Abstract
Purpose
Our aim was to evaluate if maternal age at transfer following autologous oocyte cryopreservation is associated with live birth rate (LBR).
Methods
We performed a retrospective cohort study of all patients who thawed autologous oocytes and then underwent a single frozen euploid embryo transfer between 2011 and 2021 at a large urban university-affiliated fertility center. Each oocyte thaw patient was matched 2:1 to in vitro fertilization (IVF) patients who underwent single embryo transfer < 1 year after retrieval. Primary outcome was LBR. Secondary outcomes included implantation rates (IR) and spontaneous abortion rates (SABR).
Results
A total of 169 oocyte thaw patients were matched to 338 IVF patients. As expected, oocyte thaw patients were older (median age 42.5 vs. 37.6 years, p < 0.001) and waited longer between retrieval and transfer than in vitro fertilization patients (median time 59 vs. 1 month, p < 0.001). In univariate analysis, implantation and LBR differed among oocyte thaw and IVF patients (p < 0.05), but SABR did not (p = 0.57). Transfer outcomes in oocyte thaw patients did not differ based on transfer age group (IR: p = 0.18; SABR: p = 0.12; LBR: p = 0.24). In a multiple logistic regression model, age at transfer was not predictive of live birth when controlling for age at retrieval, embryo morphology, and day of blastulation.
Conclusions
Maternal age at transfer after oocyte cryopreservation is not predictive of LBR; this suggests that “an aging womb” does not impair LBR after oocyte thaw and empowers patients to return for transfer when ready for childbearing.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Maternal age is the most important prognostic factor for live birth from assisted reproductive technology, with advancing maternal age leading to lower oocyte yield and higher aneuploidy rates [1, 2]. Despite this unavoidable age-related fertility decline, birthing trends indicate that women are postponing childbearing to age ≥ 35 years with increasing frequency for personal, financial, and professional reasons [1, 3]. Delayed family planning may result in significant consequences, including subfertility or infertility, inability to achieve one’s ideal family size, and adverse pregnancy outcomes [4, 5].
Autologous oocyte cryopreservation (OC) is a widely used and viable method for fertility preservation that provides women with the option to postpone childbearing while preserving the option of future genetic children. Prior studies have shown comparable euploidy and pregnancy rates with OC compared to fresh in vitro fertilization (IVF) [6,7,8,9]. Moreover, a recent study of OC patients who cryopreserved oocytes at a median age of 38 years and subsequently returned for oocyte thaw a median of 4 years later reported a 39% live birth rate (LBR) among all patients—which is comparable to age-matched IVF data from the SART national database [10]. Of note, this study reported a 70% LBR among patients who cryopreserved oocytes at age < 38 years and cryopreserved ≥ 20 metaphase II oocytes (M2s) with no association between cryopreservation duration and LBRs from cryopreserved oocytes [10].
The concept of fertility preservation and OC depends on the idea that oocyte age remains frozen at the retrieval age as well as the premise that maternal age at time of embryo transfer will not impact LBR. However, there are conflicting data from donor oocyte IVF transfer cycles as to the incidence and impact of uterine aging on cycle outcomes [11,12,13,14,15,16]. Such studies suggest that the uterus has diminished ability to foster the implantation and growth of an embryo—a term known as age-related uterine receptivity. Studies in donor oocyte IVF transfer cycles have shown lower LBRs after euploid embryo transfer in women > 35 years [11, 14, 16], and have demonstrated decreased pregnancy rates in donor egg recipients > 40 years [2, 12, 15, 16]. However, other studies found no relationship between maternal age and uterine receptivity [17,18,19]. Functional studies have reported changes in uterine function in older women, some of which have been associated with changes in patient health that also occur with age and are therefore relevant to the patient population who delay pregnancy [18, 20,21,22,23,24]. While research has sought to understand the impact of age-related uterine receptivity by evaluating donor oocyte and IVF patients, the impact of maternal transfer age after OC remains unknown. This is a particularly important area to investigate because most women who undergo OC intend to delay childbearing until they have reached advanced maternal age.
Despite the growing popularity of OC, the impact of maternal transfer age after OC on LBR remains unanswered. If a negative relationship between uterine receptivity and age exists, then transfer age may matter for OC patients. Since the first generation of OC patients are currently returning in numbers suitable for evaluation, our objective was to evaluate if maternal age at transfer following OC is associated with LBR.
Materials and methods
Design
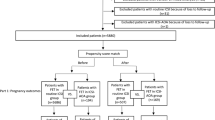
We performed a retrospective cohort study of all patients who thawed autologous oocytes and then underwent single frozen euploid embryo transfer (STEET) with embryos created from their thawed oocytes between January 1, 2011, and December 31, 2021, at New York University Langone Fertility Center. This study was performed with New York University Institutional Review Board approval (S13-00389).
Subjects
The first STEET from each patient who thawed autologous oocytes was included. Patients were excluded if (1) STEETs were less than 1 year after OC, (2) STEETs involved mosaic, aneuploid, or unbiopsied embryos, (3) oocyte cryopreservation was performed via slow freeze methodology, (4) oocyte cryopreservation occurred at an outside institution, (5) STEETs involved fresh embryos, or (6) OC was performed as part of a research study, for a medical reason, due to no sperm, due to a natural disaster (Hurricane Sandy), in combination with embryo cryopreservation, or for use with a gestational carrier.
Each autologous oocyte thaw patient was matched to two IVF patients who underwent frozen STEET from January 1, 2011, to December 31, 2021. The first STEET from each IVF patient was included. We excluded STEETs from non-autologous oocytes or previously frozen oocytes, STEETs with mosaic, aneuploid or untested embryos, fresh STEET cycles, and STEETs that occurred greater than 1 year after retrieval. Each autologous oocyte thaw patient was matched to two IVF patients using a random number generator based on age at retrieval.
All patients included underwent an evaluation of their uterine cavity using saline sonogram or hysterosalpingogram before embryo transfer. Anomalies and intracavitary lesions were addressed prior to embryo transfer.
Variables and data collection
Data regarding OC, oocyte thaw, IVF retrieval cycles, and embryo transfer cycles were obtained from the electronic medical record. Patient age and date of oocyte retrieval were collected for OC data. Collected thaw data included the following: date of oocyte thaw; patient age; number of total oocytes and M2s thawed and surviving thaw; number of embryos for PGT-A; cryopreservation; and ploidy results. Collected transfer data included the following: date of STEET; patient age; embryo morphology; day of trophectoderm biopsy, and ploidy result; and implantation and live birth outcomes. Embryos were grouped into four categories based on Gardner’s morphological grading system and associated predicted LBRs from our laboratory. The four categories were as follows: (1) “good,” which corresponded to blastocysts with predicted LBRs of 58% of more; (2) “fair,” which corresponded to blastocysts with predicted LBRs of 42 to 57%; and (3) “poor,” which corresponded to blastocysts with predicted LBRs of less than 42%. Implantation was defined as ≥ 1 intrauterine gestational sac on ultrasound. The primary outcome was LBRs, defined as the total number of live births in our cohort. Secondary outcomes were implantation rates (IR), defined as the total number of implantations in our cohort, and spontaneous abortion rates (SABR), defined as the total number of spontaneous abortions prior to 20 weeks gestational age in our cohort.
Ovarian stimulation and laboratory protocols
Ovarian stimulation protocols were selected based on the patient’s ovarian reserve and age. Oocyte retrieval was performed via ultrasound-guided transvaginal aspiration 35 h after trigger administration.
In OC cycles, vitrification was used to cryopreserve oocytes using previously described techniques [8, 10]. Oocytes were later thawed using previously described techniques, and intracytoplasmic sperm injection (ICSI) was used to fertilize the surviving oocytes [8, 10].
In IVF cycles, conventional insemination was used unless ICSI was indicated based on male history or semen parameters, which is standard practice in our laboratory.
In both oocyte thaw and IVF cycles, embryos were cultured until trophectoderm biopsy and cryopreservation on days 5–7. The endometrium was prepared for embryo transfer using previously described techniques [8, 10].
Statistical analysis
Continuous variables were assessed for normality using the Kolmogorov-Smirnoff test and determined not to be normally distributed. Mann–Whitney U tests were used to compare continuous variables. Categorical variables were analyzed using chi-squared tests and Fisher’s exact tests. Logistic regression was used for modeling and adjustment of covariates to evaluate the outcome of live birth. An alpha error of < 0.05 was considered statistically significant. Descriptive results are reported as counts, percentages, medians ± interquartile ranges (IQR) for ages, and median with range.
Results
A total of 169 OC patients, with their first single frozen euploid embryo transfer from OC, were included. OC patients cryopreserved a median of 19 oocytes (range, 5–61) and 18 M2s (range, 4–57), which led to a median of 4 blastocysts (range, 1–20) for PGT-A biopsy and a median of 2 euploid embryos (range, 1–8).
Table 1 shows demographic and embryo characteristics for OC patients and IVF patients. Median age at retrieval was statistically similar at 37.8 years in OC patients and 37.5 years in IVF controls as expected based on our matching criteria. Median age at transfer was 42.5 years (range, 32.3–52.3 years) among OC patients and 37.6 years (range, 29.3–43.8 years) among IVF controls, which did differ between groups (p < 0.001). Of note, 99.4% (168/169) of OC patients were of advanced maternal age (35 years or older) at transfer, 83.4% (n = 141/169) were 40 years or older at transfer, and 14.8% (n = 25/169) were older than 45 years at transfer. OC patients underwent transfer a median of 4 years and 11 months following retrieval (range, 16 months–12 years 2 months) while IVF controls underwent transfer a median of 2 months (range, 1–11 months) following retrieval (p < 0.001). Of note, 46.7% of OC patients returned after 5 years. While 100% of OC patients used ICSI to fertilize oocytes, 20% (68/338) of IVF patients used ICSI to fertilize oocytes and 80% (270/338) of IVF patients used insemination to fertilize oocytes. In terms of embryo characteristics, OC patients had fewer embryos biopsied on day 5, and more embryos biopsied on days 6 and 7 (p < 0.001) than IVF patients. Also, embryo morphology differed between groups, as IVF controls had significantly more embryos with “good grades,” and OC patients had more fair or poor embryos transferred (p < 0.001).
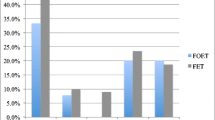
Transfer outcomes differed significantly between OC patients and IVF controls (Fig. 1). IR and LBR were significantly higher in IVF controls than in OC patients (p < 0.05). Spontaneous abortion rates did not differ between groups (p = 0.57). In multiple logistic regression models comparing OC and IVF patient, controlling for age at retrieval, embryo morphology, day of TE biopsy, and ICSI/conventional insemination as independent variates, age at transfer was not predictive of LBR (p = 0.22), IR (p = 0.87), or SABR (p = 0.13).
Autologous oocyte cryopreservation (OC) and in vitro fertilization (IVF) transfer outcomes. Notes: (1) There were a total of 169 OC patients; among these patients, there were 112 implantations, 12 spontaneous abortions, 1 elective termination, and 99 live births. (2) There were a total of 338 IVF patients; among these patients, there were 260 implantations, 29 spontaneous abortions, 2 elective terminations, and 228 for live births
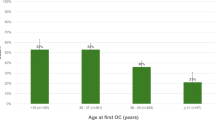
Finally, we evaluated transfer outcomes in our OC patients based on age at transfer. Across our four transfer age groups (< 40 years, 40–42 years, 42.1 to 44 years, and ≥ 44 years), we found no significant differences in IR (p = 0.18), SABR (p = 0.12), or LBR (p = 0.24) (Fig. 2). Univariate analysis comparing age of retrieval (< 37 years compared to ≥ 37 years) similarly revealed that transfer age was not associated with IR, SABR, or LBR across the four transfer age groups (Table 2). In multiple logistic regression of OC patients, controlling for age at retrieval, embryo morphology, and day of TE biopsy as independent variates, age at transfer across the four selected age groups was not predictive of LBR (p = 0.23) or IR (p = 0.98); However, age at transfer was predictive of SAB (p < 0.05), attributable to the SABR in patients 42 to 44 years (14%) compared to the three other age groups (< 40 years, 4%; 40 to 42 years, 2%; ≥ 44 years, 7%). We evaluated the older cohort of OC patients at transfer (age at transfer ≥ 42 years), and we found no difference in LBR, SABR, or IR when compared to OC patients who were < 42 years at transfer (Table 3). This effect persisted when we analyzed our oldest cohort (age at transfer ≥ 45 years, n = 25), with no associated in LBR, SABR, or IR. As such, we found that transfer outcomes among OC patients were not associated with age at transfer.
Discussion
As more women choose delayed childbearing and fertility preservation, OC outcomes based on transfer age are imperative for counseling. These outcomes will help women to make informed decisions about their family planning by providing them with accurate information about whether age of return impacts LBRs from OC. This study is the first study to evaluate the association between transfer age and outcomes after planned OC. In our analysis, transfer outcomes were similar in oocyte thaw patients regardless of transfer age. These findings support the notion that OC patients can return for transfer when ready for childbearing.
While the scope of our study focused on clinical outcomes following embryo transfer from an OC cycle, prior studies clarify additional important patient counseling tools related to oocyte thaw outcomes. Cascante et al. evaluated 15 years of oocyte thaw outcomes, and found that 1% of OC patients had no oocyte survival and 2% of OC patients had no 2PN fertilization (n = 543 patients) [10]. Among patients who thawed all oocytes, 15% had no usable embryos and 36% had no euploid/untested embryos to transfer (10). Additionally, the question regarding number of oocytes needed to yield 1 euploid embryo remains unanswered; a recent abstract found that an average of 10, 11, 14, and 35 M2s are required to yield 1 euploid embryo at ages < 35 years, 35–37 years, 38–40 years, and > 41 years, respectively, from autologous oocyte cycles [25]. Another area for future investigation in OC patients relates to the impact of partner sperm age. A recent abstract found a lower cumulative live birth rate among OC patients when partner sperm age was > 50 years, which may indicate a potential negative risk for OC patients with older male partners [26]. However, in a multiple logistic regression model controlling for OC age, the authors found that partner sperm age was not predictive of cumulative live birth rate.
We matched OC patients to IVF controls based on age of retrieval, as oocyte age critically impacts LBRs and transfer outcomes [1, 2, 27,28,29,30]. However, several factors differed between our IVF and OC patients; these factors included the day of trophectoderm biopsy for PGT-A cycles, embryo morphology, and whether ICSI was performed. IVF controls had significantly more embryos biopsied on day 5, significantly better embryo morphology, and significantly fewer ICSI fertilization compared to the OC cohort. These differences may account for the higher LBR and IR seen in the IVF cohort compared to the OC cohort. Once these differences were accounted for within multiple logistic regression models, there no longer was an association between age at transfer and LBR between the IVF and OC cohorts.
Limited research has evaluated differences in blastocyst formation rates between OC and IVF patients, though prior research has shown that LBRs following euploid embryo transfers are similar regardless of whether the oocyte was previously cryopreserved [10, 31]. Importantly, we found lower rates of blastocysts and of good-quality blastocysts among OC patients compared to age-matched IVF patients. While our regression analysis matched for age of retrieval and included embryo morphology, the analysis did not include total oocytes retrieved, total oocytes available following thaw, or total embryos available, which may limit our conclusions from this finding. However, given this data, OC patients should be counseled that the chance of obtaining a good-quality blastocyst may be lower with cryopreserved oocytes than with fresh oocytes. As such, our findings may additionally assist in counseling regarding the advantages and disadvantages of elective fertility preservation. Future research must explore how the cryopreservation process impacts blastocyst formation.
Transfer outcomes in OC patients did not differ based on transfer age when comparing patients < 40 years, 40–42 years, 42.1–44 years, and > 44 years. Moreover, in our multiple logistic regression models, age at transfer was not predictive of LBR or IR. Of note, when transfer age categories were stratified to evaluated SABR, chi-squared revealed significance (p < 0.05), though pairwise comparisons revealed that 42 to 44 years were the only significant group with a 14% miscarriage rate. This effect was not persistent as other analyses.
While LBR did not change with transfer age, advanced maternal age is associated with multiple pregnancy adverse events, including increased risk of stillbirth, preterm delivery, hypertensive disorders of pregnancy, gestational diabetes, fetal growth restriction, and/or risk of cesarean Section [32,33,34]. Patients should be counseled regarding pregnancy-related risks when considering their family planning and potentially receive clearance from a Maternal Fetal Medicine specialist if they return for transfer at age ≥ 45 years.
There were multiple strengths to this study, including that our study evaluated actual clinical outcomes, instead of modeled, to aid in personalized patient counseling. We had a relatively large cohort of autologous oocyte thaws, and we had a long time-span over 10 years. Weaknesses included our retrospective design and our long time-span (10 years), which may confound our findings due to temporal changes in IVF technology and success rates. As well, our post hoc power analysis revealed that our study was significantly underpowered across our OC cohort—we had 57% power to detect a 20% difference in LBR between our OC age groups. The post hoc power analysis did reveal that our study was 82% powered to detect a 15% difference between our OC and IVF cohort groups. Our study was limited in generalizability, as the study occurred at a single institution and may not represent the younger cohort of individuals who are cryopreserving oocytes today. Our study did not include several factors that may predict OC and IVF outcomes, including race/ethnicity, reproductive history, prior conceptions, infertility diagnosis, and socioeconomic status. We additionally did not evaluate for premature births or adverse fetal or maternal outcomes that may be pertinent to our aging cohort. Also, 20% (68/338) of our IVF patients used ICSI for fertilization of oocytes, while our standard lab practice is to perform insemination. This finding suggests a high rate of male factor infertility, which may have lowered the LBR in our IVF groups, and in turn, reduced any difference between the OC and IVF groups. Finally, we had a small cohort of individuals older than 45 years at transfer (14.8%, n = 25).
Further investigation should consider the impact of transfer age on cumulative live birth rates with larger samples. Soon, we hope to provide a personalized predictive model for patient counseling that includes both LBR and age-related pregnancy risk factors based on transfer age.
In conclusion, we found that maternal age at transfer after OC was not predictive of LBR. This study is the first to evaluate the association of maternal age at transfer following OC. We found that an “aging womb” did not impair LBRs after OC, which empowers patients to return for transfer when ready for childbearing.
Data availability
The data that support the findings of this study are available upon request. The data are not publicly available as they contain information that could compromise the privacy of research participants.
References
American College Obstetricians and Gynecologists Committee on Gynecologic Practice and Practice Committee. Female age-related fertility decline. Committee Opinion No. 589. Fertil Steril 2014;101:633–4.
Centers for Disease Control and Prevention. 2019 Assisted Reproductive Technology Fertility Clinic and National Summary Report. US Dept of Health and Human Services. 2021. https://www.cdc.gov/art/artdata/index.html.
Martin JA, Hamilton BE, Osterman MJK, Driscoll AK. Births: final data for 2019. Natl Vital Stat Rep. 2021;70:1–51.
Habbema JD, Eijkemans MJ, Leridon H, te Velde ER. Realizing a desired family size: when should couples start? Hum Reprod. 2015;30:2215–21.
te Velde E, Habbema D, Leridon H, Eijkemans M. The effect of postponement of first motherhood on permanent involuntary childlessness and total fertility rate in six European countries since the 1970s. Hum Reprod. 2012;27:1179–83.
Grifo JA, Noyes N. Delivery rate using cryopreserved oocytes is comparable to conventional in vitro fertilization using fresh oocytes: potential fertility preservation for female cancer patients. Fertil Steril. 2010;93:391–6.
Hodes-Wertz B, Noyes N, Mullin C, McCaffrey C, Grifo JA. Retrospective analysis of outcomes following transfer of previously cryopreserved oocytes, pronuclear zygotes and supernumerary blastocysts. Reprod Biomed Online. 2011;23:118–23.
Goldman KN, Noyes NL, Knopman JM, McCaffrey C, Grifo JA. Oocyte efficiency: does live birth rate differ when analyzing cryopreserved and fresh oocytes on a per-oocyte basis? Fertil Steril. 2013;100:712–7.
Goldman KN, Kramer Y, Hodes-Wertz B, Noyes N, McCaffrey C, Grifo JA. Long-term cryopreservation of human oocytes does not increase embryonic aneuploidy. Fertil Steril. 2015;103:662–8.
Cascante SD, Blakemore JK, DeVore S, Hodes-Wertz B, Fino ME, Berkeley AS, Parra CM, McCaffrey C, Grifo JA. Fifteen years of autologous oocyte thaw outcomes from a large university-based fertility center. Fertil Steril. 2022;118(1):158–66.
Scott RT Jr, Ferry K, Su J, Tao X, Scott K, Treff NR. Comprehensive chromosome screening is highly predictive of the reproductive potential of human embryos: a prospective, blinded, nonselection study. Fertil Steril. 2012;97:870–5.
Gupta P, Banker M, Patel P, Joshi B. A study of recipient related predictors of success in oocyte donation program. J Hum Reprod Sci. 2012;5(3):252–7.
Harton GL, Munne S, Surrey M, et al. Diminished effect of maternal age on implantation after preimplantation genetic diagnosis with array comparative genomic hybridization. Fertil Steril. 2013;100:1695–703.
Yaron Y, Botchan A, Amit A, Kogosowski A, Yovel I, Lessing JB. Endometrial receptivity: the age-related decline in pregnancy rates and the effect of ovarian function. Fertil Steril. 1993;60(2):314–8.
Soares SR, Troncoso C, Bosch E, Serra V, Simón C, Remohí J, Pellicer A. Age and uterine receptiveness: predicting the outcome of oocyte donation cycles. J Clin Endocrinol Metab. 2005;90(7):4399–404.
Zhao J, Huang B, Li N, Wang X, Xu B, Li Y. Relationship between advanced maternal age and decline of endometrial receptivity: a systematic review and meta-analysis. Aging (Albany NY). 2023b;15(7):2460–72. https://doi.org/10.18632/aging.204555.
Abdalla HI, Wren ME, Thomas A, Korea L. Age of the uterus does not affect pregnancy or implantation rates; a study of egg donation in women of different ages sharing oocytes from the same donor. Hum Reprod. 1997;12(4):827–9.
Irani M, Zaninovic N, Rosenwaks Z, Xu K. Does maternal age at retrieval influence the implantation potential of euploid blastocysts? Am J Obstet Gynecol. 2019;220(4):379.e1-379.e7.
Sekhon L, Whitehouse M, Sandler B, Grunfeld L, Copperman A. Using new technology to ask an old question: does the uterus age? Fertil Steril. 2015;4(3):E150–E151.
Chemerinski A, Garcia de Paredes J, Blackledge K, Douglas NC, Morelli SS. Mechanisms of endometrial aging: lessons from natural conceptions and assisted reproductive technology cycles. Front Physiol. 2024;15:1332946. https://doi.org/10.3389/fphys.2024.1332946.
Li J, Wang Y, Tang R, Peng Y, Wang Y, Liu B, et al. Changes in ultrasound uterine morphology and endometrial thickness during ovarian aging and possible associated factors: findings from a prospective study. Menopause. 2020;27(7):794–800. https://doi.org/10.1097/GME.0000000000001531.
Tomari H, Kawamura T, Asanoma K, Egashira K, Kawamura K, Honjo K, et al. Contribution of senescence in human endometrial stromal cells during proliferative phase to embryo receptivity†. Biol Reprod. 2020;103(1):104–13. https://doi.org/10.1093/biolre/ioaa044.
Lv H, Zhao G, Jiang P, Wang H, Wang Z, Yao S, et al. Deciphering the endometrial niche of human thin endometrium at single-cell resolution. Proc Natl Acad Sci U S A. 2022;119(8):e2115912119. https://doi.org/10.1073/pnas.2115912119.
Devesa-Peiro A, Sebastian-Leon P, Parraga-Leo A, Pellicer A, Diaz-Gimeno P. Breaking the ageing paradigm in endometrium: endometrial gene expression related to cilia and ageing hallmarks in women over 35 years. Hum Reprod. 2022;37(4):762–76. https://doi.org/10.1093/humrep/deac010.
Kalluru S, Cascante SD, Blakemore JK, Grifo JA. How many frozen eggs do I need to get one euploid embryo? An analysis of 450 autologous oocyte thaw patients. Fertil Steril. 2023;120(4 (Supplement)):E37.
Pecoriello J, Kelley AG, Cascante SD, Blakemore JK. Beyond the egg: sperm source and sperm age do not impact cumulative live birth rates (CLBR) in oocyte cryopreservation (OC) Patients (PTS). Fertil Steril. 2023;120(4 (Supplement)):E198.
Sanders KD, Silvestri G, Gordon T, Griffin DK. Analysis of IVF live birth outcomes with and without preimplantation genetic testing for aneuploidy (PGT-A): UK Human Fertilisation and Embryology Authority data collection 2016–2018. J Assist Reprod Genet. 2021;38(12):3277–85.
Scott RTJ, Upham KM, Forman EJ, Hong KH, Scott KL, Taylor D, et al. Blastocyst biopsy with comprehensive chromosome screening and fresh embryo transfer significantly increases in vitro fertilization implantation and delivery rates: a randomized controlled trial. Fertil Steril. 2013;100(3):697–703.
Liss J, Pastuszek E, Pukszta S, Hoffmann E, Kuczynski W, Lukaszuk A, et al. Effect of next-generation sequencing in preimplantation genetic testing on live birth ratio. Reprod Fertil Dev. 2018;30(12):1720–7.
Sui YL, Lei CX, Ye JF, Fu J, Zhang S, Li L, et al. In vitro fertilization with single-nucleotide polymorphism microarray-based preimplantation genetic testing for aneuploidy significantly improves clinical outcomes in infertile women with recurrent pregnancy loss: a randomized controlled trial. Reprod Dev Med. 2020;4(1):32–41.
Practice Committee of the American Society for Reproductive Medicine. asrm@asrm.org. Evidence Evidence-based outcomes after oocyte cryopreservation for donor oocyte in vitro fertilization and planned oocyte cryopreservation: a guideline. Fertil Steril. 2021;116(1):36–47. https://doi.org/10.1016/j.fertnstert.2021.02.024.
Pregnancy at age 35 years or older: ACOG Obstetric Care Consensus No. 11. Obstet Gynecol. 2022;140(2):348–366. https://doi.org/10.1097/AOG.0000000000004873
Fuchs F, Monet B, Ducruet T, Chaillet N, Audibert F. Effect of maternal age on the risk of preterm birth: a large cohort study. PLoS ONE. 2018;13(1):e0191002. https://doi.org/10.1371/journal.pone.0191002.
Guarga Montori M, Álvarez Martínez A, Luna Álvarez C, Abadía Cuchí N, Mateo Alcalá P, Ruiz-Martínez S. Advanced maternal age and adverse pregnancy outcomes: a cohort study. Taiwan J Obstet Gynecol. 2021;60(1):119–24. https://doi.org/10.1016/j.tjog.2020.11.018.
Funding
There was no funding utilized for this research project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The studies involving human participants were reviewed and approved by New York University Grossman School of Medicine Institutional Review Board (S13-00389). Written informed consent from the participants was not required to participate in this specific study in accordance with the national legislation and the institutional requirements.
Competing interests
J. G. is a stockholder of Inception LLC. F. B. has nothing to disclose. D. M. provides consulting services to Granata Bio, Sanford Fertility and Reproductive Medicine, Gameto, and Buffalo IVF and holds a fiduciary role in ReproART and Biogenetics Corporation. S. D. C. has nothing to disclose. J. K. B. is a Medical Advisors to Anya LLC and Julie Inc.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Barrett, F.G., Cascante, S.D., McCulloh, D. et al. Maternal age at transfer following autologous oocyte cryopreservation is not associated with live birth rates. J Assist Reprod Genet 41, 1977–1984 (2024). https://doi.org/10.1007/s10815-024-03149-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-024-03149-y