Abstract
Purpose
To determine if blastocyst transfer increases the ongoing and cumulative pregnancy rates, compared with day 3 embryo transfer, in women of all ages when at least 4 zygotes are obtained.
Methods
Prospective study including patients undergoing a first IVF/ICSI treatment and assigned to cleavage stage (n = 46) or blastocyst (n = 58) embryo transfer. Supernumerary embryos were vitrified and patients failing to achieve an ongoing pregnancy after fresh embryo transfer would go through cryopreserved cycles. The main outcome measure was the ongoing pregnancy rate after the fresh IVF/ICSI transfer and the cumulative ongoing pregnancy rate. Results were also analyzed according to age (under 35 and 35 or older).
Results
A majority of patients (96.6 %) had a blastocyst transfer when at least 4 zygotes were obtained. The ongoing pregnancy rate was significantly higher in the day-5 group compared with the day-3 group (43.1 % vs. 24 %, p = 0.041). The cumulative ongoing pregnancy rate was higher (but not significantly) with blastocyst than with cleavage stage embryos (56.8 % vs. 43.4 %, p = 0.174). When analysed by age, patients 35 or older showed significantly higher ongoing pregnancy rate (48.4 % vs. 19.3 %, p = 0.016) and cumulative ongoing pregnancy rate (58 % vs. 25.8 %, p = 0.01) in the day-5 group compared to the day-3 group, while no such differences were observed in women under 35.
Conclusions
Blastocyst transfer can be suggested whenever there are at least 4 zygotes. While there are no differences in women under 35, the benefit of this option over cleavage stage transfer could be significant in women 35 or older.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The rationale for blastocyst culture is to improve both uterine and embryonic synchronicity and enable self selection of viable embryos thus resulting in higher implantation rate [1]. The meta-analysis by Papanikolaou et al., in 2008 [2], analysing eight randomized controlled trials (RCTs) concluded that the clinical pregnancy rate and live birth rate after fresh IVF were significantly higher after blastocyst-stage embryo transfer as compared to cleavage-stage embryo transfer when equal number of embryos were transferred in the two groups compared. Subsequently 12 RCTs summarized in the Cochrane review [1] demonstrated that live birth rates can be optimized by performing fresh blastocyst transfer compared to cleavage stage embryo transfers, but no differences were observed in the analysis of 23 RCTs in either clinical pregnancy rates or miscarriage rates. However, they included trials using a different number of embryos transferred in the groups compared, which might have conditioned their results. These studies suggest that, although there is not a clear increase on pregnancy rates, live birth rates after fresh IVF significantly improve using blastocysts.
A further controversy has arisen on cumulative pregnancy rates. Several authors defend that there is little advantage in performing blastocyst embryo transfer, since the cumulative pregnancy rate after fresh and frozen embryo transfers in their studies was no different whether the transfer was performed in cleavage or blastocyst stage [3–5], or even better with cleavage stage embryos [6]. All of these studies used a slow freezing protocol with a higher survival rate after thawing for cryopreserved day 2/3 embryos compared to blastocysts. The introduction of vitrification for embryo cryopreservation in our laboratories could modify these results. Vitrification has been reported to have similar survival rate and clinical pregnancy rate for embryos in day 3 and day 5 [7] and might, therefore, allow us to compare under better conditions cumulative pregnancy rates after the transfer of cleavage or blastocyst embryos.
Part of the debate is identifying patients who would benefit from extended culture to day 5. It is not clear whether this technique should be offered to unselected IVF patients or if it would benefit women depending on a minimum number of follicles, fertilized eggs or 8-cell embryos on day 3. Many authors have pointed out the higher probability of embryo transfer cancellation in the blastocyst group compared with the day-3 transfer group [2]. While systematic blastocyst transfer policy in unselected IVF patients has been reported to hold the risk of embryo transfer cancellation up to 27 % [8], the threshold of four good embryos on the third day of evolution appeared to avoid this problem, with blastocyst transfer performed in all patients (under 38 years of age) [9]. An intermediate policy, which is, deciding on blastocyst transfer when there are a minimum of 4 fertilized eggs has had conflicting results, with no blastocysts available for transfer in 23 % [10] or 10.1 % of patients [6]. The present trial will reexamine if it is possible to decide on blastocyst embryo transfer when there are at least 4 zygotes, in women of all ages, without compromising the rate of patients reaching the embryo transfer. This policy might enlarge the number of patients benefitting from the blastocyst transfer.
In summary, the aim of this prospective study was to determine, where at least 4 zygotes were obtained, whether the blastocyst transfer is beneficial in terms of increasing the ongoing pregnancy rate and cumulative pregnancy rate, compared with day 3 embryo transfer in women of all ages. The present publication is an interim analysis of the study performed when 40 % of patients had been recruited.
Materials and methods
Study design
A prospective study of day 3 versus day 5 embryo transfer was performed between June 2011 and October 2013. Eligibility inclusion criteria were: (i) any female age; (ii) first IVF or ICSI cycle; (iii) presence of normal uterine cavity; (iv) ejaculated sperm origin; (v) absence of any contraindications to pregnancy. Exclusion criteria were: (i) oocyte donation cycles; (ii) vitrified oocytes cycles; (iii) non-ejaculated sperm; (iv) PGD.
In the study period 120 patients initiated a first IVF/ICSI cycle fulfilling the embryological inclusion criteria of having at least four fertilized oocytes (with 2 pronuclei and 2 polar bodies) on the day after oocyte retrieval (day 1).
Every patient entered the study only once. Randomization was performed on day 1 of embryo culture by the embryologist using a computer-generated randomized list. The present study was approved by our institutional review board and all the patients gave their signed informed consent. Patients were randomized to receive embryo transfer either at day 3 (n = 60) or day 5 (n = 60).
Our primary outcomes were ongoing pregnancy rate per IVF cycle and cumulative ongoing pregnancy rate per patient, expressed as a percentage. The secondary outcomes were the clinical pregnancy rate and implantation rates after fresh and vitrified embryo transfers.
Ovarian stimulation
Three ovarian stimulation protocols were used for the 120 patients in the present study depending on their age and diagnosis. The long GnRH agonist protocol with intranasal nafarelin (Synarel ®, Seid, Barcelona, Spain) initiated on day 22 of the cycle; the short GnRH agonist protocol with nafarelin (Synarel ®) initiated on day 2 of the cycle; or the GnRH antagonist protocol where the ganirelix (Orgalutran 0,25 ®, Organon, Netherland) was started on day 6 of the stimulation. Recombinant daily FSH (Puregon ®, Organon, Netherland; Gonal ®, Merck-Serono Europe Ltd, UK) was started once the patient’s hormonal status was basal in the long protocol, or on day 3 or 2 of the cycle in the short agonist and antagonist protocol respectively. The initial gonadotrophin dose remained fixed for 5 days and could then be adjusted until the final day of hCG administration based on follicular growth and estradiol levels. Some patients were given added hMG when the need of LH action was perceived (hMG Lepori ®, Angelini Farmacéutica SA, Barcelona, Spain). Final oocyte maturation was induced by administration of 250mcg of coriogonadotropin alfa (Ovitrelle ®, Merck-Serono, Europe Ltd, UK) when at least 3 follicles of 18 mm were observed. Oocyte retrieval was performed under i.v. sedation 35 h after HCG inyection. The luteal phase was supported by vaginal progesterone 600 mg daily (Utrogestan ®, Seid, Barcelona, Spain; Proggefik ®, Effik, Madrid, Spain).
To assess treatment outcome, serum b-hCG was measured 12 days after cleavage-stage embryo transfer and 10 days after blastocyst transfer. Clinical pregnancy was defined by the ultrasound confirmation of an intrauterine gestational sac after 6 weeks of gestation. Ongoing pregnancy was defined when the pregnancy had completed over 20 weeks of gestation. The implantation rate was defined as the number of gestational sacs per transferred embryos.
Embryo culture, embryo evaluation and selection for transfer
The maturity of collected oocytes was determined depending on the feature of the corona radiata. The oocytes were washed in MOPS medium (Serie G5 Vitrolife, Sweden) according to their maturity, and then incubated in IVF medium (Serie G5 Vitrolife, Sweden) at 37 °C with 7.3 % CO2 and 5 % O2 in air with saturated humidity until insemination or removal of surrounding cumulus oophorus and corona radiata cells for intracytoplasmic sperm injection (ICSI). In vitro fertilization was induced by using conventional insemination, ICSI or both of them depending on semen parameters. Oocytes and embryos were cultured in sequential media of Vitrolife Sweden (G5 serie, Kungsbacka, Sweden) using IVF, G1 and G2 medium as recomended by the manufacturer. Fertilization was assessed 16 to18 h after insemination or injection. Normal fertilization was confirmed by the presence of two pronuclei (2PN) and two polar bodies. Other outcomes (i.e. no fertilization, one pronucleus or degeneration) were also recorded. Embryo quality was assessed daily until the moment of transfer and/or vitrification of the supernumerary embryos. Embryos transferred on day 3 were cultured in G1 medium until transfer. Embryos transferred on day 5 were cultured in G2-plus medium (Serie G5 Vitrolife, Sweden) from day 3.
The number, evenness of blastomeres, degree and type of fragmentation, presence of multinuclation and/or vacuoles, anomalies in the zona pellucida and cell division rate, are evaluated every day in order to grade the quality of embryos according to ASEBIR (Asociación para el Estudio de la Biología de la Reproducción) classification [11]. A top quality (grade A) day 3 embryo was defined as having 4 blastomeres on day 2 and 7 or 8 blastomeres on day 3 of equal size and less than 10 % fragmentation. The quality of blastocyst-stage embryos was assessed according to the criteria of Gardner and Schoolcraft [12] based on the degree of expansion and hatching status of the blastocoel cavity (1–6), the size of the inner cell mass (A-C) and the development of the trophectoderm (A-C). Assisted hatching was not performed before embryo transfer.
All embryo transfers were performed using a Wallace catheter (Smith Medical International Ltd. UK) with EmbryoGlue media (Vitrolife Sweden) and guided with an abdominal ultrasound scan. One or two of the best quality embryos were transferred into the uterus on day 3 or 5. When no blastocysts were available on day 5, the most advanced embryos were transferred.
Embryo cryopreservation and vitrified embryo transfers
Supernumerary embryos were evaluated in order to be cryopreserved. Day 3 embryos with at least 6 cells and <20 % fragmentation, and blastocysts with a blastocoel cavity at least grade 3, visible inner cell mass and trophoectoderm grade A-C were vitrified in the day 3 and day 5 group respectively. The vitrification followed the Irvine Scientific procedure (Vitrification Kit, Santa Ana, CA, USA).
Patients failing to achieve an ongoing pregnancy after fresh embryo transfer would go through cryopreserved cycles until all vitrified embryos were transferred or an ongoing pregnancy was achieved. For natural cycles, follicular growth was monitored and ovulation was triggered by an injection of 250mcg of coriogonadotropin alfa (Ovitrelle ®) when a preovulatory follicle was observed. For hormone replaced cycles, ovarian down regulation was induced with an injection of Leuprolide acetate 3.75 mg (Ginecrin ®, Abbott Laboratories, Spain), and estradiol patches 150mcg/day (Estradot ®, Novartis Pharma, Germany) where given after menses. Endometrium was considered prepared when its thickness was over 7 mm and serum estradiol above 100 pg/ml. Vaginal progesterone supplementation was given for lutheal support (400 mg/day and 600 mg/day in natural and hormone replaced cycles respectively). Only warmed day-3 embryos that cleaved after 24 h or thawed blastocysts that re-expanded after 3 h of culture were replaced. Replacement was performed on day 4 or 5 after ovulation or progesterone commencement for cleavage stage embryos and blastocysts respectively. Depending on the couple’s wishes one or two embryos were transferred after warming.
Clinical pregnancy, ongoing pregnancy and implantation rate after vitrified embryo transfer (VET) were defined as stated before in IVF cycles. Cumulative ongoing pregnancy obtained with fresh or vitrified embryos from the same stimulation cycle was defined when the pregnancy had completed over 20 weeks of gestation.
Statistical analysis
The study was designed to detect a difference of 15 % in ongoing pregnancy rates between the groups in which embryo transfer was performed on day 3 or on day 5. For a statistical power of 80 %, at a significance level of 0.05, a sample size of 152 IVF/ICSI cycles in each group was needed, assuming a baseline pregnancy rate of 25 %. The significance level of our interim analysis, including 40 % of the patients needed for each group, reached a statistical power of 65 %. The statistical power of the differences observed in women 35 or older was calculated assuming a cumulative ongoing pregnancy rate at that age group of 25 %, and having 31 patients to compare in day-3 and day-5 groups.
Continuous variables were compared using the independent Student’s t- test or the Mann–Whitney test according to the distribution of their values. Data are presented as mean ± SD. Categorical variables were compared with the χ2 test, using Fisher’s exact test when necessary. The significance level was set at 5 % (P < 0.05). All analyses were performed with the commercial software SPSS version 13.0.
Results
Among the 386 patients who started a first IVF/ICSI cycle between June 2011 and October 2013, 120 patients fulfilled the inclusion criteria on the first day of embryo culture and were randomized to have either a cleavage-stage embryo transfer (day-3 group n = 60) or a blastocyst stage embryo transfer (day-5 group, n = 60). There were 16 patients eligible for the study that were excluded from it: 10 patients with a perceived risk of early ovarian hyperstimulation (OHSS) had their embryo transfer performed on day 5; and 6 patients were not randomized due to organizational reasons that fixed the transfer day on a given date.
Patients demographics, stimulation characteristics and embryology data
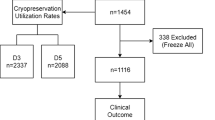
The final distribution of patients was: day-3 group n = 46; and day-5 group n = 58. There were no significant differences in terms of age, infertility diagnosis, duration of sterility, basal FSH levels, antral follicular count, E2 levels on triggering day, and total gonadotrophin dose used (Table 1). Comparable numbers of follicles, oocytes retrieved, metaphase II oocytes and 2PN zygotes were obtained in each group. All patients underwent embryo transfer and the number of embryos transferred were similar in each group (Table 2). In the day-5 group, 2 patients had 2 embryos transferred that had not reached blastocyst stage by day 5. The rate of blastocyst formation in our series was 67.7 %, that is, 245 out of the 362 embryos obtained in the day-5 transfer group reached the blastocyst stage. The percentage of patients with embryos that were vitrified (71.7 % vs. 67.2 % in day-3 and day-5; p = 0.622) and the number of embryos vitrified per patient (3.9 vs 4.9 in day-3 and day-5; p = 0.162) were similar in both groups.
Pregnancy outcomes
The different parameters concerning pregnancy outcome per patient are summarized in Table 3. The clinical pregnancy rate and implantation rate were higher in the day-5 group compared with the day-3 group but did not reach statistical significance. The proportion of miscarriages and multiple pregnancy rate were similar in both groups. The ongoing pregnancy rate at week 20 (43.1 % vs. 24 %; p = 0.041, OR, 2.4; 95 % CI 1.02–5.66) was significantly higher in the day-5 group compared with the day-3 group.
The percentage of patients not achieving an ongoing pregnancy after fresh embryo transfer who had embryos vitrified was similar in both groups (Table 4). Of those, most (87 % and 88.8 %) had vitrified embryo transfers. Three patients from the day-3 group and 2 from the day-5 group (with a mean number of 3 vitrified embryos) had not done a VET cycle at the time of closing our interim analysis. The number of embryos transferred per VET was higher in the day-3 group compared to the day-5 group (1.7 +/− 0.47 vs 1.33+/−0.48; p = 0.02). However the pregnancy rate per cycle, implantation rate and miscarriage rate was similar in both groups. Probably due to the higher number of embryos per transfer, the multiple pregnancy rate was higher in the day-3 group although it did not reach statistical significance. Ongoing pregnancy rate per patient in VET was similar in both groups, as was the cumulative ongoing pregnancy rate per patient.
Impact of age on pregnancy results
Results were analysed for each group for patients under 35 years old and patients 35 or older (Table 5). In patients under 35 no significant differences were observed in either the clinical pregnancy rate, the ongoing pregnancy rate per IVF cycle or the cumulative ongoing pregnancy rate per randomized patient in day-3 vs. day-5 group. However, in patients 35 or older, significantly higher clinical pregnancy rate (54.8 % vs. 29 %, p = 0.039, OR, 2.968; 95 % CI 1.039–8.479), ongoing pregnancy rate per IVF cycle (48.4 % vs. 19.3 %, p = 0.016, OR, 3.906; 95 % CI 1.255–12.163) and cumulative ongoing pregnancy rate per patient (58 % vs. 25.8 %, p = 0.01, OR, 3.981; 95 % CI 1.358–11.666) were observed in the day-5 group compared to the day-3 group. There were no significant differences in patient demographics, stimulation characteristics and embryology data (including the mean number of embryos transferred) in either of the age groups. Table 6 shows patient demographics and stimulation characteristics in women 35 or older. The significance level of 0.01 observed in cumulative ongoing pregnancy rate in favour of blastocyst-stage transfer had a statistical power of 85 %.
Discussion
There is an ongoing debate about the benefits of blastocyst transfer. In our study, in women with 4 or more zygotes, blastocyst transfer allowed us to obtain a higher ongoing pregnancy rate in IVF when analyzing women of all ages, and a higher clinical pregnancy rate, ongoing pregnancy rate and cumulative ongoing pregnancy rate in women 35 or older.
Our approach to decide on blastocyst transfer when there were at least 4 zygotes allowed all patients in the day-5 group to have an embryo transfer. With a blastocyst formation rate of 67.7 %, the majority of patients had at least one blastocyst for transfer (96.6 %) and only 3.4 % of patients had transfers with embryos that had not reached the blastocyst stage. These results are more promising than those presented by Coskun et al. [10] and Emiliani et al. [6] with the same approach. Coskun et al. [10] had 77 % of patients with at least one blastocyst for transfer (the rest having a less advanced embryo transferred). This was probably due to their relatively low blastocyst formation rate (28 %) which they attributed to the high rate of male factor within their patient’s population. Emiliani et al. [6] with a blastocyst formation rate of 48.3 %, had blastocysts for transfer in 89.9 % of patients. In contrast with our study, theirs included patients in all IVF and ICSI cycles or up to 3 previous IVF cycles which might have worsen their patients’ prognosis, or the use of different stimulation protocols (they only used long protocols) could have an effect on the oocyte quality. However, it is likely that the evolution of culture systems in recent years might have allowed for an improvement in our rates of blastocyst formation.
The transfer of an equal number of embryos in the blastocyst and cleavage stage groups, resulted in a higher clinical pregnancy rate and implantation rate with the former, but differences did not reach statistical significance. It is possible that increasing the number of patients compared might allow us to observe the significant difference described by Papanikolaou et al. [2]. The results in our interim analysis, however, are similar to those described in previous meta-analysis [1, 2] regarding the ongoing clinical pregnancy rate. Having a proportion of miscarriages similar in both groups, the ongoing pregnancy rate at week 20 was significantly higher in the day-5 group compared with the day-3 group. Also in accordance with their findings, the multiple pregnancy rate in both groups was similar.
To our surprise, in our study, the number of cryopreserved embryos per patient as well as the percentage of patients with embryos cryopreserved was the same after the transfer in cleavage stage or blastocyst. This is in clear contrast with most publications where cryopreservation rate per cycle and the number of embryos cryopreserved per patient is higher if transfers are performed on day 2/3 vs. day 5 [2–6]. Our high rate of blastocyst formation and only including patients from a first IVF/ICSI cycle might justify the better outcome found in our study. Additionally, in agreement with a previous publication by Cobo et al., [7] we found a survival rate after vitrification and warming similar for embryos in cleavage and blastocyst stage embryos. This allows us to compare under better conditions (than the slow cooling method) the results after cryopreservation. We found that the clinical pregnancy rate per cycle and multiple pregnancy rate were higher, but not significantly, in the day-3 compared with the day-5 group. However, these results are not clinically comparable since the number of embryos per transfer was higher in the day-3 group than in the day-5 group. This problem was also present in the large previous retrospective study by Cobo et al. [7], where they found a higher live birth rate with vitrified embryos on day-3 than day-5. Their patients in the cleavage stage group, however, not only had more embryos transferred per cycle but were also younger than those in the blastocyst transfer group. To limit the effect of the higher number of embryos per transfer in the day-3 group we also analyzed the ongoing pregnancy rate per patient after all cycles of VET and results were similar for both groups.
Finally, the cumulative ongoing pregnancy rate per patient randomized in our study, was higher in the blastocyst group, but the difference with the cleavage stage group was not significant. This result is similar to that obtained by Guerif et al. [4] and Brugnon et al. [5], and different from Rienzi et al. [3] and Emiliani et al. [6] that found a cumulative pregnancy rate per oocyte recovery higher for the day 2/3 transfer as compared with day 5 transfer. All these studies’ results are conditioned, however, by a higher number of cryopreserved embryos and higher survival rate after thawing in the day-3 group compared with the day −5 group.
Most studies have analysed the benefit of blastocyst transfer in young women. Whether comparing the impact of blastocyst transfer in unselected patients, good or poor prognosis patients, most RCTs grouped in the Cochrane review recruited women aged less than 40 years of age, with the mean age varying from 29 to 34 years [1]. While some authors have observed that there is not a significant relationship between age and blastocyst formation [10, 13] others have reported reduced blastocyst development with increasing age [14, 15]. In our study the benefit in ongoing pregnancy rate was significant when considering all patients. Additionally, when stratified by age, women 35 or older had a significantly higher clinical pregnancy rate, ongoing pregnancy rate and cumulative pregnancy rate after day 5 transfer, while women under 35 presented no significant differences in these parameters. Beesley et al. [16], in a retrospective study, had already observed a higher live birth rate in women over 35 years old with day-5 embryo transfers, whereas no difference was seen in patients <35 years old. This benefit might have to do with selecting the best embryos for transfer. Staessen et al. [17] in a PGD study for aneuploidy embryos demonstrated that, at least in women older than 36 years, 59 % of top-quality day 3 embryos were genetically abnormal, whereas only 35 % of top-quality blastocysts were aneuploid. Given that the percentage of aneuploid embryos increases with age it is logical that we observe a more pronounce benefit with older patients transferring day 5 embryos both in fresh IVF and VET.
We are aware that our interim analysis, despite observing a significant improvement in ongoing pregnancy rate per IVF cycle, lacks the originally aimed statistical strength. This would make the continuation of the study a desirable objective. However, we thought it was important to describe the observations within the age groups as the differences observed, mainly in the cumulative ongoing pregnancy rate, were very significant.
In summary, it is possible to decide on performing blastocyst transfer when having 4 or more fertilized oocytes, without risking for a transfer cancellation, or decreasing the percentage of patients with vitrified embryos and number of embryos vitrified. Vitrification allows for a similar good survival rate for both cleavage and blastocyst stage embryos when warmed. The transfer of blastocysts in fresh IVF appear to improve the ongoing clinical pregnancy rates in all patients; however a more significant improvement is observed in patients 35 years or older in clinical pregnancy rate in fresh IVF, ongoing pregnancy rate and cumulative ongoing pregnancy rate.
References
Glujovsky D, Blake D, Bardach A, Farquhar C. Cleavage stage versus blastocyst stage embryo transfer in assisted reproductive technology (Review). Cochrane Database of Syst Rev. 2012. doi:10.1002/1465 1858.CD002118.pub4.
Papanikolaou E, Kolibianakis E, Tournaye H, Venetis C, Fraterni H, Tarlatzis B, et al. Live birth rates after transfer of equal number of blastocysts or cleavage-stage embryos in IVF. A systematic review and meta-analysis. Hum Reprod. 2008;23:91–9.
Rienzi I, Ubaldi F, Iacobelli M, Ferrero S, Minasi MG, Martinez F, et al. Day 3 embryo transfer with combined evaluation at the pronuclear and cleavage stages compares favourably with day 5 blastocyst transfer. Hum Reprod. 2002;17:1852–5.
Guerif F, Lemseffer M, Bidault R, Gasnier O, Saussereau MH, Cadoret V, et al. Single day 2 embryo versus blastocyst-stage transfer: a prospective study integrating fresh and frozen embryo transfers. Hum Reprod. 2009;24:1051–8.
Brugnon F, Bouraoui Z, Ouchchane L, Gremeau AS, Peikrishvili R, Pouly JL, Janny L. Cumulative pregnancy rates after single cleavage-stage versus blastocyst-stage embryo transfer: a randomized and prospective study. Hum Reprod. 2010; i60-1.
Emiliani S, Delbaere A, Vannin AS, Biramane J, Verdoodt M, Englert Y, et al. Similar delivery rates in a selected group of patients, for day 2 and day5 embryos both cultured in sequential medium: arandomized study. Hum Reprod. 2003;18:2145–50.
Cobo A, de los Santos MJ, Castelló D, Gámiz P, Campos P, Remohí J. Outcomes of vitrified early cleavage-stage and blastocyst-stage embryos in a cryopreservation program: evaluation of 3150 warming cycles. Fertil Steril. 2012;98:1138–45.
Van der Auwera I, Debrock S, Spiessens C, Afschrift H, Bakelants E, Meuleman C, et al. A prospective randomized study: day 2 versus day 5 embryo transfer. Hum Reprod. 2002;17:1507–12.
Papanikolaou E, D’haeseleer E, Verheyen G, Van de Velde H, Camus M, Van Steirteghem A, et al. Live birth rate is significantly higher after blastocyst transfer when at least four embryos are available on day 3 of embryo culture. A randomized prospective study. Hum Reprod. 2005;20:3198–203.
Coskun S, Hollanders J, Al-Hassan S, Al Sufyan H, Al-Mayman H, Jaroudi K. Day 5 versus day 3 embryo transfer: a controlled randomized trial. Hum Reprod. 2000;15:1947–52.
ASEBIR. Clinical Embryology Papers: ASEBIR criteria for the morphological evaluation of human oocytes, early embryos and blastocysts. www.asebir.com
Gardner DK, Schoolcraft WB. In vitro culture of human blastocysts. In: Jansen R, Mortimer D, editors. Towards reproductive certainty: Infertility and genetics beyond 1999. The plenary proceedings the 11th World Congress on In Vitro Fertilization and Humarn Reproductive Genetics. New York: Parthenon Pub. Group; 1999. p. 378–88.
Gardner DK, Schoolcraft WB, Wagley L, Schlenker T, Stevens J, Hesla J. A prospective randomized trial of blastocyst culture and transfer in in-vitro fertilization. Hum Reprod. 1998;13:3434–40.
Janny L, Menezo YJR. Maternal age effect on early human embryonic development and blastocyst formation. Mol Reprod Dev. 1996;45:31–7.
Pantos K, Athanasiou V, Stefanidis K, Stavrou D, Vaxevanoglou T, Chronopoulou M. Influence of advanced age on the blastocyst development rate and pregnancy rate in assisted reproductive technology. Fertil Steril. 1999;71:1144–6.
Beesley R, Robinson R, Propst A, Arthur N, Retzloff M. Impact of day 3 or day 5 embryo transfer on pregnancy rates and multiple gestations. Fertil Steril. 2009;91:1717–20.
Staessen C, Plateau P, Van Assche E, Miciels A, Torunaye H, Camus M, et al. Comparison of blastocyst transfer with or without preimplantation genetic diagnosis for aneuploidy screening in couples with advanced maternal age: a prospective randomized controlled trial. Hum Reprod. 2004;19:2849–58.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule The transfer of blastocysts significantly improve the clinical and ongoing pregnancy rate as well as thecumulative pregnancy rate in women 35 years or older.
Rights and permissions
About this article
Cite this article
Fernández-Shaw, S., Cercas, R., Braña, C. et al. Ongoing and cumulative pregnancy rate after cleavage-stage versus blastocyst-stage embryo transfer using vitrification for cryopreservation: Impact of age on the results. J Assist Reprod Genet 32, 177–184 (2015). https://doi.org/10.1007/s10815-014-0387-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-014-0387-9