Abstract
Ecklonia cava subsp. kurome (EK) and E. cava subsp. stolonifera (ES) are rich in phlorotannins and water-soluble polysaccharides (alginates, laminarans and fucoidans). Total phenolic content (TPC), antioxidant (O2− radical scavenging and Fe-reducing) and anti-glycation (bovine serum albumin-fructose model) capacities of dried EK and ES were higher than those of Eisenia bicyclis and Eisenia nipponica in vitro (p < 0.05). To clarify the effect of EK and ES on gut microbiota, 5% (w/w) EK or ES was administered to Institute of Cancer Research mice fed a high-sucrose and low-dietary fibre diet for 14 days and their caecal microbiota was analysed using 16S rDNA (V4) amplicon sequencing. The amplicon sequence variant (ASV) numbers of the EK and ES groups were 4- and 2-fold lower than those of the no fibre (NF) diet group. Bacteroidota containing alginate- and laminaran-degrading microbes, such as Bacteroides acidifaciens- and B. intestinalis-like bacteria, were more abundant in EK and ES groups than in the NF group. Faecalibaculum rodentium- and Akkermansia muciniphila-like bacteria were also more abundant in EK and ES groups than in the NF group. These EK- and ES-responsive indigenous gut bacteria have been regarded as beneficial commensal for host health. Further studies on EK and ES including investigation of dose-dependent effects are needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The brown algal genera, Eisenia and Ecklonia, of the Lessoniaceae family are known as “kajime” in Japan. Eisenia bicyclis, Eisenia nipponica, Ecklonia cava subsp. kurome (EK), and E. cava subsp. stolonifera (ES) are rich in phlorotannins (phenolic compounds) and water-soluble polysaccharides (alginates, laminarans and fucoidans) and considered to have various health benefits (Ganesan et al. 2019; Rajan et al. 2021). Strong antioxidant, anti-glycation, anti-allergenic, anti-diabetic, antiviral and antimicrobial properties of the Eisenia and Ecklonia spp. are attributed to phlorotannins and water-soluble polysaccharides (Kuda and Ikemori 2009; Kuda et al. 2016, 2021; Zhang et al. 2022). In each region, the local specialty “kajime” species have traditionally been consumed but are not used otherwise, and large casts of these seaweeds along the coastline can be a problem to the environment and fisheries.
During respiration, living organisms generate reactive oxygen species (ROS) such as superoxide anion (O2-) radicals, hydrogen peroxide (H2O2), hydroxyl (OH) radicals, nitric oxide (NO) and singlet oxygen (1O2) throughout the body (Prasad et al. 2019). Although ROS are vital to the immune system, their overproduction results in inflammation and various lifestyle, chronic, and degenerative conditions, including respiratory, neurodegenerative, and digestive diseases, as well as cancer (Liu et al. 2018). Glycation is a non-enzymatic reaction between amino and carbonyl groups, which can occur in exogenous (foods) or endogenous environments (the body). Endogenous advanced glycation end products (AGEs) are a heterogeneous class of compounds formed via a complex Maillard reaction, which includes a series of oxidation steps and non-enzymatic reactions that result in the production of reactive carbonyl compounds and the glycoxidation of proteins, lipids, and nucleic acids (Vijaykrishnaraj and Wang 2021). AGEs contribute to the development of various chronic and age-related diseases, such as diabetic retinopathy, diabetic nephropathy, cardiovascular disease and Alzheimer’s disease (Kuzan 2021). Moreover, an increase in glycated serum albumin level is associated with diabetes and used as a biomarker for glycaemic control (Rabbani and Ahn 2019).
Several hundred species of bacteria colonise the gut and are important components of the human gut microbial ecosystem (Michels et al. 2022). Moreover, their metabolism can affect host health; for example, by mitigating or exacerbating lifestyle-related and chronic diseases (Barone et al. 2022). The gut microbiota is affected by many factors, such as lifestyle conditions and diet, with the latter having a pronounced and rapid impact (Ecklu-Mensah et al. 2022). The gut microbiome is rapidly and significantly affected by the type of diet within a few days to a week, before the appearance of lifestyle disease markers and/or symptoms (David et al. 2014; Zmora et al. 2019).
Among the brown algal soluble polysaccharides, alginate (viscous polymers of guluronic and mannuronic acids in the cell matrix) and laminaran (storage polysaccharide with 1 → 3 glycosidic bonds and 1 → 6 branching linkages) can be digested well by the human gut microbiota (Harada et al. 2021). Eisinia bicyclis alginate and laminaran are known to increase excretion frequency, faecal weight, and caecal alginate- and laminaran-degrading bacteria, including Bacteroides acidifaciens and Bacteroides intestinalis in mice fed a high-sucrose and low-fibre diet (Takei et al. 2019, 2020). Some studies have indicated that brown algal polysaccharides suppress carcinogenesis not only by improving the gut microbiota composition but also by stimulating their metabolism and generating metabolites such as short-chain fatty acids, which have anti-cancer, anti-type 2 diabetes and anti-inflammation effects (You et al. 2020; Yao et al. 2022). Additionally, several reports show that phlorotannin extracted from some brown algae have ameliorative effects on diabetes and mouse and rat models of obesity with modulation of their gut microbiota (Yuan et al. 2019).
EK and ES are not only rich in water-soluble polysaccharides but are also high in phlorotannins, which may directly or indirectly affect the gut microbiota and the health of the host. The present study tested the effect of these brown algae on the gut microbiota in mice fed a high-sucrose and low-fibre diet. Their caecal microbiota was analysed using 16S rDNA (V4) amplicon sequencing. The results show how EK and ES alter gut microbiota composition and illustrate their potential application as functional food materials to benefit human gastrointestinal health.
Materials and methods
Dried products
Dried product of Eisinia bicyclis was obtained from Suzuki Nori Co. (Choshi, Japan). Dried products of Eisinia nipponica and Ecklonia cava subsp. kurome (EK) harvested in Ise and Kyushu, Japan, respectively, were purchased from Satsumaya Co. (Chiba, Japan). Dried product of Ecklonia cava subsp. stolonifera (ES) was purchased from Ofune Kaisan Co. (Aomori, Japan). These brown algae were harvested, lightly rinsed in water, and immediately subjected to sun-drying. The dried seaweeds were pulverised using a blender and sieved through a 1.0-mm mesh.
Antioxidant and anti-glycation properties of the aqueous extract solutions
TPC and antioxidant capacities
To obtain aqueous extract solutions, the dried products (0.2 g) were added to 9.8 mL of distilled water. The suspensions were autoclaved at 121 °C for 15 min, cooled to 20 ℃, and centrifuged at 2,950 × g for 10 min at 4 °C. The obtained supernatant was defined as the aqueous extract solution. TPC of the aqueous extract solutions was determined using the Folin–Ciocalteu method, as previously reported (Kuda and Ikemori 2009). TPC was expressed in terms of phloroglucinol equivalents per mL (PGEq mL−1). To evaluate the antioxidant properties of the aqueous extract solution, 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity, superoxide anion (O2-) radical scavenging activity and ferric (Fe)-reducing power were determined as previously reported (Kuda et al. 2021). The antioxidant properties were calculated and recorded as μmol (+)-catechin/aqueous extract solution equivalents (CatEq mL−1).
Anti-glycation capacities
Anti-glycation activity assays were performed as previously described (Eda et al. 2016) using bovine serum albumin (BSA)-fructose (Fru) and BSA-methylglyoxal (MGO) models and chemicals purchased from Fujifilm Wako Pure Chemicals (Japan). Briefly, 0.5 mL of fructose (1.5 mol L−1) or MGO (60 mmol L−1) was mixed with 0.5 mL each of aqueous extract solution and 0.5 mL of sodium phosphate buffer (50 mmol L−1 with 0.02% sodium azide; pH 7.4) in screw-capped test tubes, followed by incubation at 37 °C for 2 h. BSA (0.5 mL; 30 mg mL−1) was added to each test tube, followed by incubation at 37 °C for 5 days. Fluorescent AGEs were monitored using a multiple microplate reader (SH-9000; Corona Electric, Hitachi, Japan) with excitation and emission wavelengths of 340 nm and 420 nm, respectively, for the BSA-Fru model and 340 nm and 380 nm, respectively, for the BSA-MGO model. The anti-glycation activity was calculated as follows:
where, Fl 0d and Fl 5d represent the fluorescence intensities after 0 and 5 days of reaction, respectively.
Effects of EK and ES on mice fed a high-sucrose and low-dietary fibre diet
Animal care
Animal experiments were performed in accordance with the ‘Fundamental Guidelines for Proper Conduct of Animal Experiments and Related Activities in Academic Research Institutions’ under the jurisdiction of the Ministry of Education, Culture, Sports, Science and Technology, Japan. The study protocol was approved by the Animal Experiment Committee of Tokyo University of Marine Science and Technology (Approval No. R3-1).
Eighteen 5-week-old male Institute of Cancer Research (ICR) mice (25 ± 2 g) were purchased from Tokyo Laboratory Animals Science Co. (Tokyo, Japan) and housed in metal wire cages with a 12-h dark/light cycle at 22 ± 2 ℃. The mice were acclimatized to a powder control diet without cellulose (50% (w/w) sucrose, 20% corn starch, 20% milk casein, 5% corn oil, 3.5% AIN-93 mineral mix, 1% AIN-76 vitamin mix, 0.3% DL-methionine and 0.2% choline bitartrate) and provided distilled water ad libitum. After 7 days, the mice were divided into three groups: no fibre (NF), EK, and ES. Mice in the EK and ES groups were fed a diet in which 5% corn starch was replaced with EK and ES powder, respectively, for 14 days. During feeding days 11–13, the excretion frequency and faecal weight were measured.
At the experimental endpoint, the mice were exsanguinated via the abdominal vein under isoflurane anaesthesia (Fujifilm Wako Pure Chemicals), and the liver, kidneys, spleen, and epididymal fat pads were removed and weighed. After ligation with yarn, the caecum was excised and placed on ice until the microbial analysis was performed.
Analysis of caecal microbiota
The caecal contents (0.1 g) were diluted using 4.9 mL of phosphate-buffered saline (Nissui Pharmaceutical, Japan), and the bacterial cell count was determined via dielectrophoretic impedance measurements (DEPIM) (Shikano et al. 2019) using a bacterial counter (PHC Ltd., Japan); 16S rDNA (V4) amplicon sequencing was performed by Fasmac Co. Ltd. (Atsugi, Japan). Briefly, DNA was extracted from 0.1 g of caecal samples and 1 mL of faecal culture suspensions using an MPure bacterial DNA Extraction Kit (MP Bio Japan, Japan). The DNA library was prepared using a two-step polymerase chain reaction (PCR) method (Sinclair et al. 2015).
The V4 region was amplified using the forward 515f and reverse 806r primers and ExTaq HS DNA polymerase (Takara Bio, Japan) in the first PCR (94 °C for 2 min; followed by 20 cycles of 94 °C for 30 s, 50 °C for 30 s, and 72 °C for 30 s, and finally, 72 °C for 5 min). After purification of the products using the AMPure XP Kit (Beckman Coulter Life Science, Japan), individual DNA fragments were tagged in the second PCR (94 °C for 2 min; followed by 8 cycles of 94 °C for 30 s, 60 °C for 30 s, and 72 °C for 30 s, and finally, 72 °C for 5 min) using the same polymerase kit. The DNA libraries were multiplexed and loaded onto an Illumina MiSeq system (Illumina, USA).
Reads with a mismatched sequence in the start region were filtered using the FASTX Toolkit (http://hannonlab.cshl.edu/fastx_toolkit/), and 235–260 base-pair reads were selected. Chimeras in the selected reads were identified and omitted using the QIIME2 bioinformatics pipeline (https://qiime2.org/). A feature table was generated using the dada2 denoise-paired option in the QIIME 2 plugin (Poncheewin et al. 2020), and the sequences were clustered into amplicon sequence variants (ASVs) using the SILVA 138 database (https://www.arb-silva.de/). The obtained sequences were deposited in the DDBJ Sequence Read Archive (DRA) under the accession number DRA014942 (https://ddbj.nig.ac.jp/).
Plasma lipid levels, caecal bile acid content and caecal antioxidant capacities
Plasma triacylglycerol (TG) and total cholesterol (TC) levels were determined using commercial kits according to the manufacturer’s instructions (Triglyceride E-Test Wako and Total Cholesterol E-Test Wako, respectively; Fujifilm Wako Pure Chemicals). Total bile acid (TBA) content in the diluted caecal suspension for the direct cell count was determined using a commercial kit (TBA-Test Wako, Fujifilm Wako Pure Chemicals). TPC, O2− radical scavenging and Fe-reducing capacities of the diluted caecal suspension were determined in the same manner as described above.
Statistical analysis
Analysis of variance, followed by Tukey’s and Dunnett’s post hoc tests for in vitro and in vivo experiments, respectively, were performed using the statistical software MEPHAS (http://www.gen-info.osaka-u.ac.jp/testdocs/tomocom/). Statistical significance was set at p < 0.05. Alpha diversity of the caecal microbiota was determined using Shannon–Wiener (H') and Simpson (D) indices (Kim et al. 2017). Principal component analysis (PCA) of ASV abundance was performed using MetaboAnalyst (https://www.metaboanalyst.ca/) to estimate any differences in the composition of the caecal microbiota among the test groups.
Results
TPC, antioxidant capacities and anti-glycation activity in the aqueous extract solutions
TPC and antioxidant properties
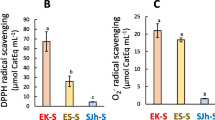
TPC in the aqueous extract solutions of the two Ecklonia (EK and ES) was 3.5 μmol PGEq mL−1 (175 μmol PGEq g−1 dry sample equivalent) (Fig. 1A). These were higher than that of the Eisenia extract solutions (p < 0.05). DPPH radical scavenging capacity was the highest in the ES solution (Fig. 1B). In contrast, O2− radical scavenging capacity was the highest in the EK solution (Fig. 1C). Fe-reducing power of the two Ecklonia solutions were high (Fig. 1D). Results of the O2− radical scavenging capacity and Fe-reducing power correlated with the TPC results expressed as r2 = 0.60 and 0.95, respectively (Fig. 1E).
Total phenolic content (TPC, A), DPPH radical (B) and superoxide anion radical (C) scavenging capacities, and Fe-reducing power (D) of aqueous extract solutions from dried products of Eisenia bicyclis (EB), Eisenia nipponica (EN), Ecklonia cava subsp. kurome (EK) and E. cava subsp. stolonifera (ES). Values represent mean ± standard error of the mean (SEM, n = 3). a−c Values with different superscript letters indicate significant differences at p < 0.05. (E) shows co-efficiency between TPC and antioxidant capacities
Anti-glycation activity
Concentration of the all algae extract solutions depending on anti-glycation activity in both BSA-Fru and BSA-MGO models is shown in Fig. 2A, B. Anti-glycation activities of the Ecklonia samples in the 5 mg sample equivalent mL−1 working solution were higher than that of Eisenia samples, same as the antioxidant capacities shown in Fig. 1. Co-efficiency between the anti-glycation activity and TPC (Fig. 2C) was more pronounced in the BSA-MGO model (r2 = 0.977) compared with BSA-Fru model (r2 = 0.800).
Anti-glycation capacity of aqueous extract solutions from dried products of Eisenia bicyclis (EB), Eisenia nipponica (EN), Ecklonia cava subsp. kurome (EK) and E. cava subsp. stolonifera (ES) in bovine serum albumen (BSA)-fructose (A) and BSA-methylglyoxal (B) models. * Dried sample equivalent in the working solution of the models. Values represent mean ± standard error of the mean (SEM, n = 3). a−c, a’−c’ Values with different superscript letters indicate significant differences at p < 0.05. (C) shows co-efficiency between TPC and the anti-glycation capacity
Body, faecal and organ weights of mice fed EK and ES
No disease symptoms or abnormalities were observed in any of the mice during the feeding period. The mean initial body weight of mice was 37 g (Table 1) and body weight gain tended to be lower in mice fed EK and ES than that in mice fed control diet (NF), though this difference was not significant. In mice fed EK or ES, the excretion frequency was twofold higher than that in mice fed NF. Faecal and caecal weights of mice in the EK and ES groups were approximately 3- and 2-fold, respectively, higher than those of mice in the NF group. Caecal wall tissue weight was also higher in the EK and ES groups than that in the NF group. Epididymal fat pads weight was lower in EK-fed mice than those in NF-fed mice.
Plasma lipid levels, caecal bile acid content, TPC and antioxidant capacities
Although plasma TG and TC levels tended to be lower in the EK and ES groups compared with the NF group, the difference was not significant (Fig. 3A, B). The average caecal TBA concentration in the NF group was 3.0 μmol g−1 and decreased to 1.0 μmol g−1 with EK or ES administration (Fig. 3C). TPC, O2− radical scavenging and the Fe-reducing power of the caecal content were higher in mice fed Ecklonia, particularly EK (Fig. 3D-F).
Plasma triacylglycerol and cholesterol levels (A, B), caecal bile acid content (C), caecal TPC (D) and caecal antioxidant capacities (E, F) of mice fed a diet containing no fibre (NF), 5% (w/w) Ecklonia cava subsp. kurome (EK) or E. cava subsp. stolonifera (ES). Box plot represents the third quartile, median, first quartile, and minimum values of six mice per treatment group. Asterisk represents significant difference from the NF group (p < 0.05)
Caecal microbiota
Total bacterial counts and microbiome diversity
The direct total bacterial cell count in the caecum of the NF-fed mice measured using DEPIM was approximately 10.9 log cells g–1 (Fig. 4A). The number of bacteria per gram was lower in the EK group than in the NF group, but there was no difference between the two groups in the overall cell number in the caecum. The total number of reads was 50,000–100,000 (Fig. 4B), and the average number of ASVs in the NF group was 158, which is 4- and 2-fold higher than that in the EK and ES groups, respectively (Fig. 4C). However, there were no significant differences in the indices of α-diversity indices (Shannon–Wiener index (H′) and Simpson’s index (D)) (Fig. 4D, E). The PCA results (Fig. 4F) revealed differences in the gut microbiota composition among all three groups.
Direct cell count (A), total amplicon sequence read number (B), amplicon sequence variant (ASV) number (C), alpha diversity indices (D, E) and principal component analysis (PCA, F) of caecal microbiota in mice fed a diet containing no fibre (NF), 5% (w/w) Ecklonia cava subsp. kurome (EK) or E. cava subsp. stolonifera (ES). (A–E) Box plot represents the third quartile, median, first quartile, and minimum values of six mice per treatment group. Asterisk represents significant difference from the NF group (p < 0.05)
Microbiota at the phylum, family and genus levels
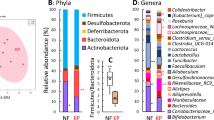
The caecal microbiota profile at the phylum, family and genus levels, expressed by relative abundance, is shown in Fig. 5. The predominant phylum in the NF group was Firmicutes (68% ± 4%), followed by Actinobacteriota (21% ± 4.0%), Bacteroidota (7.0% ± 2.2%) and Desulfobacterota (3.6% ± 0.6%) (Fig. 5A). Bacteroidota counts were higher in the EK (32% ± 6%) and ES (19% ± 2%) groups compared with the NF group. Actinobacteria was suppressed in the EK (< 1.0%) and ES (11% ± 2.0%) groups. Though Verrucomicrobiota was very low in the NF group, it was dominant in the EK (6.0% ± 1.2%) and ES (4.1% ± 0.4%) groups. Proteobacteria was one of dominant phyla in the EK group (9.4% ± 2.9%). Firmicutes was suppressed in the EK group (45% ± 10%). Desulfobacterota was suppressed in the EK and ES groups (< 1.0%).
Composition of the caecal microbiota at the phylum (A), family (B) and genus (C) levels in mice fed a diet containing no fibre (NF), 5% (w/w) Ecklonia cava subsp. kurome (EK) or E. cava subsp. stolonifera (ES). La, Er, and Ri are genera that belong to Lachnospiraceae, Erysipelotrichaceae, and Rikenellaceae families, respectively. Asterisk represents significant difference from the NF group (p < 0.05)
Among Firmicutes, the predominant family was Erysipelotrichaceae (36% ± 6%) in the NF group, followed by Streptococcaceae (13% ± 2%), Lachnospiraceae (6.7% ± 1.9%) and Lactobacillaceae (5.6% ± 1.6%) (Fig. 5B). These families other than Erysipelotrichaceae were significantly lower in the EK and ES groups than those in the NF group. In the phylum Bacteroidota, Bacteroidaceae (25% ± 5.0%) and Tannerellaceae (6.9% ± 2.3%) were dominant in the EK group, whereas Bacteroidaceae was dominant in the ES group. Rikenellaceae, Prevotellaceae and Muribaculaceae were also detected as members of Bacteroidota. Almost all Actinobacteriota, Proteobacteria and Verrucomicrobiota were Bifidobacteriaceae, Enterobacteriaceae and Akkermansiaceae, respectively.
Among Erysipelotrichaceae found in the NF group, Allobaculum (30% ± 7%) and Faecalibaculum (5.1% ± 2.1%) were the predominant genera (Fig. 5C). The Allobaculum count was significantly suppressed in the EK (< 1.0%) and ES (4.9% ± 0.8%) groups. In contrast, Faecalibaculum abundance was significantly higher in the EK (31% ± 12%) and ES (46% ± 7%) groups compared with the NF group. Almost all Tannerellaceae and Rikenellaceae were Parabacteroides and Alistipes, respectively.
ASV level
Figure 6 shows a heat map of the top 30 ASVs by abundance with a defined name using BLASTn. In total, 22 and 17 ASVs differed between the NF and EK or ES groups, respectively. In the phylum Firmicutes, almost all Allobaculum and Faecalibaculum included one ASV each (ID-1 and ID-0) were dominant in the NF and Ecklonia groups, respectively. ASV ID-0 was defined with BLASTn as Faecalibaculum rodentium (similarity = 100%)-like bacteria. Clostridium disporicum (100%)- and Thomasclavelia ramosa (100%)-like bacteria were EK-responsive bacteria. In the phylum Bacteroidota, several ASVs that were high in the EK group were defined as Parabacteroides goldsteinii (100%)-, Phocaeicola vulgatus (100%)-, Bacteroides acidifaciens (100%)- and Bacteroides intestinalis (100%)-like bacteria. Alistipes finegoldii (100%)- and the B. intestinalis-like bacteria were also ES-responsive bacteria. Akkermansia ASV ID-8 was defined as A. muciniphila (100%)-like bacteria. Bifidobacterium, a dominant genus in the NF group, also included an ASV that was defined as B. pseudolongum (100%)-like bacteria.
Discussion
Phlorotannins are phenolic compounds with strong antioxidant properties that are found in brown algae including Eisenia and Ecklonia spp. (Shrestha et al. 2020). Additionally, polysaccharides (fucoidan, alginate and laminaran) are known to show free radical scavenging activities, including O2- radical scavenging (Kuda et al. 2021). As oxidative pathways are involved in AGE formation, the anti-glycation activity of various ROS-scavenging natural medicinal and food materials have been investigated (Dil et al. 2019; Vijaykrishnaraj and Wang 2021). Our results of high TPC, antioxidant and anti-glycation capacities of Eisenia spp. and Ecklonia spp. are consistent with previous studies (Kuda et al. 2007, 2016, 2021; Kuda and Ikemori 2009; Zhang et al. 2022). Additionally, this experiment clearly showed that the activities of the Ecklonia samples were higher than that of the Eisenia samples. The following animal experiments were conducted only for the Ecklonia samples.
Increase in faeces and caecal content is known to be a typical effect of dietary fibre administration. Enlargement of the caecum and reduction in fat tissue weight due to soluble and fermentable fibres rather than insoluble fibres has been documented (Nakata et al. 2016). Furthermore, the anti-obesity effect of polyphenol compounds of E. kurome and E. stolonifera has been reported (Xu et al. 2012; Hu et al. 2016). Suppression of blood lipid levels, as previously reported, was not observed in this experiment, but this may be due to the short duration of administration and lack of fasting treatment. In this regard, dietary fibre can bind bile acids in the digestive tract, reducing their re-entry into the enterohepatic circulation (He et al. 2022). Reduced bile acid concentration promotes bile acid synthesis in the liver, which can lead to a reduction in blood cholesterol (Jia et al. 2021). Control of ROS in the gut is crucial to maintain gut microbiota homeostasis and subsequently, gut health (Wu et al. 2021).
Phlorotannins in E. bicyclis, EK and ES, have been shown to exhibit antimicrobial activity (Jimenez-Lopez et al. 2021). These phlorotannins might suppress a part of the caecal microbiota and decrease the ASV number. However, the administration of diet containing 5% (w/w) E. bicyclis to ICR mice does not cause an extreme reduction in ASV number (Takei et al. 2019). Differences in the phlorotanninn content of the algae may be reflected in differences in the ASV number.
L. johnsonii, L. murinus, F. rodentium, P. goldsteinii, P. vulgatus, B. acidifaciens, B. intestinalis and B. pseudolongum were isolated from the caecal content of ICR mice obtained from the same breeder and identified using 16S rRNA sequencing and BLASTn in our previous studies (Takei et al. 2019; Lee et al. 2022b). Some of the EK- and ES-responsive endogenous gut bacterial candidates in the present study might be the aforementioned species.
B. acidifaciens and B. intestinalis are established as murine gut commensal alginate- and laminaran-responsive and degrading bacteria, respectively (Takei et al. 2020). Furthermore, T. ramose (formerly Erysipelatoclostridium ramosum) isolated from human faeces can ferment laminaran in vitro (Harada et al. 2021). B. intestinalis can also be isolated from human faeces (Bakir et al. 2006). B. acidifaciens has beneficial effects on host health, such as amelioration of liver injury and improvement of gut immune function (Nakajima et al. 2020; Wang et al. 2022). Alginate and laminaran have shown antioxidant and immunomodulation capacities with fermentation by B. acidifaciens and B. intestinalis, respectively (Harada et al. 2021). Additionally, alginate and laminaran cultures with degrading bacterial strains Bacteroides xylanisolvens and Bacteroides uniformis isolated from human faeces showed similar activities (Lee et al. 2022a).
Among Erysipelotrichaceae, Allobaculum is positively associated with multiple dietary lifestyle diseases, including obesity and diabetes (Zheng et al. 2019); it was found to be significantly suppressed in the EK and ES groups in this study. In contrast, the beneficial effects of F. rodentium, which may be a murine EK- and ES-responsive bacterial species, include protection against intestinal tumour growth and modulation of intestinal epithelial homeostasis (Zagato et al. 2020; Cao et al. 2022). A decrease in caecal Allobaculum and increase in F. rodentium has been reported in ICR mice fed a phlorotannin-rich brown alga Sargassum horneri (Lee et al. 2022b). However, in ICR mice fed alginate, laminaran or E. bicyclis, the effects on caecal Allobaculum and F. rodentium are not clear (Takei et al. 2019, 2020). The changes in Allobaculum and F. rodentium may be due to rich phlorotannins in EK and ES. Further investigation is needed to confirm this hypothesis.
Akkermansia is an gut commensal considered an important genus for maintaining the mucus layer of the host (Cheng and Xie 2021). Its abundance may have a negative correlation with obesity and diabetes (Xu et al. 2020). Prebiotics that increase Akkermansia are currently being explored; several reports have demonstrated that polyphenols increase gut Akkermansia (Rodríguez-Daza et al. 2021). Additionally, Akkermansia is reported to be increased in mice fed a high-fat diet supplemented with fucoidan (Zheng et al. 2020). The fucoidin content of EK and ES is low (Kuda et al. 2007) and unlikely responsible for the increase in Akkermansia we observed. Previous studies on mice fed E. bicyclis, alginate or laminaran did not report an increase in Akkermansia (Takei et al. 2019, 2020). Therefore, the increase in Akkermansia in the EK and ES groups may be due to phlorotannin contents of the brown algae.
Although the inhibitory effects of various polyphenols on Escherichia coli and Helicobacter have been reported (Nagayama et al. 2002; Ayala et al. 2014), their abundance was high in the EK group. In a previous study, E. coli was shown to exhibit resistance against 80% (v/v) ethanol extract solution of EK and ES; however, Pseudomonas aeruginosa, Corynebacterium glutamicum, Staphylococcus aureus and Bacillus cereus were found to be susceptible to the alcohol solution (Kuda et al. 2007). Therefore, the in vivo effects of the polyphenols may depend on their type and dose.
This study was conducted to examine the possible use of the brown algae, EK and ES, as functional food materials. These are abundant, yet underutilised species that are nuisance species along coastlines as beach casts. The increase in alginate- and laminaran-degrading bacteria F. rodentium and Akkermansia show that these algae have beneficial effects due to their fermentable dietary fibres and phlorotannins. However, due to increase in Enterobacteriaceae and Helicobacter in the EK group, its excessive ingestion may result in gut dysbiosis. These results are instructive for the utilisation of these brown algae as functional ingredients for human gastrointestinal health. In the future, the role of phlorotannins from EK and ES in shifting the composition of the gut microbiota, particularly with respect to F. rodentium and Akkermansia is required.
Conclusion
EK and ES are rich in phlorotannins and typical water-soluble polysaccharides. TPC, O2− and DPPH radical scavenging, Fe-reducing power and anti-glycation capacities in vitro of the dried EK and ES were shown to be higher than those of E. bicyclis and E. nipponica. To clarify their effect on gut microbiota, 5% (w/w) EK or ES was administered to ICR mice fed a high-sucrose and low-dietary fibre diet. The ASV number of the EK and ES groups were 4- and twofold lower than that of the NF group. Alginate- and laminaran-degrading candidates such as B. acidifaciens- and B. intestinalis-like bacteria were higher in the EK and ES groups than the NF group. F. rodentium- and Akkermansia muciniphila-like bacteria were also higher in EK and ES groups than the NF group. These EK- and ES-responsive gut indigenous bacterial species may be beneficial commensals for host health. In the case of mice fed EK, Enterobacteriaceae was also higher than that in mice fed NF. Further studies including dose-dependent effects are needed.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Ayala G, Escobedo-Hinojosa WI, de La Cruz-Herrera CF, Romero I (2014) Exploring alternative treatments for Helicobacter pylori infection. World J Gastroenterol 20:1450–1469
Bakir MA, Kitahara M, Sakamoto M, Matsumoto M, Benno Y (2006) Bacteroides intestinalis sp. nov., isolated from human faeces. Int J Syst Evol Microbiol 56:151–154
Barone M, D’Amico F, Rampelli S, Brigidi P, Turroni P (2022) Age-related diseases, therapies and gut microbiome: A new frontier for healthy aging. Mech Ageing Dev 206:111711
Cao YG, Bae S, Villarreal J, Moy M, Chun E, Michaud M, Lang JK, Glickman JN, Lobel L, Garrett WS (2022) Faecalibaculum rodentium remodels retinoic acid signaling to govern eosinophil-dependent intestinal epithelial homeostasis. Cell Host Microbe 30:1295–1310
Cheng D, Xie MZ (2021) A review of a potential and promising probiotic candidate—Akkermansia muciniphila. J Appl Microbiol 130:1813–1822
David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ (2014) Diet rapidly and reproducibly alters the human gut microbiome. Nature 505:559–563
Dil FA, Ranjkesh Z, Goodarzi MT (2019) A systematic review of antiglycation medicinal plants. Diab Metab Syndr: Clin Res Rev 13:1225–1229
Ecklu-Mensah G, Gilbert J, Devkota S (2022) Dietary selection pressures and their impact on the gut microbiome. Cell Mol Gastroenterol Hepatol 13:7–18
Eda M, Kuda T, Kataoka M, Takahashi H, Kimura B (2016) Anti-glycation properties of the aqueous extract solutions of dried algae products harvested and made in the Miura Peninsula, Japan, and effect of lactic acid fermentation on the properties. J Appl Phycol 28:367–3624
Ganesan AR, Tiwari U, Rajauria C (2019) Seaweed nutraceuticals and their therapeutic role in disease prevention. Food Sci Human Wellness 8:252–263
Harada M, Kuda T, Nakamura S, Gayang L, Takahashi H, Kimura B (2021) In vitro antioxidant and immunomodulation capacities of low-molecular weight-alginate- and laminaran-responsible gut indigenous bacteria. LWT-Food Sci Technol 151:112127
He Y, Wang B, Wen L, Wang F, Yu H, Chen D, Su X, Zhang C (2022) Effects of dietary fiber on human health. Food Sci Human Wellness 11:1–10
Hu X, Tao N, Wang X, Xiao J, Wang M (2016) Marine-derived bioactive compounds with anti-obesity effect: A review. J Funct Foods 21:372–387
Jia W, Wei M, Rajani C, Zheng X (2021) Targeting the alternative bile acid synthetic pathway for metabolic diseases. Prot Cell 12:411–425
Jimenez-Lopez C, Pereira AG, Lourenço-Lopes C, Garcia-Oliveira P, Cassani L, Fraga-Corral M, Prieto MA, Simal-Gandara J (2021) Main bioactive phenolic compounds in marine algae and their mechanisms of action supporting potential health benefits. Food Chem 341:128262
Kim B, Shin J, Guevarra RB, Lee JH, Kim DW, Seol K, Lee JH, Kim HB, Isaacson R (2017) Deciphering diversity indices for a better understanding of microbial communities. J Microbiol Biotechnol 27:2089–2093
Kuda T, Ikemori T (2009) Minerals, polysaccharides and antioxidant properties of aqueous solutions obtained from macroalgal beach-casts in the Noto Peninsula, Ishikawa, Japan. Food Chem 112:575–581
Kuda T, Kunii T, Goto H, Suzuki T, Yano T (2007) Varieties of antioxidant and antibacterial properties of Ecklonia stolonifera and Ecklonia kurome products harvested and processed in the Noto peninsula, Japan. Food Chem 103:900–905
Kuda T, Eda M, Kataoka M, Nemoto M, Kawahara M, Oshio S, Takahashi H, Kimura B (2016) Anti-glycation properties of the aqueous extract solutions of dried algae products and effect of lactic acid fermentation on the properties. Food Chem 192:1109–1115
Kuda T, Nishizawa M, Toshima D, Matsushima K, Yoshida S, Takahashi H, Kimura B, Yamagishi T (2021) Antioxidant and anti-norovirus properties of aqueous acetic acid macromolecular extracts of edible brown macroalgae. LWT-Food Sci Technol 141:110942
Kuzan A (2021) Toxicity of advanced glycation end products (Review). Biomed Rep 14:46
Lee G, Harada M, Midorikawa Y, Yamamoto M, Nakamura A, Takahashi H, Kuda T (2022a) Effects of alginate and laminaran on the microbiota and antioxidant properties of human faecal cultures. Food Biosci 47:101763
Lee G, Midorikawa Y, Kuda T, Harada M, Fujita S, Takahashi H, Kimura B (2022b) In vitro antioxidant and anti-glycation properties of Sargassum horneri from golden tides on the South Korean coast and the effect on gut microbiota of mice fed a high-sucrose and low-fibre diet. J Appl Phycol 34:2211–2222
Liu Z, Ren Z, Zhang J, Chuang CC, Kandaswamy E, Zhou T, Zuo L (2018) Role of ROS and nutritional antioxidants in human diseases. Front Physiol 9:477
Michels N, Zouiouich S, Vanderbauwhede B, Vanacker J, Ruiz BII, Huybrechts I (2022) Human microbiome and metabolic health: An overview of systematic reviews. Obesity Rev 23:e13409
Nagayama K, Iwamura Y, Shibata T, Hirayama I, Nakamura T (2002) Bactericidal activity of phlorotannins from the brown alga Ecklonia kurome. J Antimicrob Chemother 50:889–893
Nakajima A, Sasaki T, Itoh K, Kitahara T, Takema Y, Hiramatsu K, Ishikawa D, Shibuya T, Kobayashi O, Osada T, Watanabe S, Nagahara A (2020) A soluble fiber diet increases Bacteroides fragilis group abundance and immunoglobulin a production in the gut. Appl Environ Microbiol 86:e00405-e420
Nakata T, Kyoui D, Takahashi H, Kimura B, Kuda T (2016) Inhibitory effects of laminaran and alginate on production of putrefactive compounds from soy protein by intestinal microbiota in vitro and in rats. Carbohydr Polymers 143:61–69
Poncheewin W, Hermes GDA, van Dam JCJ, Koehorst JJ, Smidt H, Schaap PJ (2020) NG-Tax 2.0: A semantic framework for high-throughput amplicon analysis. Front Genet 10:1366
Prasad A, Pospíšil P, Tada M (2019) Reactive oxygen species (ROS) detection methods in biological system. Front Physiol 10:1316
Rabbani G, Ahn S (2019) Structure, enzymatic activities, glycation and therapeutic potential of human serum albumin: A natural cargo. Int J Biol Macromol 123:979–990
Rajan DJ, Mohan K, Zhang S, Ganesan AR (2021) Dieckol: a brown algal phlorotannin with biological potential. Biomed Pharmacother 142:111988
Rodríguez-Daza M, Pulido-Mateos EC, Lupien-Meilleur J, Guyonne D, Desjardins Y, Roy D (2021) Polyphenol-mediated gut microbiota modulation: Toward prebiotics and further. Front Nutr 8:689456
Shikano A, Kuda T, Shibayama J, Toyama A, Ishida Y, Takahashi H, Kimura B (2019) Effects of Lactobacillus plantarum Uruma-SU4 fermented green loofah on plasma lipid levels and gut microbiome of high-fat diet fed mice. Food Res Int 121:817–824
Shrestha S, Zhang W, Smid SD (2020) Phlorotannins: A review on biosynthesis, chemistry and bioactivity. Food Biosci 39:100832
Sinclair L, Osman OA, Bertilsson S, Eiler A (2015) Microbial community composition and diversity via 16S rRNA gene amplicons: Evaluating the Illumina platform. PLoS One 10:e0116955
Takei M, Kuda T, Fukunaga M, Toyama A, Goto M, Takahashi H, Kimura B (2019) Effects of edible algae on caecal microbiomes of ICR mice fed a high-sucrose and low–dietary fibre diet. J Appl Phycol 31:3969–3978
Takei MN, Kuda T, Taniguchi M, Nakamura S, Takahashi H, Kimura B (2020) Detection and isolation of low molecular weight alginate- and laminaran susceptible gut indigenous bacteria from ICR mice. Carbohydr Polym 238:116205
Vijaykrishnaraj M, Wang K (2021) Dietary natural products as a potential inhibitor towards advanced glycation end products and hyperglycemic complications: A phytotherapy approaches. Biomed Pharmacother 144:112336
Wang H, Wang Q, Yang C, Guo M, Cui X, Jing Z, Liu Y, Qiao W, Qi H, Zhang H, Zhang X, Zhao N, Zhang M, Chen M, Zhang S, Xu H, Zhao L, Qiao M, Wu Z (2022) Bacteroides acidifaciens in the gut plays a protective role against CD95-mediated liver injury. Gut Microb 1:e2027853
Wu S, Bekhit AEA, Wu Q, Chen M, Liao X, Wang J, Ding Y (2021) Bioactive peptides and gut microbiota: Candidates for a novel strategy for reduction and control of neurodegenerative diseases. Trends Food Sci Technol 108:164–176
Xu H, Kitajima C, Ito H, Miyazaki T, Baba M, Okayama T, Okada Y (2012) Antidiabetic effect of polyphenols from brown alga Ecklonia kurome in genetically diabetic KK-Ay mice. Pharmaceut Biol 50:393–400
Xu Y, Wang N, Tan H, Li S, Zhang C, Feng Y (2020) Function of Akkermansia muciniphila in obesity: Interactions with lipid metabolism, immune response and gut systems. Front Microbiol 11:219
Yao W, Qiu H, Cheong K, Zhong S (2022) Advances in anti-cancer effects and underlying mechanisms of marine algae polysaccharides. Int J Biol Macromol 221:472–485
You L, Gong Y, Li L, Hu X, Brennan C, Kulikouskaya V (2020) Beneficial effects of three brown seaweed polysaccharides on gut microbiota and their structural characteristics: An overview. Int Food Sci Technol 55:1199–1206
Yuan Y, Zheng Y, Zhou J, Geng Y, Ping Z, Li Y, Zhang C (2019) Polyphenol-rich extracts from brown macroalgae Lessonia trabeculata attenuate hyperglycemia and modulate gut microbiota in high-fat diet and streptozotocin-induced diabetic rats. J Agric Food Chem 67:12472–12480
Zagato E, Pozzi C, Bertocchi A, Schioppa T, Saccheri F, Guglietta S et al (2020) Endogenous murine microbiota member Faecalibaculum rodentium and its human homologue protect from intestinal tumour growth. Nature Microbiol 5:511–524
Zhang S, Pei R, Li M, Su H, Sun H, Ding Y, Su M, Huang C, Chen X, Du Z, Jin C, Zang Y, Li J, Xu Y, Chen X, Zhang B, Ding K (2022) Cocktail polysaccharides isolated from Ecklonia kurome against the SARS-CoV-2 infection. Carbohydr Polym 275:118779
Zheng Z, Lyu W, Ren Y, Li X, Zhao S, Yang H, Xiao Y (2019) Allobaculum involves in the modulation of intestinal ANGPTLT4 expression in mice treated by high-fat diet. Front Nutri 8:690138
Zheng L, Chen X, Cheong K (2020) Current trends in marine algae polysaccharides: The digestive tract, microbial catabolism, and prebiotic potential. Int J Biol Macromol 151:344–354
Zmora N, Suez J, Elinav E (2019) You are what you eat: diet, health and the gut microbiota. Nature Rev Gastroenterol Hepatol 16:35–56
Acknowledgements
Dried E. bicyclis sample was kindly obtained from Mr. Seiichi Suzuki; Suzuki Nori Co., Choshi, Japan. We would like to thank Editage https://www.editage.com for English language editing.
Author information
Authors and Affiliations
Contributions
Sae Fujita and Gayang Lee: Conceptualisation, Methodology, Investigation, Validation, Formal analysis. Hajime Takahashi and Ayaka Nakamura: Supervision, Bioinformatics. Kazuya Koga and Natsumi Handa: Investigation. Takashi Kuda: Conceptualisation, Methodology, Validation, Formal analysis, Resources, Data curation, Writing – review & editing, Visualisation, Supervision, Project administration. Yumeng Xia: Methodology, Investigation, Validation, Formal analysis.
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fujita, S., Lee, G., Takahashi, H. et al. Ecklonia cava subsp. kurome and E. cava subsp. stolonifera can aid regulation of gut microbiota in mice fed a high-sucrose and low-dietary fibre diet. J Appl Phycol 35, 1365–1375 (2023). https://doi.org/10.1007/s10811-023-02966-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-023-02966-5