Abstract
The traditional and local edible brown algae Ecklonia cava subspp. kurome (EK) and stolonifera (ES) are rich in minerals, phlorotannins (brown algal polyphenols), and water-soluble dietary fibres (alginate, laminaran, and fucoidans). These dried powders increase not only alginate- and laminaran-degrading Bacteroides acidifaciens- and B. intestinalis-like bacteria but also Faecalibaculum. To elucidate the effect of EK and ES ingredients, other than dietary fibres, on gut microbiota, 100 mL of ethanolic/aqueous extract solutions (EK-S, ES-S; final solvent was distilled water) were prepared from 100 g of dried samples. EK-S and ES-S contained total phenolic contents of 160 and 120 μmol phloroglucinol equivalent mL-1, respectively. A diet containing 5% (v/w) EK-S or ES-S was fed to ICR mice for 14 days. Amplicon sequencing of the 16S rDNA (V4) gene revealed that EK-S and ES-S increased Faecalibaculum abundance 2-fold and more, and this was the predominant genus (37–38%) in mice. However, the abundances of B. acidifaciens- and B. intestinalis-like bacteria did not increase. Akkermansia muciniphila-like bacteria were higher in EK-S-fed mice. Faecalibaculum and Akkermansia are regarded as desirable gut commensals correlated with anti-inflammatory and immunomodulatory effects on the host’s health. These results suggest that phlorotannins have beneficial functional effects on indigenous gut bacteria responsible for host health.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hundreds of bacterial species inhabit the human gut and are an important component of the gut microbial ecosystem (Collela et al. 2023). In addition, their metabolism can affect host health. For example, they may alleviate or exacerbate lifestyle-related and chronic diseases (Swaby et al. 2023). The gut microbiota is influenced by many factors including lifestyle and diet, the latter of which has a pronounced and immediate impact (Tcherni-Buzzeo 2023). The gut microbiota are rapidly and significantly affected by diet within days to a week prior to the appearance of lifestyle disease markers and symptoms (David et al. 2014; Zmora et al. 2019).
The body generates reactive oxygen species (ROS) such as superoxide anion (O2-) radicals, hydrogen peroxide (H2O2), hydroxyl (OH) radicals, nitric oxide (NO), and singlet oxygen (1O2) during breathing (Andrés et al. 2023). ROS are essential for the immune system, but their overproduction causes inflammation and a range of lifestyle-related, chronic, and degenerative diseases, including respiratory, neurodegenerative, gastrointestinal, and cancer diseases (Sharma and Mehdi 2023). There are several reports on antioxidant components in foods that inhibit ROS generation (Pammi et al. 2023). Though it is generally recognised that adequate fibre intake has a positive effect on the intestinal microbiota, desirable effects of dietary antioxidants, such as astaxanthin for increased Akkermansia and curcumin for increased Roseburia, have been reported (Naliyadhara et al. 2023).
The brown algae of the family Lessoniaceae, genera Eisenia and Ecklonia, are known as 'kajime' in Japan. 'Kajime', a speciality of various regions, has traditionally been used in meals and is otherwise not used. Therefore, large amounts of seaweed drift onto the coastline, which have a negative impact on the environment and fishing industry. Ecklonia cava subspp. kurome (EK) and stolonifera (ES) are rich in water-soluble polysaccharides (alginate, laminaran, and fucoidan), phlorotannins (brown algal polyphenols), and minerals and may have various health benefits (Kuda and Ikemori 2009).
We found that studies in which dried EK and ES powders were administered to mice fed a high-sucrose, low-fibre diet resulted in a marked increase in Bacteroides and Faecalibaculum in the caecum and a decrease in Allobaculum (Fujita et al. 2023). In this study, EK and ES feeding led to a significant increase in cecum volume and a decrease in bile acids in the cecum. Although both genera belong to Erysipelotrichaceae, Faecalibaculum is regarded as an immunomodulatory murine gut commensal (Zagato et al. 2020) and Allobaculum is an obesity-positive related commensal (Zheng et al. 2021). The increase in Bacteroides, but not Faecalibaculum, was also observed when fermentable polysaccharides in brown algae, namely alginate and laminaran, were given (Takei et al. 2020). Therefore, the increase in Faecalibaculum might have been influenced by components other than polysaccharides, such as phlorotannins and minerals.
Although there are reports on the beneficial effects of phlorotannins, such as bifidogenic activity (Vázquez-Rodríguez et al. 2021), these results were obtained from faecal cultures and not in vivo. Different mineral mixture contents in semi-refined diets and salt addition have been reported to affect the caecal microbiota of mice, but no variation in Faecalibaculum or Allobaculum has been observed (Xia et al. 2022). In the present study, to investigate the Faecalibaculum-promoting and Allobaculum-suppressing compounds in EK and ES, ethanolic/aqueous extracts of the algae were administered to mice fed a high-sucrose and low-fibre diet. The caecal microbiota were analysed using 16S rDNA (V4) amplicon sequencing. The results showed that EK and ES phlorotannins altered the gut microbial composition and had potential applications as functional food ingredients for enhancing human gastrointestinal health.
Materials and methods
Preparation of ethanolic/aqueous extracts
Dried Ecklonia cava subsp. kurome products harvested in Ise and Kyushu, Japan, were purchased from Satsumaya Co. (Chiba, Japan). Dried Ecklonia cava subsp. stolonifera products were purchased from Ofune Kaisan Co. (Aomori, Japan). Dried Saccharina japonica holdfast (SJh) was obtained from a fishing port in Fukushima (Hokkaido, Japan). Brown algae was harvested, lightly rinsed in water, and immediately subjected to sun-drying.
The dried brown algae were pulverised using a blender (Oster Urban Blender 450 W; Coleman Japan Co., Tokyo, Japan) and sieved through a 1.0-mm mesh. Dried algae powder (100 g) was added to 500 mL of 50% (v v-1) ethanol and shaken at 150 rpm at 50 °C for 30 min twice. After filtration through a No. 2 filter paper (Advantec Toyo Kaisha, Japan) using an aspirator, 200 mL ethanol was added to the filtered solution (400 mL). This was then centrifuged at 6000 × g for 10 min at 4 °C. The resulting supernatant was concentrated to 100 mL using a rotary evaporator. The extracted solutions were labelled EK-S, ES-S, and SJh-S and were stored at -20 °C until further use.
Major ions and antioxidant properties
Major ions
Among the major five cations (Na+, K+, NH4+, Ca2+, and Mg2+) and three anions (Cl-, PO43-, and SO42-), K+, NH4+, Ca2+, Cl-, PO43, and SO42- were measured using commercially available kits from the series of Reagent Set for Water Analyzer (LR-K, LR-NH4-A, LR-Ca-B, LR-Cl, LR-PO4, and LR-SO4, respectively; Kyoritsu Chemical-Check Lab., Corp., Japan) (Takei et al. 2017). Na+ was measured using an Na+ ion meter (LAQUA Twin B-721; Horiba, Japan). Mg2+ concentration was determined using a commercial kit (Magnesium B-Test Wako; Fujifilm Wako Pure Chemical Industries, Ltd., Japan).
Total phenolic content (TPC) and antioxidant capacities
The TPC of the aqueous extract solutions was determined using the Folin–Ciocalteu method as previously reported (Kuda and Ikemori 2009). TPC was expressed as phloroglucinol equivalents per millilitre (PGEq mL-1). To evaluate the antioxidant properties of the aqueous extract solutions, 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical-scavenging activity, superoxide anion (O2-) radical-scavenging activity, and ferric (Fe)-reducing power were determined as previously reported (Kuda et al. 2021). The antioxidant properties were measured and recorded as μmol (+)-catechin/aqueous extract solution equivalents per millilitre (CatEq mL-1).
Effects of the ethanolic/aqueous extracts on mice fed a high-sucrose and low-dietary fibre diet
Animal care
Animal experiments were performed in accordance with the ‘Fundamental Guidelines for Proper Conduct of Animal Experiments and Related Activities in Academic Research Institutions’ under the jurisdiction of the Ministry of Education, Culture, Sports, Science and Technology, Japan. The study protocol was approved by the Animal Experiment Committee of Tokyo University of Marine Science and Technology (Approval No. R3-1).
Thirty 5-week-old male Institute of Cancer Research (ICR) mice (25 ± 2 g) were purchased from Tokyo Laboratory Animals Science Co. (Tokyo, Japan) and housed in metal wire cages with a 12-h dark/light cycle at 22 ± 2 °C. The mice were acclimatised to a powder control (CT) diet [50% (w w-1) sucrose, 15% corn starch, 5% cellulose, 20% milk casein, 5% corn oil, 3.5% AIN-93 mineral mix, 1% AIN-76 vitamin mix, 0.3% DL-methionine, and 0.2% choline bitartrate] and provided with distilled water ad libitum. After 7 days, the mice were divided into five groups: CT, NF (no fibre), EK, ES, and SJh. The CT group was fed a diet supplemented with 5% (v w-1) distilled water (DW). The NF, EK, ES, and SJh groups were fed a diet in which cellulose from the CT diet was replaced with corn starch, with added 5% (v w-1) DW, EK-S, ES-S, or SJh-S extracts for 14 days. During feeding days 11–13, the defaecation frequency and faecal weight were measured.
At the experimental endpoint, the mice were exsanguinated via the abdominal vein under isoflurane anaesthesia, and the liver, kidneys, spleen, and epididymal fat pads were removed and weighed. After ligation with yarn, the caecum was excised and placed on ice until microbial analysis was performed.
Plasma lipid and glucose levels and caecal bile acid content
Plasma triacylglycerol (TG), total cholesterol (TC), and glucose (Glu) levels were determined using commercial kits according to the manufacturer’s instructions (Triglyceride E-Test Wako, Total Cholesterol E-Test Wako, and Glucose C II-Test Wako, respectively; Fujifilm Wako Pure Chemicals). The total bile acid (TBA) content in the diluted caecal suspension for the direct cell count was determined using a commercial kit (TBA-Test Wako, Fujifilm Wako Pure Chemicals).
Analysis of caecal microbiota
The caecal contents (0.1g) were diluted using 4.9 mL of phosphate-buffered saline (Nissui Pharmaceutical, Japan), and the bacterial cell count was determined via dielectrophoretic impedance measurements (DEPIM) (Shikano et al. 2019) using a bacterial counter (PHC Ltd., Japan); 16S rDNA (V4) amplicon sequencing was performed by Fasmac Co. Ltd. (Atsugi, Japan). Briefly, DNA was extracted from 0.1 g of caecal samples and 1 mL of faecal culture suspensions using an MPure bacterial DNA Extraction Kit (MP Bio Japan, Japan). A DNA library was prepared using a two-step polymerase chain reaction (PCR) method (Sinclair et al. 2015).
The V4 region was amplified using the forward 515f and reverse 806r primers and ExTaq HS DNA polymerase (Takara Bio, Japan) in the first PCR (94 °C for 2 min; followed by 20 cycles of 94 °C for 30 s, 50 °C for 30 s, and 72 °C for 30 s; and finally, 72 °C for 5 min). After purification of the products using the AMPure XP Kit (Beckman Coulter Life Science, Japan), individual DNA fragments were tagged in the second PCR (94 °C for 2 min; followed by 8 cycles of 94 °C for 30 s, 60 °C for 30 s, and 72 °C for 30 s; and finally, 72 °C for 5 min) using the same polymerase kit. The DNA libraries were multiplexed and loaded onto an Illumina MiSeq system (Illumina, San Diego, CA, USA).
Reads with a mismatched sequence in the start region were filtered using the FASTX Toolkit (http://hannonlab.cshl.edu/fastx_toolkit/) and 235–260 base-pair reads were selected. Chimeras in the selected reads were identified and omitted using the QIIME2 bioinformatics pipeline (https://qiime2.org/). A feature table was generated using the dada2 denoise-paired option in the QIIME 2 plugin (Poncheewin et al. 2020), and the sequences were clustered into amplicon sequence variants (ASVs) using the SILVA 138 database (https://www.arb-silva.de/). The obtained sequences were deposited in the DDBJ Sequence Read Archive (DRA) under the accession number DRA DRA017128 (https://ddbj.nig.ac.jp/).
Statistical analysis
Analysis of variance, followed by Tukey’s post hoc test for in vitro and in vivo experiments, respectively, were performed using the statistical software MEPHAS (http://www.gen-info.osaka-u.ac.jp/testdocs/tomocom/). Statistical significance was set at p < 0.05. Alpha diversity of the caecal microbiota was determined using Shannon–Wiener (H') and Simpson (D) indices (Kim et al. 2017). To estimate the beta diversity and detect key bacterial groups, principal coordinate analysis (PCoA) and linear discriminant analysis (LDA) effect size (LEfSe) were performed using the MicrobiomeAnalyst web service (https://www.microbiomeanalyst.ca/).
Results
Major ions and antioxidant properties
Major ions
The salinities and major ions in the ethanolic/aqueous solutions are summarised in Table 1. The salinities of EK-S, ES-S, and SJh-S were 2.2 ± 0.1%, 4.8 ± 0.1%, and 5.6 ± 0.4% (w/v), respectively. Among the major cations, the K+ concentration in the SJh-S and ES solutions (795 ± 66 and 635 ± 87 μmol mL-1) was higher than that in EK solution (95 ± 9 μmol mL-1). The Na+ concentration was the highest in ES-S (405 ± 20 μmol mL-1). The ratio of K+ to Na+ in the SJh-S solution (4.6 ± 0.2) was much higher than that in the other solutions. Ca2+ and Mg2+ in ES-S (34 ± 1, 16 ± 2 μmol mL-1) were also higher than those in EK-S and SJh-S. Although the dominant anion in the ES-S and SJh-S was Cl- (268 ± 5, 349 ± 12 μmol mL-1), SO4- was high in EK-S (131 ± 19 μmol mL-1).
TPC and antioxidant properties
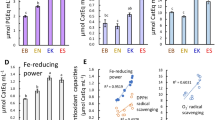
TPC levels in the ethanolic/aqueous extract solutions of EK-S and ES-S were 160 and 120 μmol PGEq mL-1, respectively (Fig. 1A). The DPPH radical-scavenging capacity was high in the EK-S and ES-S solutions (Fig. 1B). The O2- radical-scavenging capacity and Fe-reducing power were the highest in EK-S, followed by ES-S (Fig. 1C, D). The DPPH and O2- radical-scavenging capacities and Fe-reducing power correlated with the TPC results, expressed as r2 = 0.957, 0.981, and 0.993, respectively .
Total phenolic content (TPC, A), DPPH and superoxide anion radical- scavenging capacities (B, C), and Fe-reducing power (D) of ethanolic/aqueous extract solutions from dried products of Ecklonia cava subsp. kurome (EK-S), E. cava subsp. stolonifera (ES-S), and Saccharina japonica holdfast (Sjh-S). Values represent the means ± standard deviation (SD, n = 3). a-c Values with different superscript letters indicate significant differences at p < 0.05.
Body, faecal, and organ weights, and levels of plasma lipids, plasma glucose, and caecal bile acid
No disease symptoms or abnormalities were observed in any of the mice during the feeding period. The mean initial body weight of mice was 35–36 g (Table 2), and there was no significant difference in body weight gain (9.1–11.4 g). Defaecation frequency and faecal weight in the NF group were 2-fold lower than those in the CT group. Although faecal weight increased with EK-S and ES-S administration, there were only minor differences. There was no significant difference in organ weights between the NF group and mice fed the extract solutions.
Although there were no significant differences in the plasma lipid and glucose levels, the TC and glucose tended to be lower in the EK group than in the NF group. EK-S feeding reduced the caecal TBA by 5 fold.
Caecal microbiota
Total bacterial counts and microbiome diversity
The direct total bacterial cell count in the caecum of the mice measured using DEPIM was approximately 10.3–10.7 log cells g-1, which tended to be lower in the EK group, although the difference was not significant (Fig. 2A). The total number of reads was 65,000–79,000 (Fig. 2B), which was lower in the CT group than in the other groups. The average number of ASVs in the CT group was 150, which was 1.5–2-fold higher than those in the NF, EK, and SJh groups (Fig. 2C). Compared with the CT diet, alpha diversity indices [Shannon–Wiener index (H′) and Simpson’s index (D)] were lowered with diets not containing cellulose (Fig. 2D,E).
Direct cell count (A), total amplicon sequence read number (B), amplicon sequence variant (ASV) number (C), and alpha diversity indices (D, E) of the caecal microbiota in mice fed a control diet (CT), no fibre diet (NF), and NF containing 5% (v/w) Ecklonia cava subsp. kurome (EK), E. cava subsp. stolonifera (ES), or Saccharina japonica holdfast (SJh) ethanolic/aqueous solution. (A–E) The box plot represents the third quartile, median, first quartile, and minimum values of six mice per treatment group. a,b Values with different superscript letters indicate significant differences at p < 0.05.
Beta-diversity and LEfSe
PCoA at the genus level (Fig. 3A) revealed that the composition of the gut microbiota of the EK and ES groups, not the Sjh group, differed from that of the NF group. EK consumption further altered the composition of the gut microbiome compared to ES consumption. Figure 3B shows that the LDA score was 4.0 and higher at the genus level. The LEfSe results suggest that Faecalibaculum, Bacteroides, Akkermansia, Parasutterlla, Parabacteroides, and Alistipes were EK- and ES-responsible gut indigenous genera.
Results of principal coordinate analysis (PCoA) (A) and linear discriminant analysis (LDA) effect size (LEfSe) plot (B) at the genus level and composition of the caecal microbiota at the phylum (C), family (D), and genus (E) levels in mice fed a control diet (Cont., CT), no fibre diet (NF), and NF containing 5% (v/w) Ecklonia cava subsp. kurome (EK), E. cava subsp. stolonifera (ES), or Saccharina japonica holdfast (SJh) ethanolic/aqueous solution. a-c Values with different superscript letters indicate significant differences at p < 0.05.
Microbiota at the phylum, family, and genus levels
The caecal microbiota profile at the phylum, family, and genus levels, expressed by relative abundance, is shown in Fig. 3C-E. The predominant phylum in the CT group was Firmicutes (81 ± 2%), followed by Actinobacteriota (10 ± 3%), Desulfobacteriota (4.7 ± 2.1%), and Bacteroidota (2.7 ± 1.4%) (Fig. 3C). The NF diet increased Actinobacteriota (23 ± 3%) and decreased Desulfobacteriota (0.31 ± 0.13%) and Bacteroidota (0.43 ± 0.13%). Compared with the NF diet, the EK and ES diets increased Bacteroidota. (9.0 ± 2.3% and 7.7 ± 1.3%), Although not very abundant, EK-S increased Verrucomicrobiota from 0.31 ± 0.13% to 1.8 ± 0.3%.
Among the Firmicutes, the predominant family in the CT group was Erysipelotrichaceae (27 ± 8%), followed by Lachnospiraceae (17 ± 6%), Lactobacillaceae (15 ± 6%), and Streptococcaceae (15 ± 2%) (Fig. 3D). Further, the NF diet reduced Lachnospiraceae (1.9 ± 0.5%). Compared to the NF group, EK-S and ES-S decreased Streptococcaceae from 9.9 ± 1.6% to 5.3 ± 0.9% and 4.6 ± 0.5%, respectively. Among the phylum Bacteroidota increased by EK-S and ES-S, Bacteroidaceae was dominant, followed by Muribaculaceae. Almost all Actinobacteriota, Verrucomicrobiota, and Proteobacteria consisted of Bifidobacteriaceae, Akkermansiaceae, and Sutterellaceae species, respectively.
Among the Erysipelotrichaceae found in the NF group, Allobaculum (24 ± 2%) and Faecalibaculum (18 ± 4%) were predominant (Fig. 3E). The Allobaculum count was suppressed in the EK (13 ± 1%) and ES (11 ± 4%) groups. In contrast, Faecalibaculum abundance was significantly higher in the EK (39 ± 2%) and ES (38 ± 7%) groups than in the NF group.
ASV level
Figure 4 shows a heat map of the top 30 ASVs with a high abundance and significant differences between groups by abundance with a defined name using BLASTn in National Center for Biotechnology Information (https://blast.ncbi.nlm.nih.gov/Blast.cgi). In total, 10, 5, and 1 ASVs differed between the NF and EK, ES, and SJh groups, respectively. In the phylum Firmicutes, all three Allobaculum ASVs (ID-2, 22, and 23) were dominant in the NF and SJh groups. In contrast, three Faecalibaculum ASVs (ID-0, 16, and 17), which were assumed to be F. rodentium-like bacteria, were dominant in the EK and ES groups. In the phylum Bacteroidota, Phocaeicola vulgatus-like bacteria (ID-8 and 35) were abundant in the EK and ES groups. Parabacteroides goldsteinii-like bacteria (ID-33) were high in EK. An ASV of Akkermansia (ID-19) and an ASV of Sutterellaceae (ID-25) that were high in EK were defined as A. muciniphila- and Parasutterella excrementihominis-like bacteria, respectively. Three Bifidobacteria, which were increased in the NF diet group, were defined as B. pseudolongum-like bacteria.
Heat map of the top 30 selected abundant ASVs in the caecal microbiota of mice fed control diet (CT), no fibre diet (NF), and NF containing 5% (v/w) Ecklonia cava subsp. kurome (EK), E. cava subsp. stolonifera (ES), or Saccharina japonica holdfast (SJh) ethanolic/aqueous solution. a-c Values with different superscript letters indicate significant differences at p < 0.05.
Discussion
The ratio of K+ to Na+ (K:Na) is important for individuals who take diuretics to control hypertension and experience excessive K+ excretion (Little et al. 2023). Various plants that require salt tolerance possess transport systems that accumulate K+ in their bodies (Chen et al. 2019). These results suggest that SJh (Table 1) is a particularly promising K+-supplying food.
The high TPC and strong antioxidant properties of EK-S and ES-S observed in the present study (Fig. 1) corroborate with previously reported results for hot water extracts of EK and ES (Kuda and Ikemori 2009; Fujita et al. 2023). Phlorotannins are phenolic compounds found in brown algae, which are known for their powerful antioxidant effects, along with other properties, such as anticancer, antiviral, anti-inflammatory, and anti-diabetic abilities (Kuda et al. 2021; Shrestha et al. 2021; Pradhan and Ki 2023). These activities are related to body, liver, and spleen weights. Furthermore, the significant reduction in the gut bile acids by EK, also observed in this experiment, may be related to enterohepatic circulation and lipid metabolism (Collins et al. 2022). However, in this study, owing to the short administration period, the only significant difference was in faecal weight compared to that in the NF group, with an increasing trend in caecal weight (Table 2).
The effects of these diets on the intestinal microbiota are known to occur earlier than those caused by lifestyle changes in various disease conditions (David et al. 2013). In a previous study, 5% (w w-1) EK and ES powder-containing diets significantly increased caecal weight that was 2.4 and 1.7 fold higher than that of the NF group (Fujita et al. 2023). The main factors contributing to the increase in caecal weight are assumed to be changes in osmotic pressure owing to the water-holding capacity of soluble dietary fibres (alginate and laminaran) and stimulation of intestinal epithelial cells by gut fermentation (Nakata et al. 2016); moreover, the effect of phlorotannins on intestinal metabolism may be involved.
The high and low abundances of the caecal Actinobacteriota (Bifidobacterium) and Bacteroidota, respectively, in the NF mice in our study have also been observed in a previous report (Kuda et al. 2017). Bifidobacterium was decreased and Bacteroidota was increased by EK, ES, and SJh powders (Fujita et al. 2023; Midorikawa et al. 2023). In this study, although Bacteroidota were slightly increased by EK-S and EK-S, Bifidobacterium was not reduced, suggesting that these bacterial groups are more influenced by dietary fibre than by the polyphenols in brown algae (Fig. 3). Among the Bacteroidaceae species, Bacteroides acidifaciens-, Bacteroides intestinalis-, and Phocaeicola vulgatus-like bacteria were increased by EK and ES (Fujita et al. 2023). Alginate and laminaran intake increase caecal B. acidifaciens and B. intestinalis, respectively, but not P. vulgatus (Takei et al. 2020). The increased ASVs of P. vulgatus-like bacteria (ID-8 and 35 in Fig. 4) can be considered one of the phlorotannin-responsible gut indigenous bacteria (RIB) in ICR mice. P. vulgatus-like bacteria are also increased by other brown algae rich in phlorotannins, such as Sargassum horneri (Lee et al. 2022). There are several reports on the correlation between the health functions of polyphenols, such as quercetin, and gut P. vulgatus abundance (Etxeberria et al. 2015), but there is no research on the relationship between phlorotannins and gut P. vulgatus in vivo. P. vulgatus is a human and rodent gut indigenous bacteria, which is beneficial in immunoregulation, obesity management, and coronary artery disease interventions (Xu et al. 2023).
Among Erysipelotrichaceae, Allobaculum is positively associated with multiple dietary- and lifestyle-related diseases, including obesity and diabetes (Zheng et al. 2021). This genus was suppressed by EK, ES, and SJh (Fujita et al. 2023; Midorikawa et al. 2023), and did not decrease with alginate and laminaran (Takei et al. 2020). In the present study, although Allobaculum tended to be low in EK-S and EK-S (Fig. 3C), there may have been a combined effect of polyphenols and dietary fibres in brown algae. In contrast, caecal Faecalibaculum, which was estimated to be F. rodentium-like bacteria, was increased by TPC-rich EK, ES, and S. horneri, but not by alginate or laminaran (Takei et al. 2020; Lee et al. 2022; Fujita et al. 2023). These results suggest that F. rodentium-like bacteria is the most common phlorotannin-RIB in ICR mice. Faecalibaculum rodentium is regarded as a murine gut commensal that has a protective effect against intestinal tumours and is positively correlated with antioxidant and anti-inflammatory foods and compounds, such as Sichuan pepper and glycine (Zagato et al. 2020; Zhang et al. 2021; Xia et al. 2023).
Akkermansia is an important genus that maintains the mucosal layer in its host (Cheng and Xie 2021). Its abundance may negatively correlate with obesity and diabetes (Xu and Feng 2020). Prebiotics that increase Akkermansia are currently under research, with several reports indicating the Akkermansia-increasing effects of polyphenols (Rodríguez-Daza et al. 2021). In a previous study, EK, ES, and S. horneri powders increased Akkermansia abundance, which was more significant than that observed in the present study (Lee et al. 2022; Fujita et al. 2023). Akkermansia increased in mice fed a high-fat diet supplemented with fucoidan (Zheng et al. 2020). Furthermore, Parasutterella, which was abundant in the EK-S group, is an anaerobic genus belonging to Betaproteobacteria. Although the roles of Parasutterella in the gut environment are unclear, the relative abundance of P. excrementihominis has been associated with different host health outcomes, such as inflammatory bowel disease, obesity, diabetes, and fatty liver disease (Ju et al. 2019).
As previously mentioned, EK and ES powders significantly increased F. rodentium-like bacteria and Akkermansia and decreased Allobaculum in the murine caecum. However, F. rodentium-like bacteria and Akkermansia were not increased by brown algal soluble dietary fibres such as alginate and laminaran. The results of this study showed an increase in F. rodentium-like bacteria following EK-S and ES-S intake. Additionally, Akkermansia can be considered a phlorotannin-RIB. These results evidenced that phlorotannins and RIBs have beneficial functional properties that improve host health. These findings can guide the usage of brown algae as a functional food for human gastrointestinal health. Based on the results of this study, an analysis of the composition of phlorotannins in different EK and ES species and the effect of different types of phlorotannins on the gut microbiota will be performed in the future.
Conclusion
To clarify the effects of EK, ES, and SJh on gut microbiota, their ethanolic/aqueous extracts were used. Amplicon sequencing revealed that the diets containing 5% (v w-1) EK-S and ES-S, but not SJh-S, increased Faecalibaculum significantly. This effect was observed in the diet containing 5% (w/w) EK and ES dried powder, but not with alginate, laminaran, or SJh. In contrast, the alginate- and/or laminaran-degrading bacteria, B. acidifaciens- and B. intestinalis-like bacteria, were dominant in mice fed the EK, ES, and SJh diets. Akkermansia muciniphila-like bacteria were also abundant in the EK-S mice than in the NF mice. These suggest that phlorotannins and RIBs have beneficial functional properties on host health. Hence, brown algae has potential use as a functional food for improving human gastrointestinal health.
Data availability
The obtained sequences were deposited in the DDBJ Sequence Read Archive (DRA) under the accession number DRA DRA017128 (https://ddbj.nig.ac.jp/). The other datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Andrés CMC, de la Lastra JMP, Juan CA, Plou FJ, Pérez-Lebeña E (2023) From reactive species to disease development: Effect of oxidants and antioxidants on the cellular biomarkers. J Biochem Mol Toxycol 37:e23455
Chen T, Wong W, Xu K, Xu Y, Ji D, Chen C, Xie C (2019) K+ and Na+ transport contribute to K+/Na+ homeostasis in Pyropia haitanensis under hypersaline stress. Algal Res 40:101526
Cheng D, Xie MZ (2021) A review of a potential and promising probiotic candidate—Akkermansia muciniphila. J Appl Microbiol 130:1813–1822
Colella M, Charitos IA, Ballini A, Cafiero C, Topi S, Palmirotta R, Luigi Santacroce L (2023) Microbiota revolution: How gut microbes regulate our lives. World J Gastroenterol 29:4368–4383
Collins SL, Stine JG, Bisanz JE, Okafor CD, Patterson AD (2022) Bile acids and the gut microbiota: metabolic interactions and impacts on disease. Nat Rev Microbiol 21:236–247
David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ (2014) Diet rapidly and reproducibly alters the human gut microbiome. Nature 505:559–563
Etxeberria U, Arias N, Boqué N, Macarulla MT, Portillo MP, Martínez JA, Milagro FI (2015) Reshaping faecal gut microbiota composition by the intake of trans-resveratrol and quercetin in high-fat sucrose diet-fed rats. J Nutr Biochem 26:651–660
Fujita S, Lee G, Takahashi H, Nakamura A, Koga K, Handa N, Kuda T, Xia Y (2023) Ecklonia cava subsp. kurome and E. cava subsp. stolonifera can aid regulation of gut microbiota in mice fed a high-sucrose and low-dietary fibre diet. J Appl Phycol 35:1365–1375
Ju T, Kong JY, Stothard P, Willing BP (2019) Defining the role of Parasutterella, a previously uncharacterized member of the core gut microbiota. ISME J 13:1520–1534
Kim B, Shin J, Guevarra RB, Lee JH, Kim DW, Seol K, Lee J, Kim HB, Issacson RE (2017) Deciphering diversity indices for a better understanding of microbial communities. J Microbiol Biotechnol 27:2089–2093
Kuda T, Ikemori T (2009) Minerals, polysaccharides and antioxidant properties of aqueous solutions obtained from macroalgal beach-casts in the Noto Peninsula, Ishikawa, Japan. Food Chem 112:575–581
Kuda T, Nishizawa M, Toshima D, Matsushima K, Yoshida S, Takahashi H, Kimura B (2021) Antioxidant and anti-norovirus properties of aqueous acetic acid macromolecular extracts of edible brown macroalgae. LWT-Food Sci Technol 141:110942
Kuda T, Yokota Y, Shikano A, Takei M, Takahashi H, Kimura B (2017) Dietary and lifestyle disease indices and caecal microbiota in high fat diet, dietary fibre free diet, or DSS induced IBD models in ICR mice. J Funct Foods 35:605–614
Lee G, Midorikawa Y, Kuda T, Harada M, Fujita S, Takahashi H, Kimura B (2022) In vitro antioxidant and anti-glycation properties of Sargassum horneri from golden tides on the South Korean coast and the effect on gut microbiota of mice fed a high-sucrose and low-fibre diet. J Appl Phycol 34:2211–2222
Little R, Murali SK, Poulsen SB, Grimm PR, Assmus A, Cheng L, Ivy JR, Hoom EJ, Matchkoy V, Welling PA, Fenton RA (2023) Dissociation of sodium-chloride cotransporter expression and blood pressure during chronic high dietary potassium supplementation. JCL Insight 8:e156437
Midorikawa Y, Kuda T, Xia Y, Nishizawa M, Yamagishi T, Takahashi H, Lee G (2023) Effects of Saccharina japonica holdfast powder on microbiota in the caecum of mice fed a high-sucrose and low-fibre diet and in human faecal cultures. Waste Biomass Valoriz 14:3539–3552
Nakata T, Kyoui D, Takahashi H, Kimura B, Kuda T (2016) Inhibitory effects of laminaran and alginate on production of putrefactive compounds from soy protein by intestinal microbiota in vitro and in rats. Carbohydr Polym 143:61–69
Naliyadhara N, Kumar A, Gangwar SK, Devanarayan TN, Hegde M, Alqahtani MS, Abbas M, Sethi G, Kunnumakkara A (2023) Interplay of dietary antioxidants and gut microbiome in human health: What has been learnt thus far? J Funct Foods 100:105365
Pammi SSS, Suresh B, Giri A (2023) Antioxidant potential of medicinal plants. J Crop Sci Biotechnol 26:13–26
Poncheewin W, Hermes GDA, van Dam JCJ, Koehorst JJ, Smidt H, Schaap PJ (2020) NG-Tax 2.0: A Semantic framework for high-throughput amplicon analysis. Front Genet 10:1366
Pradhan B, Ki J (2023) Antioxidant and chemotherapeutic efficacies of seaweed-derived phlorotannins in cancer treatment: A review regarding novel anticancer drugs. Phytother Res 37:2067–2091
Rodríguez-Daza M, Pulido-Mateos EC, Lupien-Meilleur J, Guyonne D, Desjardins Y, Roy D (2021) Polyphenol-mediated gut microbiota modulation: Toward prebiotics and further. Front Nutr 8:689456
Sharma V, Mehdi MM (2023) Oxidative stress, inflammation and hormesis: The role of dietary and lifestyle modifications on aging. Neurochem Int 164:105490
Shikano A, Kuda T, Shibayama J, Toyama A, Ishida Y, Takahashi H, Kimura B (2019) Effects of Lactobacillus plantarum Uruma-SU4 fermented green loofah on plasma lipid levels and gut microbiome of high-fat diet fed mice. Food Res Int 121:817–824
Shrestha S, Zhang W, Smid SD (2021) Phlorotannins: A review on biosynthesis, chemistry and bioactivity. Food Biosci 39:100832
Sinclair L, Osman OA, Bertilsson S, Eiler A (2015) Microbial community composition and diversity via 16S rRNA gene amplicons: Evaluating the Illumina platform. PloS One 10:e0116955
Swaby A, Atallah A, Varol O, Cristea A, Quail DF (2023) Lifestyle and host determinants of antitumor immunity and cancer health disparities. Trends Cancer 9:1019–1040
Takei M, Kuda T, Eda M, Shikano A, Takahashi A, Takahashi H, Kimura B (2017) Antioxidant and fermentation properties of aqueous solutions of dried algal products from the Boso Peninsula, Japan. Food Biosci 19:85–91
Takei MN, Kuda T, Taniguchi M, Nakamura S, Takahashi H, Kimura B (2020) Detection and isolation of low molecular weight alginate- and laminaransusceptible gut indigenous bacteria from ICR mice. Carbohydr Polym 238:116205
Tcherni-Buzzeo M (2023) Dietary interventions, the gut microbiome, and aggressive behavior: Review of research evidence and potential next steps. Aggressive Behav 49:15–32
Vázquez-Rodríguez B, Santos-Zea L, Heredia-Olea D, Acevedo-Pacheco L, Gutiérrez-Uribe JA, Cruz-Suárez LE (2021) Effects of phlorotannin and polysaccharide fractions of brown seaweed Silvetia compressa on human gut microbiota composition using an in vitro colonic model. J Funct Foods 84:104596
Xia Y, Kuda T, Nakamura S, Takahashi H, Kimura B (2022) Detection of low‑mineral‑ and high‑salt responsible caecal indigenous bacteria in ICR mice. 3 Biotech 12:59
Xia Y, Kuda T, Yamamoto M, Yano T, Nakamura A, Takahashi H (2023) Effect of Sichuan pepper on gut microbiota in mice fed a high-sucrose and low-dietary fibre diet. Appl Microbiol Biotechnol 107:2627–2638
Xu M, Lan R, Qiao L, Lin X, Hu D, Zhang S, Yang J, Zhou J, Ren Z, Li X, Liu G, Liu L, Xu J (2023) Bacteroides vulgatus ameliorates lipid metabolic disorders and modulates gut microbial composition in hyperlipidemic rats. Microbiol Spectrum 11:02517–22
Zagato E, Pozzi C, Bertocchi A, Schioppa T, Saccheri F, Guglietta S, Fosso B, Meloncchi L, Nizzoli G, Troisi J, Marzano M, Oresta B, Spadoni I, Atarashi K, Carloni S, Dornasa G, Asnicar F, Segata N, Guglielmetti S, Honda K, Pesole G, Vermi W, Penna G, Rescigno M (2020) Endogenous murine microbiota member Faecalibaculum rodentium and its human homologue protect from intestinal tumour growth. Nature Microbiol 5:511–524
Zhang Y, Mu T, Jia H, Yang Y, Wu Z (2021) Protective effects of glycine against lipopolysaccharide-induced intestinal apoptosis and inflammation. Amino Acids 54:353–364
Zheng L, Chen X, Cheong K (2020) Current trends in marine algae polysaccharides: The digestive tract, microbial catabolism, and prebiotic potential. International J Biol Macromol 151:344–354
Zheng Z, Lyu W, Ren Y, Li X, Zhao S, Yang H, Xiao Y (2021) Allobaculum involves in the modulation of intestinal ANGPTLT4 expression in mice treated by high-fat diet. Front Nutr 8:600138
Zmora N, Suez J, Elinav E (2019) You are what you eat: diet, health and the gut microbiota. Nat Rev Gastroenterol Hepatol 16:35–56
Acknowledgements
We would like to thank Editage (https://www.editage.com) for English language editing.
Funding
This study was conducted without funding.
Author information
Authors and Affiliations
Contributions
Sae Fujita and Takashi Kuda: Conceptualisation, Methodology, Validation, Formal analysis, Investigation, Resources, Data curation, Writing – original draft, Visualisation. Ayaka Nakamura and Hajime Takahashi: Conceptualisation, Methodology, Supervision. Yumeng Xia and Kazuya Koga: Formal analysis, Investigation.
Corresponding author
Ethics declarations
Ethical approval
Animal experiments were performed in accordance with the ‘Fundamental Guidelines for Proper Conduct of Animal Experiments and Related Activities in Academic Research Institutions’ under the jurisdiction of the Ministry of Education, Culture, Sports, Science and Technology, Japan. The study protocol was approved by the Animal Experiment Committee of Tokyo University of Marine Science and Technology (Approval No. R3-1).
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fujita, S., Koga, K., Nakamura, A. et al. Effects of Ecklonia cava subspp. kurome and stolonifera ethanolic/aqueous extracts on caecal microbiota in mice fed a high-sucrose and low-dietary fibre diet. J Appl Phycol (2024). https://doi.org/10.1007/s10811-024-03278-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10811-024-03278-y