosomes in Robertsonian translocations. Ann Hum Genet England. 1989;53:49–65.
Zhao W-W, Wu M, Chen F, Jiang S, Su H, Liang J, et al. Robertsonian translocations: an overview of 872 Robertsonian translocations identified in a diagnostic laboratory in China. PLoS One United States. 2015;10:e0122647.
Mack H, Swisshelm K. Robertsonian translocations. Brenner’s Encycl Genet. 2nd Ed; 2013;301–5.
Roux C, Tripogney C, Morel F, Joanne C, Fellmann F, Clavequin MC, et al. Segregation of chromosomes in sperm of Robertsonian translocation carriers. Cytogenet Genome Res Switzerland. 2005;111:291–6.
Chantot-Bastaraud S, Ravel C, Siffroi JP. Underlying karyotype abnormalities in IVF/ICSI patients. Reprod Biomed Online Netherlands. 2008;16:514–22.
Ogur G, Van Assche E, Vegetti W, Verheyen G, Tournaye H, Bonduelle M, et al. Chromosomal segregation in spermatozoa of 14 Robertsonian translocation carriers. Mol Hum Reprod England. 2006;12:209–15.
Lamotte A, Martinez G, Devillard F, Hograindleur J-P, Satre V, Coutton C, et al. Is sperm FISH analysis still useful for Robertsonian translocations? Meiotic analysis for 23 patients and review of the literature. Basic Clin Androl. 2018;28:5.
Douet-Guilbert N, Bris M-JL, Amice V, Marchetti C, Delobel B, Amice J, et al. Interchromosomal effect in sperm of males with translocations: report of 6 cases and review of the literature. Int J Androl. England. 2005;28:372–9.
Ferfouri F, Selva J, Boitrelle F, Gomes DM, Torre A, Albert M, et al. The chromosomal risk in sperm from heterozygous Robertsonian translocation carriers is related to the sperm count and the translocation type. Fertil Steril. United States. 2011;96:1337–43.
Wang B, Nie B, Tang D, Li R, Liu X, Song J, et al. Analysis of meiotic segregation patterns and interchromosomal effects in sperm from 13 Robertsonian translocations. Balkan J Med Genet. 2017;20:43–50.
LeMaire-Adkins R, Radke K, Hunt PA. Lack of checkpoint control at the metaphase/anaphase transition: a mechanism of meiotic nondisjunction in mammalian females. J Cell Biol United States. 1997;139:1611–9.
Pylyp LY, Zukin VD, Bilko NM. Chromosomal segregation in sperm of Robertsonian translocation carriers. J Assist Reprod Genet. Netherlands. 2013;30:1141–5.
Syme RM, Martin RH. Meiotic segregation of a 21;22 Robertsonian translocation. Hum Reprod England. 1992;7:825–9.
Mennicke K, Diercks P, Schlieker H, Bals-Pratsch M, al Hasani S, Diedrich K, et al. Molecular cytogenetic diagnostics in sperm. Int J Androl England. 1997;20(Suppl 3):11–9.
Pierron L, Irrmann A, de Chalus A, Bloch A, Heide S, Rogers E, et al. Double chromosomal translocation in an infertile man: one-step FISH meiotic segregation analysis and reproductive prognosis. J Assist Reprod Genet. Netherlands; 2019;36:973–978.
WHO. Examination and processing of human semen. World Health Edition, F; 2010;286.
Ko DS, Cho JW, Lee HS, Kim JY, Kang IS, Yang KM, et al. Preimplantation genetic diagnosis outcomes and meiotic segregation analysis of robertsonian translocation carriers. Fertil Steril [Internet]. Amsterdam: Elsevier Inc.; 2013; 99(5):1369-76.
Escudero T, Abdelhadi I, Sandalinas M, Munne S. Predictive value of sperm fluorescence in situ hybridization analysis on the outcome of preimplantation genetic diagnosis for translocations. Fertil Steril United States. 2003;79(Suppl 3):1528–34.
Alfarawati S, Fragouli E, Colls P, Wells D. Embryos of robertsonian translocation carriers exhibit a mitotic interchromosomal effect that enhances genetic instability during early development. PLoS Genet. 2012;8:e1003025.
Findikli N, Gultomruk M, Boynukalin FK, Ogur C, Caferler J, Bahceci M. Possible impact of interchromosomal effect at the blastocyst stage in cases undergoing PGT-A for translocations. Reprod Biomed Online [Internet]. Amsterdam: Elsevier; 2019;38(1):e56–7.
Tulay P, Gultomruk M, Findikli N, Yagmur E, Bahceci M. Is the interchromosomal effect present in embryos derived from Robertsonian and reciprocal translocation carriers particularly focusing on chromosome 10 rearrangements? Zygote England. 2015;23:908–15.
Xie Y, Xu Y, Wang J, Miao B, Zeng Y, Ding C, et al. Preliminary analysis of numerical chromosome abnormalities in reciprocal and Robertsonian translocation preimplantation genetic diagnosis cases with 24-chromosomal analysis with an aCGH/SNP microarray. J Assist Reprod Genet Netherlands. 2018;35:177–86.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vujisic, S., Korac, P., Pavlica, M. et al. Chromosomal segregation in sperm of the Robertsonian translocation (21;22) carrier and its impact on IVF outcome. J Assist Reprod Genet 37, 231–238 (2020). https://doi.org/10.1007/s10815-019-01648-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-019-01648-x