Abstract
Purpose
Prior studies suggest that pregnancy outcomes after autologous oocyte cryopreservation are similar to fresh in vitro fertilization (IVF) cycles. It is unknown whether there are differences in pregnancy and perinatal outcomes between cryopreserved oocytes and cryopreserved embryos.
Methods
This is a retrospective cohort study comparing pregnancy and perinatal outcomes between oocyte and embryo cryopreservation at a university-based fertility center. We included 42 patients and 68 embryo transfers in patients who underwent embryo transfer after elective oocyte preservation (frozen oocyte-derived embryo transfer (FOET)) from 2005 to 2015. We compared this group to 286 patients and 446 cycles in women undergoing cryopreserved embryo transfer (frozen embryo transfer (FET)) from 2012 to 2015.
Results
Five hundred fourteen transfer cycles were included in our analysis. The mean age was lower in the FOET vs FET group (34.3 vs 36.0 years), but there were no differences in ovarian reserve markers. Thawed oocytes had lower survival than embryos (79.1 vs 90.1%); however, fertilization rates were similar (76.2 vs 72.8%). In the FOET vs FET groups, clinical pregnancies were 26.5 and 30%, and live birth rates were 25 and 25.1%. Miscarriages were higher in the FET group, 8.1 vs 1.5%. There were no differences in perinatal outcomes between the two groups. The mean gestational age at delivery was 39.1 vs 38.6 weeks, mean birth weight 3284.2 vs 3161.1 gms, preterm gestation rate 5.9 vs 13.4%, and multiple gestation rate 5.9 vs 11.6%.
Conclusions
In our study, live birth rates and perinatal outcomes were not significantly different in patients after oocyte and embryo cryopreservation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Age-associated aneuploidy is a well-established etiology for the natural decline in fertility [1]. There has been increasing momentum to educate women about their reproductive potential early enough for them to make informed decisions [2]. Oocyte cryopreservation (OC) has emerged as an important option that allows women to maintain their reproductive autonomy. It is increasingly evident that most women prefer to cryopreserve oocytes rather than embryos for the purposes of delaying childbearing [3]. As the technology of OC has continued to improve, survival of frozen oocytes has been reported to be around 82.3% [4], which approaches that of vitrified embryos, reported to be approximately 84.3 to 89.4% in two recent randomized controlled trials [5, 6]. However, studies comparing outcomes in patients undergoing OC and cryopreserved embryo transfers are lacking.
Data comparing OC and fresh in vitro fertilization (IVF) in both autologous and donor cycles suggest that OC yields comparable pregnancy and perinatal outcomes to fresh oocytes [7]. In fresh IVF cycles, supraphysiologic estradiol levels in controlled ovarian hyperstimulation (COS) are thought to prematurely luteinize the endometrium, creating a dyssynchronous environment that may negatively impact implantation and pregnancy rates [8, 9]. Since OC and embryo cryopreservation cycles typically utilize programmed cycles for embryo transfer, this potential confounder should be obviated. Additionally, information about pregnancy and perinatal outcomes in women undergoing autologous elective OC are very limited, especially in an older population.
Our primary aim was to evaluate live birth rates in patients undergoing transfers with embryos derived from cryopreserved oocytes in comparison to outcomes from cryopreserved embryo transfers. Secondary outcomes included thaw survival, fertilization, implantation, miscarriage, and perinatal outcomes. We hypothesized that pregnancy and perinatal outcomes in assisted reproduction after autologous OC were comparable to those after embryo cryopreservation.
Materials and methods
Study design
We conducted a retrospective cohort study of patients undergoing IVF and subsequent embryo transfer at a university-based fertility clinic. In this study, we included all patients undergoing embryo transfer after previous elective OC performed at our fertility center, “frozen oocyte-derived embryo transfer” (FOET), from 2005 to 2015. For our comparison group, we included patients undergoing transfer of previously cryopreserved embryos from January 2012 to 2015, “frozen embryo transfer” (FET). We excluded cycles with fresh embryo transfer and donor oocytes.
Controlled ovarian stimulation and oocyte retrieval
All patients having oocyte retrieval underwent COS with injectable gonadotropins using one of three protocols—long GnRH agonist, microdose GnRH agonist flare, or antagonist protocol. Ovulation was triggered using either hCG or GnRH agonist. Oocyte retrieval was performed under trans-vaginal ultrasound guidance 34 or 35 h after trigger injection.
Cryopreservation
For OC cycles, mature or metaphase II (MII) oocytes were cryopreserved on the day of retrieval or 1 day after retrieval if they were immature (germinal vesicle or metaphase I) and subsequently became mature oocytes overnight. For embryo cryopreservation cycles, mature oocytes were fertilized with sperm via conventional insemination, intracytoplasmic sperm injection (ICSI), or a combination of the two. Fertilized embryos were either cryopreserved in the zygote or 2PN stage or grown to the cleavage or blastocyst stage and cryopreserved. Both slow-freeze and vitrification methods were utilized for cryopreservation of both oocytes and embryos between 2005 and 2013. In our clinic, the method of cryopreservation transitioned exclusively to vitrification after 2013. Vitrification was performed using Cryotech straws using a dimethylsulfoxide-ethylene glycol-sucrose solution for cryoprotectant (Irvine Scientific, Santa Ana, CA).
Embryo transfers
For patients desiring embryo transfer after OC, the physician and patient determined the number of oocytes to thaw based on an estimate of the number of embryos that they planned to transfer. The intent was to only thaw as many oocytes as would be needed to accomplish the embryo transfer, without the need for cryopreservation of supernumerary embryos. The embryologist thawed cryopreserved oocytes, and all MII oocytes that survived the thaw were fertilized using ICSI. Embryos were cultured and grown to cleavage stage or blastocyst stage embryos prior to transfer.
Our second cohort included patients undergoing transfer after embryo cryopreservation. Embryos were thawed after previous cryopreservation at the 2PN, cleavage, or blastocyst stage. Previously frozen 2PN embryos were grown to the cleavage or the blastocyst stage and subsequently transferred. Embryos previously cryopreserved at the cleavage or blastocyst stage were thawed and transferred on the same day as the thaw. All surviving embryos were transferred. As with FOET cycles, the intent was always to only thaw as many embryos as would be needed to accomplish the embryo transfer. All patients underwent programmed cycles for endometrial preparation as previously described [10]. Embryo transfers were performed using transabdominal ultrasound guidance.
Data collection
Candidates for the study were identified from the SART database based on either having a FET (non-donor) between 2012 and 2015 or having an embryo transfer derived from autologous cryopreserved oocytes. Baseline demographics were abstracted from medical records including age at the time of oocyte retrieval, BMI, gravidity, parity, infertility diagnosis, and medical comorbidities. We also collected information including hormone parameters, cycle data, and pregnancy data. We analyzed the database for outlying data points and used statistical software to assess the ranges and distribution of the inputs in order to verify the accuracy of data. Patients with significant missing variables were excluded from the analysis.
Data analysis
We excluded all donor egg cycles from our analysis. We also excluded fresh IVF cycles and those requiring surgical sperm extraction for severe male factor. For all analyses, age was defined as age at the time of oocyte retrieval for both FOET and FET cycles. We compared thaw survival between cryopreserved oocytes and cryopreserved embryos. This analysis was stratified for the cryopreservation method based on previous literature demonstrating differential survival between slow-freeze and vitrification techniques [11, 12]. We compared fertilization rates for thawed oocytes or freshly retrieved oocytes for FETs. For assisted reproductive technology (ART) outcomes, serum bHCG levels were determined at 4 weeks of gestational age. Implantation rate per embryo was calculated by the sum of all intrauterine gestational sacs divided by the total number of embryos transferred. For FOET cycles, implantation rate per embryo was calculated the same way. The implantation rate per oocyte was calculated by the sum of all intrauterine gestational sacs divided by the total number of oocytes surviving the thaw, regardless of fertilization status. Miscarriage was defined as pregnancy loss after establishment of a gestational sac on ultrasound. Clinical pregnancy was defined as having a positive fetal heartbeat on ultrasound. Our primary outcome, live birth, was defined as a delivery of a viable infant ≥24-week gestation. Birth outcomes, such as gestational age, birth weight, singletons, multiple gestations, sex, and mode of delivery, were collected.
Statistical approach
Data were assessed for normality and determined to be parametric. Student’s t test was used for analysis of continuous variables for demographics and the perinatal outcomes of gestational age at delivery and neonatal weight, with values depicted as mean ± standard deviation. Pearson’s chi-squared or Fisher’s exact test was used to analyze the categorical demographic data and dichotomous perinatal outcomes such as preterm gestations and multiple gestations, with values written as numbers (percentages). To control for more than one cycle per patient, repeated measures linear regression was used for analysis of interval outcome variables such as oocyte or embryo survival, fertilization, and implantation rates. Repeated measures logistic regression was used for bivariate analysis of ART outcomes between the FOET and FET groups and to calculate odds ratios for positive bHCG, biochemical pregnancies, miscarriages, clinical pregnancies, and live births.
A multivariable model was constructed to analyze the relationship between FOET and FET cycles with respect to ART outcomes. Generalized estimating equations were used to estimate the odds ratios controlling for confounding variables. Outcome variables included positive bHCG, biochemical pregnancies, miscarriages, clinical pregnancies, and live births. Predictor variables were chosen based on clinical relevance and include age, body mass index (BMI), cryopreservation method, stage of ET (cleavage vs blastocyst), number of embryos transferred, and duration of cryopreservation. Given the different distribution of cleavage and blastocyst transfers between the FOET and FET groups, we conducted a subanalysis of cleavage stage embryos as implantation rates differ from blastocyst embryos [13, 14] (see Supplemental Table 1 for subanalysis of cleavage stage transfers). A subanalysis was not performed on blastocyst transfers alone given the small number of transfer cycles in the FOET group (n = 7). The number of embryos transferred was based on patient wishes while complying with the American Society for Reproductive Medicine (ASRM) guidelines [15]. We also constructed a multivariable model for comparison of perinatal outcomes between FOET and FET cycles. Variables for outcomes included gestational age, preterm birth, neonatal weight, and multiple gestations. Predictor variables for this model included age, BMI, cryopreservation method, stage of ET (cleavage vs blastocyst), number of embryos transferred, and duration of cryopreservation. We also controlled for multiple gestations when evaluating gestational age, preterm birth, and neonatal weight.
A power analysis was performed using chi-squared test for comparison of proportions, α of 0.05, and a two-sided significance level. Our study had an 80% power to detect a 14% difference in live birth rates between FET and FOET groups, assuming a 25% live birth rate in the FET group.
All p values were based on two-tailed tests, with statistical significance indicated by p < 0.05. STATA 13 (StataCorp, College Station, TX, USA) was used for all analyses.
Results
Patient characteristics
We included 328 patients and 514 transfer cycles in our final analysis. Forty-two patients and 68 cycles were embryo transfers resulting from fertilization of cryopreserved oocytes. Two hundred eighty-six patients and 446 cycles were frozen embryo transfers from oocytes that were fertilized at the time of oocyte retrieval. A comparison of demographic data revealed that the mean age for FOET patients at oocyte retrieval was younger than the mean age for FET cycles (34.3 ± 4.5 vs 36.0 ± 4.3 years, p < 0.02). The mean age at which patients underwent embryo transfer was similar in the FOET vs FET group at 36.1 ± 6.2 vs 36.8 ± 4.6 years, p = 0.43. Women in the FOET group returned to use their oocytes after a mean duration of 22.2 months (1–87 months), while the FET group returned after an average of 13.5 months (1–76 months), p < 0.001. Other demographic parameters, including BMI, paternal age, and ovarian reserve markers, were not significantly different between groups. The most common diagnoses for the FOET group were male factor (41.5%), tubal factor (39%), situational/elective (26.8%), and diminished ovarian reserve (26.8%). Meanwhile, the most common diagnoses for the FET group were male factor (43.4%), diminished ovarian reserve (40.2%), tubal factor (19.2%), and ovulatory dysfunction (16.4%) (see Table 1 for a comparison of demographics).
IVF cycle parameters and outcomes
We analyzed IVF cycle parameters, including gonadotropin dosing, length of stimulation, peak estradiol level, oocytes retrieved, and embryos transferred. The total gonadotropin dose, length of stimulation, and peak estradiol levels were similar between groups. The mean number of oocytes retrieved in the FOET vs FET group was 19 ± 12.85 vs 17.5 ± 8.7, p = 0.34. The number of embryos transferred was 2.49 ± 1.2 vs 2.35 ± 1.14 in the FOET vs FET groups, p = 0.97. There were more blastocyst transfers performed in the FET group, likely due to the clinical preference of transferring cleavage stage embryos derived from cryopreserved oocytes. The FOET group had 61 cleavage stage transfer cycles and 7 blastocyst stage transfer cycles, while the FET group had 299 cleavage transfer cycles and 147 blastocyst transfer cycles. In the FOET group, 12/42 (28.6%) cycles utilized vitrification and 30/42 (71.4%) employed a slow-freeze protocol. In the FET group, 75/286 (26.2%) cycles utilized vitrification and 211/286 (73%) cycles employed a slow-freeze protocol. There was no significant difference in proportion of slow-freeze and vitrification cycles between groups (χ2 = 0.51, p = 0.47) (see Table 2 for a summary of cycle outcomes).
Thaw survival and fertilization
Thaw survival was significantly lower in oocytes at 79.1% (385/487) compared to 90.1% in embryos (1174/1303), p < 0.001. This difference persisted even when controlling for the cryopreservation method. The standard practice for the cryopreservation method is currently vitrification, and in this group, 71.3% (112/157) survival was seen in oocytes vs 94.9% (205/216) in embryos, p < 0.001. Meanwhile, for the slow-freeze group, 82.7% (273/330) of oocytes survived the thaw, and 89.7% (913/1017) of embryos survived the thaw. Despite the differential survival between oocytes and embryos, fertilization rates were similar (76.2 vs 72.8%, p = 0.31) (see Table 3 for thaw survival and fertilization rates).
Pregnancy outcomes
We performed a bivariate analysis of pregnancy outcomes between FOET and FET cycles. In our cohort, miscarriages were higher in the FET group at 36 (8.1%) vs 1 (1.5%) in the FOET group, p = 0.08. The overall implantation rate for the FOET group was 12.6% (20 sacs/159 embryos transferred) vs 17.6% in the FET group (184 sacs/1043 embryos transferred). The implantation rate per oocyte was 5.2% (20 sacs/385 oocytes surviving the thaw). Comparing clinical pregnancies, there were 18 (26.5%) in the FOET and 134 (30%) in the FET groups, p = 0.55. There were 17 (25%) live births in the FOET and 112 (25.1%) in the FET groups. Our multivariable analysis confirmed no significant differences between the FOET and FET groups with respect to miscarriage, clinical pregnancies, and live births in both cleavage and blastocyst stage groups when controlling for covariates (see Table 4 for pregnancy outcomes).
We next performed a subanalysis using the same statistical methodology on cleavage stage transfers. We did not perform this analysis on blastocyst stage transfers due to the small number of blastocyst transfer cycles in the FOET group (n = 7). When examining cleavage stage transfers, there were 16/61 (26.2%) pregnancies in the FOET group and 74/299 (24.7%) pregnancies in the FET group, which was not significantly different with both unadjusted (p = 0.44) and adjusted analyses (p = 0.35) (see Supplementary Table 1 for the subanalysis on cleavage stage transfers).
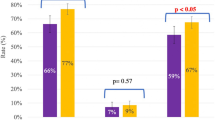
When examining the cohort of women aged 38 years and older, pregnancy outcomes were not significantly different, with the most advanced-aged pregnancy and live birth resulting from oocytes cryopreserved at 41 years old. Comparing the FOET and FET groups, the clinical pregnancy rate was 3/14 (21.4%) vs 39/175 (22.3%), live birth rates were 3/14 (21.4%) vs 31/175 (17.7%), and miscarriages were 0/14 (0%) and 15/175 (8.6%), respectively. Using a generalized estimated equation model controlling for confounders, there was no significant difference in cleavage stage clinical pregnancy rates between groups (p = 0.69) or live birth rates (p = 0.35). Given that there were no miscarriages in the FOET group likely due to low sample size, it is difficult to make conclusions about statistical differences regarding miscarriages in this age cohort (see Fig. 1 for pregnancy outcomes for women ≥38 years at the time of OC).
Perinatal outcomes
Perinatal outcomes, including gestational age at delivery, preterm births, multiple gestations, and neonatal weight, were not significantly different between groups. The mean gestational age at delivery was 39.1 ± 1.5 and 38.6 ± 2.5 weeks for FOET vs FET groups, respectively (p = 0.3). The mean neonatal weight was 3284.2 ± 691 vs 3161.1 ± 765 g for these two groups (p = 0.59). Out of 17 live births, there was one preterm delivery (5.9%) in the FOET group. Out of 112 live births in the FET group, there were 15 (13.4%) preterm deliveries (p = 0.33). There were one set of twins in the FOET group and 13 sets of twins in the FET group, 5.8 vs 3.5%, p = 0.45. No neonatal deaths were reported. We did not have long-term developmental outcomes for analysis. After adjusting for potential confounding variables, there were still no differences in perinatal outcomes between the FOET and FET groups (see Table 5 for perinatal outcomes).
Discussion
In our study, clinically meaningful outcomes of live birth rate and perinatal outcomes were similar between women undergoing transfers of embryos derived from oocyte and embryo cryopreservation. Additionally, fertilization, implantation, clinical pregnancy, and perinatal outcomes were not significantly different between groups. We found a higher rate of miscarriage in the FET group even when controlling for potential confounders. We believe that this is likely due to overall low incidence of miscarriage in our study from low numbers rather than an inherent difference in OC and embryo cryopreservation. Another potential explanation is that poorer-quality oocytes may not survive the thaw process, leading to a decrease in failed pregnancies due to embryos derived from these oocytes.
In 2013, ASRM removed the experimental label from OC, as previous studies demonstrated that births from vitrified oocytes did not increase the risk of aneuploidy or developmental problems [16, 17]. Since then, elective OC has become more commonplace. The first live birth reported from a frozen embryo was in 1984 and from an oocyte was in 1986 [18, 19]. Early studies demonstrated lower fertilization, implantation, and live birth rates from frozen oocytes [20]. However, advances in slow-freeze technique and eventually the widespread use of vitrification have led to better oocyte survival and pregnancy outcomes [21,22,23].
Our results support findings from current studies, which demonstrate comparable outcomes between OC and fresh IVF cycles [7, 24]. There are few but encouraging studies evaluating the outcomes of OC compared to fresh IVF cycles. For donor oocyte cycles, randomized controlled trials comparing vitrified and fresh IVF cycles validated the use of OC as a viable option due to similar fertilization, clinical pregnancy, and live birth rates [7, 25, 26]. The few studies examining outcomes in autologous OC cycles demonstrate comparable fertilization rates, ongoing pregnancy rates, and live birth rates between fresh and vitrified oocytes [17, 27, 28]. Doyle, et al. published a study examining autologous OC outcomes in the USA. This study confirmed previous findings and reported a vitrified-warmed oocyte to live born child efficiency of 6.4% [24]. Promising live birth data have also been published in the cancer population, validating OC as a feasible method for fertility preservation in women planning to undergo chemotherapy or radiation [29].
OC in the setting of fertility preservation may be preferable in many circumstances. Women may be single and have unanticipated relationship changes or ethical reservations about cryopreserving embryos. In the field of ART, circumstances include the following: oocytes may need to be cryopreserved in the setting of failed surgical sperm extraction, unavailability of a male partner, or ovarian hyperstimulation syndrome. OC gives women the freedom and flexibility to utilize their oocytes regardless of future relationship status. A previous study on decisional modeling and cost-effectiveness found that OC prior to age 38 might reduce the cost of a live birth from $55,060 to $39,946 [30]. As more women return to utilize their cryopreserved oocytes, aggregation of long-term data may help to better characterize the outcomes and effectiveness of this practice.
Limitations of our study are the retrospective nature and small number of patients. Findings such as low miscarriage rates in the FOET group are likely due a small sample size rather than differences in OC and embryo cryopreservation. Our average oocyte survival rate was lower than what is reported in the literature [4] likely due to the fact that we captured OC cycles over the span of 10 years, during which our clinic transitioned from slow-freeze to vitrification cryopreservation protocols. Additionally, there were differences in the FOET and FET groups, most notably the distribution of age and proportion of cleavage and blastocyst stage transfers. We controlled for these factors as confounders with statistical modeling, but an age-matched cohort study or randomized controlled trial would serve as better models for verifying outcomes. We did, however, perform a subanalysis on cleavage stage transfers, which showed similar outcomes when using the multivariable model. We acknowledge that there are differences in outcomes between slow-freeze and vitrification, with regard to thaw survival and pregnancy outcomes [31]. In our study, there was no difference in proportion of cryopreservation methods between the FOET and FET groups, and we used this covariate to control for differences in our multivariate statistical model. Lastly, OC is typically done in women who have unproven fertility; thus, larger studies may show that elective OC may have better outcomes than patients undergoing embryo cryopreservation for infertility.
To our knowledge, this is the first study to directly compare pregnancy and perinatal outcomes of elective autologous OC with autologous embryo cryopreservation. Our study encompasses a wide range of ages of patients, and includes outcomes in older women, which have largely been excluded from previous studies in the existing literature. While our numbers are small, we believe that our study provides valuable information since there are limited data on outcomes of women who have undergone elective OC, likely due to the lag time for women coming back to utilize their oocytes. Further studies are needed, with larger cohorts focusing solely on vitrified oocyte and embryo outcomes in order to make definitive conclusions about the efficacy and outcomes of OC vs embryo cryopreservation.
Conclusions
Based on our findings, there were no significant differences in clinical pregnancy rates, live birth rates, or perinatal outcomes between OC as compared to embryo cryopreservation. This option allows women to maintain their sense of reproductive autonomy and optimize their options for future family building.
References
Faddy MJ, Gosden RG, Gougeon A, Richardson SJ, Nelson JF. Accelerated disappearance of ovarian follicles in mid-life: implications for forecasting menopause. Hum Reprod. 1992;7:1342–6.
Cil AP, Turkgeldi L, Seli E. Oocyte cryopreservation as a preventative measure for age-related fertility loss. Semin Reprod Med. 2015 Nov;33(6):429–35.
Mertes H, Pennings G. Social egg freezing: for better, not for worse. Repro Biomed Online. 2011;23:824–9.
Rienzi L, Gracia C, Maggiulli R, LaBarbera AR, Kaser KJ, Ubaldi FM, et al. Oocyte, embryo and blastocyst cryopreservation in ART: systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Hum Reprod Update. 2017;1;23(2):139–55.
Debrock S, Peeraer K, Fernandez Gallardo E, De Neubourg D, Spiessens C, D’Hooghe TM. Vitrification of cleavage stage day 3 embryos results in higher live birth rates than conventional slow freezing: a RCT. Hum Reprod. 2015;30(3):1820–30.
Fasano G, Fontenelle N, Vannin AS, Biramane J, Devreker F, Englert Y, et al. A randomized controlled trial comparing two vitrification methods versus slow-freezing for cryopreservation of human cleavage stage embryos. J Assist Reprod Genet. 2014 Feb;31(2):241–7.
Cobo A, Meseguer M, Remohí J, Pellicer A. Use of cryo-banked oocytes in an ovum donation programme: a prospective, randomized, controlled, clinical trial. Hum Reprod. 2010;25(9):2239–46.
Richter KS, Shipley SK, Vearry I, Tucker MJ, Wildra EA. Cryopreserved embryo transfers suggest that endometrial receptivity may contribute to reduced success rates of later developing embryos. Fertil Steril. 2006;86:862–6.
Roque M, Lattes K, Serra S, Psych IS, Geber S, Carreras R, et al. Fresh embryo transfer versus frozen embryo transfer in in vitro fertilization cycles: a systematic review and meta-analysis. Fertil Steril. 2013;99(1):156–62.
Paulson RJ. Hormonal induction of endometrial receptivity. Fertil Steril. 2011;96:530–5.
Cobo A, Diaz C. Clinical application of oocyte vitrification: a systematic review and meta-analysis of randomized controlled trials. Fertil Steril. 2011 Aug;96(2):277–85.
Loutradi KE, Kolibianakis EM, Venetis CA, Papanikolau EG, Pados G, Bontis I, et al. Cryopreservation of human embryos by vitrification or slow freezing: a systematic review and meta-analysis. Fertil Steril. 2008;90(1):186–93.
Fernández-Shaw S, Cercas R, Braña C, Villas C, Pons I. Ongoing and cumulative pregnancy rate after cleavage-stage versus blastocyst-stage embryo transfer using vitrification for cryopreservation: impact of age on the results. J Assist Reprod Genetic. 2015 Feb;32(2):177–84.
Glujovsky D, Blake D, Farquhar C, Bardach A. Cleavage stage versus blastocyst stage embryo transfer in assisted reproductive technology. Cochrane Database Syst Rev. 2012;11(7):CD002118.
American Society for Reproductive Medicine: Criteria for number of embryos to transfer: a committee opinion. Practice Committee of the American Society for Reproductive Medicine. Practice Committee of Society for assisted reproductive technology. Fertil Steril. 2013 Jan;99(1):44–6.
Grifo JA, Noyes N. Delivery rate using cryopreserved oocytes is comparable to conventional in vitro fertilization using fresh oocytes: potential fertility preservation for female cancer patients. Fertil Steril. 2010;93(2):391–6.
Forman EJ, Li X, Ferry KM, Scott K, Treff NR, Scott RT. Oocyte vitrification does not increase the risk of embryonic aneuploidy or diminish the implantation potential of blastocysts created after intracytoplasmic sperm injection: a novel, paired randomized controlled trial using DNA fingerprinting. Fertil Steril. 2012;98:644–9.
New York Times. First baby of a frozen embryo. NY Times Web. 1984;11:A16.
Chen C. Pregnancy after human oocyte cryopreservation. Lancet. 1986;1:884–6.
Oktay K, Cil AP, Bang H. Efficiency of oocyte cryopreservation: a meta-analysis. Fertil Steril. 2006;86:70–80.
Cobo A, Domingo J, Pérez S, Crespo J, Remohí J, Pellicer A. Vitrification: an effective new approach to oocyte banking and preserving fertility in cancer patients. Clin Transl Oncol. 2008;10:268–73.
Smith GD, Serafini PC, Fioaravanti J, Yadid I, Coslovsky M, Hassun P, et al. Prospective randomized comparison of human oocyte cryopreservation with slow-rate freezing or vitrification. Fertil Steril. 2010;94:2088–95.
Levi Setti PE, Porcu E, Patrizio P, Vigliano V, de Luca R, d’Alicia P, et al. Human oocyte cryopreservation with slow freezing versus vitrification. Results from the National Italian Registry data, 2007-2011. Fertil Steril. 2014 Jul;102(1):90–5.
Doyle JO, Richter KS, Lim J, Stillman RJ, Graham JR, Tucker MJ. Successful elective and medically indicated oocyte vitrification and warming for autologous in vitro fertilization, with predicted birth probabilities for fertility preservation according to number of cryopreserved oocytes and age at retrieval. Fertil Steril. 2016;105(2):459–66.
Solé M, Santaló J, Boada M, Clua E, Rodríguez I, Martínez F, et al. How does vitrification affect oocyte viability in oocyte donation cycles? A prospective study to compare outcomes achieved with fresh versus vitrified sibling oocytes. Hum Reprod. 2013;28(8):2087–92.
Trokoudes KM, Pavlides C, Zhang X. Comparison outcome of fresh and vitrified donor oocytes in an egg-sharing donation program. Fertil Steril. 2011;95(6):1996–2000.
Chang C, Elliot TA, Wright G, Shapiro DB, Toledo AA, Nagy ZP. Prospective controlled study to evaluate laboratory and clinical outcomes of oocyte vitrification obtained in in vitro fertilization patients aged 30 to 39 years. Fertil Steril. 2013;99(7):1891–7.
Rienzi L, Romano S, Albricci L, Magguilli R, Capalbo A, Baroni E, et al. Embryo development of fresh ‘versus’ vitrified metaphase II oocytes after ICSI: a prospective randomized sibling-oocyte study. Hum Reprod. 2010;25(1):66–73.
Druckenmiller S, Goldman KN, Labella PA, Fino ME, Bazzocchi A, Noyes N. Successful oocyte cryopreservation in reproductive-aged cancer survivors. Obstet Gynecol. 2016;127(3):474–80.
Devine K, Mumford SL, Goldman KN, Hodes-Wertz B, Druckenmiller S, Propst AM, et al. Baby budgeting: oocyte cryopreservation in women delaying reproduction can reduce cost per live birth. Fertil Steril. 2015;103(6):1446–53.
Li Z, Wang YA, Ledger W, Edgar DH, Sullivan EA. Clinical outcomes following cryopreservation of blastocysts by vitrification or slow freezing: a population-based cohort study. Hum Reprod. 2014;29(12):2794–801.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This study was approved by the institutional review board at the University of Southern California (HS-15-00628). Written informed consent was not required from participants for this retrospective study.
Funding
No external funding was received for this study.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 15 kb)
Rights and permissions
About this article
Cite this article
Ho, J.R., Woo, I., Louie, K. et al. A comparison of live birth rates and perinatal outcomes between cryopreserved oocytes and cryopreserved embryos. J Assist Reprod Genet 34, 1359–1366 (2017). https://doi.org/10.1007/s10815-017-0995-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-017-0995-2