Abstract
The cyanobacterium Synechocystis sp. strain FS 78 was recently isolated from chromium polluted paddy fields in the North of Iran. In the present work, the effect of chromium (VI) was studied at extreme alkaline conditions (pH 11) on the growth, pigment composition, function of photosystems and phycobilisomes. We monitored the cyanobacterium viability in the presence of chromium (VI) from 0.5 to 5 µM every hour up to 6 h inoculation time. An increase in growth, pigment production, photosystems and phycobilisomes activities was observed in cells grown at low concentration (0.5 µM) of chromium (VI). The toxicity of higher chromium concentrations (3.5 and 5 µM) was observed after 1 h of inoculation. Confocal laser microscopy analysis showed the toxic effect of elevated chromium (VI) concentration on viability, probably due to the damage of the photosystem and phycobilisome structures, which on the long-term was able to recover. We concluded that the combination of the three factors (extreme alkaline pH, different concentrations of Cr(VI) and time) can affect cyanobacterial behaviours individually or in combination.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, polluted environments with heavy metals have increased as a consequence of the industrial development and agricultural activities (Sirunyan et al. 2019; Hussein et al. 2019). Industrial effluents are directly disposed into rivers or canals that extensively used to irrigate the paddy fields, by which toxic metals enter into the agricultural fields (Tiwari et al. 2020). Industrial wastewater—organic compounds and toxic metals—such as chromium, cadmium, cobalt, copper, iron, lead, magnesium, mercury, nickel and zinc plays an important role in the environment and the diversity of terrestrial organisms. Nonetheless, when reaching a threshold level, they can have devastating effects (Hazarika et al. 2015; Ali et al. 2019). Among the industrial waste, chromium, one of the most common heavy metals, is known as the fourth major toxic metals after lead, cadmium and mercury (Tang et al. 2020). Chromium is principally present in several oxidations states, but the most stable and common forms are Cr(III) and Cr(VI), being Cr(VI) the most toxic (Miranda et al. 2012).
Cyanobacteria are widely distributed across terrestrial and aquatic environments (Dutta and Bhadury 2020). They are photosynthetic organisms and exhibit abundant growth and limited growth requirements even under stressed environmental conditions. They can play a fundamental role in the soil biological cycle by the production of organic matter and oxygen release with tremendous applied capabilities (Qu et al. 2015; Munagamage et al. 2020). In paddy fields, cyanobacteria exclusively enhance the soil fertility and productivity and act as bio-fertilizers (Tiwari et al. 2020). The presence of Cr(VI) can induce diverse alterations on the growth, photosynthesis and biochemical and physiological characteristics of cyanobacteria (Rocchetta et al. 2007; Tiwari et al. 2018). Under natural conditions in rice fields, cyanobacteria are exposed to the combined influence of several factors, such as pH, irradiance, salinity, temperature and dissolved inorganic carbon fluctuations, which vary both daily and over the crop cycle (Quesada and Fernández-Valiente 1996). However, many fluctuating environmental parameters can influence growth, molecular resource allocation and photosynthesis through complex acclamatory strategies (Zabochnicka-Świątek and Krzywonos 2014; Cheng et al. 2017). Therefore, the survival of cyanobacteria in natural environments depends upon their ability to acclimate to the variable conditions of environmental factors (Shokravi et al. 2014). Among them, pH is a key factor that influences the bioavailability of cyanobacteria (Pawlik-Skowrońska et al. 1997; Hinners et al. 2015; Tang et al. 2020). Most cyanobacteria grow in environments that are neutral to alkaline and in laboratory cultures, the optimal pH ranges from 7.5 to 10 (Kaushik 1994; Shokravi and Soltani 2011). Up to now, only a very limited effort has been devoted to study extreme alkalinity conditions (Ihnken et al. 2014; Touloupakis et al. 2016; Pathak et al. 2018; Krausfeldt et al. 2019) but never in combination with chromium. Another important factor in the efficiency of growth and photosynthesis is the longevity (age of culture) of the organism which is exposed to culture containing chromium or other elements. Our knowledge about the effect of time in chaotic environments like paddy-fields is negligible. The initial hours of salinity and alkalinity stress may create a significant effect in physiological and morphological activities on the subsequent hours (especially the first 24 h) (Amirlatifi et al. 2018; Abbasi et al. 2019). Regarding the role of environmental factors and time, few studies have been carried out on the effect of chromium to cyanobacteria. Moreover, most studies focused on the effect of chromium on cyanobacteria under non-extreme alkaline conditions for long periods of time (at daily or weekly basis) (Hörcsik 2006; Sirunyan et al. 2019; Roestorff and Chirwa 2019). Sen et al. 2017 and 2018 predicted that the growth of biomass with time are based on the one-factor-at-a-time (OFAT) methodology. They suggested that operating variables such as initial concentration of Cr(VI), initial solution pH, inoculum size and culture time affect the performance of the cyanobacterial consortium in removal of Cr(VI). Therefore, the study of environmental fluctuations in short-time regime on cyanobacteria is essential to serve the sustainable development economy in near future.
In the present study, we have selected the unicellular cyanobacteria Synechocystis sp. for its fast growth rate, environmental stability and tolerance to heavy metal pollution (Pan et al. 2009; Li et al. 2019). Synechocystis sp. FS 78 was isolated from chromium-rich polluted paddy fields located in the Golestan province in North-East of Iran, by the authors in 2019 (unpublished results). Based on a preliminary survey, this strain showed the high tendency in extremely alkaline conditions (pH > 10). Therefore, we consider it a suitable model for studying cyanobacteria interactions at extremely high alkalinity and chromium concentrations. Up to now, no work has yet attempted to assess directly the ecophysiology of native terrestrial cyanobacteria (croplands and paddy-fields) under different Cr(VI) concentrations at pH 11. The objective of the present study was to investigate the combined effect of Cr(VI) concentration and pH 11 on the survival, growth, photosystem and phycobilisome responses of Synechocystis sp. FS 78 under controlled laboratory conditions.
Methods and materials
Isolation of strain
The edaphic cyanobacterium Synechocystis sp. FS 78 was isolated from chromium polluted paddy fields in the Golestan province (36°50´ N, 54° 27´ E) near the Caspian Sea (North of Iran) by the authors in 2019. The soil samples were cultured, and after colonization sample was diluted (repeatedly subculture) with agar plate in BG-11 medium to achieve unialgal cultures of the stain (Kaushik 1987). The pH was adjusted in 7.8 by NaOH and was placed in a culture chamber at 30 °C under fluorescent white light (60 μmol photons m−2 s−1). The culture was refreshed and repeated every week by picking single colonies in a new medium until a single pure colony and axenic cultures had been obtained, which was checked by light and phase-contrast microscopy. The sample was identified morphologically in terms of aggregation forms, cell shape (and dimension) and morphological variation using light, fluorescence and phase-contrast microscopy. Identification and determination were made according to Desikachary (1959), Anagnostidis and Komárek (1990) and John et al. (2002). Strain after identification as Synechocystis sp. strain FS 78 was coded and preserved (long time preservation) in the algae museum of the institute of applied sciences of Shahid Beheshti University, Tehran, Iran. The cyanobacterium culture was maintained in liquid BG-11 medium (Li et al. 2019) at 30 °C under constant fluorescent white light (60 μmol photons m−2 s−1) (Poza-Carrión et al. 2001; Pan et al. 2009).
Incubation condition and Cr(VI) treatment
The cells were harvested at an exponential growth phase and then transferred to culture media with different Cr(VI) concentrations at pH 11. Cr(VI) solution was prepared by dissolving K2Cr2O7 in deionized water (Miranda et al. 2012). The samples were treated with Cr(VI) at final concentrations of 0.5, 2, 3.5 and 5 μM. A negative control was prepared of culture medium without addition of chromium. Culture media were buffered with 10-mM Bis–Tris Propane (BTP) and titrated to the desired pH (pH 11) with KOH (Poza-Carrión et al. 2001; Summerfield et al. 2013). Measurements were performed every hour from immediately after inoculation (time zero) to 6 h.
Growth curves and analysis
Growth measurements were analysed under the different Cr(VI) concentrations at alkaline condition according to Abbasi et al. 2019. The in vivo absorbance spectra were measured from 380 to 800 nm using a Hitachi-557 double-beam spectrophotometer. The absorbance spectra were normalized to biomass (OD 750) according to Tang and Vincent 1999. Growth curves were followed by optical density (750 nm) for 6 h after inoculation according to Leganés et al. (1987) and Śliwińska-Wilczewska et al. (2019). Growth rate (µ) and doubling times (G) were calculated both by cell count and absorbance according to Kaushik 1987.
Spectroscopy
The operation of photosystems and phycobilisome characteristics were measured and analysed spectrofluorimetrically (including confocal spectra) according to Inoue-Kashino et al. (2005), Vermaas et al. (2008) and Zorz et al. (2015). Room temperature fluorescence emission spectra of the cells were recorded by Synergy HTX (Multi-Mode Microplate Reader, USA) following Tiwari and Mchanty (1996) and Fraser et al. (2013). Emission and excitation spectra were recorded at λex: 440–550 nm and λem 630–760 nm, respectively. The fluorescence intensity of single cell was measured using λscan of confocal laser microscope system (Leica TCS-SP5 CLSM -Leica Microsystems Heidelberg GmbH, Mannheim, Germany). CLSM enables us to obtain high-resolution images and study different physiological processes including intensity of fluorescence emitted (as spectral unmixing) from single in vivo cyanobacteria cells (Grigoryeva and Chistyakova 2019). Photosynthetic pigments excitation was carried out with an argon laser at 405 nm. The fluorescence emission spectrum was collected by detection channels of wavelengths between 420 and 730. Analysis of the lambda scan data was carried out using the Leica Confocal Software.
Survivability
The fluorescence intensity of living and dead cells was determined by CSLM dual laser (CLSM-DL) according to Millach et al. 2017. CLSM-DL allows the capturing of different and specific wavelengths within the spectrum at the same time. In order to better differentiate between living and dead cells, high-resolution images were obtained by single and overlapping inspection. Confocal fluorescence images (514 × 514 pixels) were recorded using an oil immersion objective (× 63). The spectra were normalized to their maximum, and mean values and standard deviations were calculated (Ramírez et al. 2011; Sugiura and Itoh 2012).
Statistical analysis
Statistical analysis was performed using one-way analysis of variance (ANOVA) by SPSS-24 software. ANOVA test shows a significant difference between treatments with p < 0.05. All the experiments were carried out in six replicates and data presented as mean values of six independent replicates. The Polar plot was used to investigate the relation in the data by MATLAB software.
Results
Growth
Comparison of the growth curve and growth rate of Synechocystis sp. FS 78 showed that extreme alkaline conditions (pH 11) were more favourable to growth and had a significant effect on biomass production compared with pH 7 and 9 (results not shown). Moreover, we showed in Fig. 1 that PSII (Fig. 1a) and phycobilisome activity (Fig. 1b) were higher at pH 11. From the time-course study results, the effect of low concentrations Cr(VI) (0.5 µM) showed a significant effect (p < 0.05) on growth and biomass production of Synechocystis sp. FS 78 from the first hour after inoculation (Fig. 2). The better condition for growth and matter production under pH 11 causes relatively short lag phase and high exponential phase under control (without adding chromium) and 0.5 µM Cr(VI) concentration (Fig. 2). During lag phase the cells acclimatize themselves in a new environment by synthesizing new enzymes and orienting the metabolite pathway in a certain manner suitable for adaptation to new environment (Sen et al. 2017). Therefore, cells are able to adapt and grow—during lag phase—under these conditions. The growth rate and biomass production decreased with increasing Cr(VI) concentrations (3.5 and 5 µM) for the duration of 0–4 h after inoculation. Concentrations of 3.5 and 5 µM Cr(VI) caused negative growth from the first hour, which results from the toxic shock applied to the strain. No changes upon time were observed at the relatively high concentration of Cr(VI) (3.5 and 5 µM). On its side, the concentration of 2 µM Cr(VI) caused a relatively mild effect (no toxic shock or high exponential growth is registered).
Absorption spectra
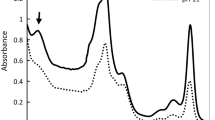
The results of the absorption spectra (Fig. 3) confirmed the growth curves (Fig. 2). The main visible peaks of absorption were observed at 440 nm (chlorophyll blue), 680 nm—PSII (chlorophyll red) and 630 nm—PBS (phycocyanin) in all the treatments (Fig. 3). Also, a shoulder has appeared at 480 nm which was related to carotenoids, and it was detectable especially at 0.5 µM Cr(VI). Comparison of overlay in vivo absorption spectra revealed the dynamism of PS II, phycobilisomes and light-harvesting complexes depending on the Cr(VI) concentrations and time. Low Cr(VI) concentration (0.5 µM) led to a significant increase in all parts of the photosynthesis apparatus compared with the other conditions especially at 2 h after inoculation (Fig. 3b). The absorption peaks of different parts of the photosynthetic apparatus decreased at concentrations higher than 0.5 µM Cr(VI), while this reduction was time independent. The absence of the shift and stability of red chlorophyll (680 nm) peak region was noticeable in all treatments. In the presence of 3.5 and 5 μM Cr(VI) concentration, this stability has been maintained although the peak size significantly decreased (after 2 h). Phycocyanin maintained its structure up to 3.5 µM Cr(VI) concentration. However, in the presence of 5 µM Cr(VI), the structure was disrupted after 2 h. It can be concluded that after the light-harvesting complex, phycobilisomes were more sensitive to high Cr(VI) concentrations. The Polar plot presented the relationships between the relative intensities of carotenoid, PBS and PSII under different Cr(VI) concentrations and time (Fig. 4). A significant correlation was observed in low Cr(VI) concentrations (0.5 μM), where PBS activity, biosynthesis of carotenoids and PSII increased from 2 to 6 h after inoculation. The highest PBS activity and biosynthesis of carotenoids seemed compatible by the pattern of growth under 0.5 μM Cr(VI) concentrations (Fig. 2), whereas high Cr(VI) concentrations (3.5 and 5 μM) led to a significant decline.
In-vivo absorption spectra of cyanobacterial strain Synechocystis sp. FS 78 obtained under different chromium concentration and time at pH11 which normalized to optical density (OD 750). (a) Immediately after inoculation, (b) 2 h after inoculation, (c) 4 h after inoculation, (d) 6 h after inoculation
Spectrofluorimetry
To understand the impact of Cr(VI) on cyanobacteria growth, photosynthesis activity was measured by fluorescence emission upon excitation at 550 nm. The results obtained from PBS function (Fig. 5) confirmed the results of growth curves and in vivo absorption spectra (Figs. 2 and 3). Fluorescence emission significantly increased when cells grew at 0.5 μM Cr(VI) concentration (in particular after 2 h). PBS activity decreased under control condition immediately after inoculation compared with 0.5 μM Cr(VI), while higher Cr(VI) concentration (5 µM) led to lower PBS activity from the first hour after inoculation, which was found not dependent on time. This result confirmed the toxic shock in the growth curves at the initial time (Fig. 2). Fluorescence emission intensities for PSI and PSII activity under different Cr(VI) concentrations were scanned by CLSM (Fig. 6). The pattern of PSI and PSII operation followed the PBS activity. We observed the maximum and minimum activities of photosystems at 0.5 and 5 μM Cr(VI) concentrations, respectively. PSII activity significantly increased at 0.5 µM Cr(VI) concentration and was time-independent, while PSI activity was less affected by Cr(VI) concentrations. Increasing Cr(VI) concentration (≥ 2 μM) caused no significant change in the activity of photosystems (results not shown).
Survivability
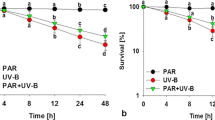
We assessed the viability of Synechocystis sp. FS 78 under different Cr(VI) concentrations for 6 h by registering the autofluorescence from chlorophyll α to differentiate viable and non-viable cells, as previously described by Millach et al. (2017). In Fig. 7, we identified the injured cells (green, Fig. 7a), viable cells (red, Fig. 7c) and the overlap of both autofluorescence (Fig. 7e). The fluorescence spectra taken from viable cells (Fig. 7d) showed the activity of photosynthetic cells compared with non-viable cells (Fig. 7b). The studied strain seems relatively resistant to the toxicity of high Cr(VI) at the initial time of inoculation (< 2 h) while increasing time led to more inactive cells. We observed high number of dead cells at 5 µM Cr(VI) concentration after 4 h of incubation (Figs. 7a, b). The toxic effect of high Cr(VI) concentration on growing cells was probably due to the damage of photosystem and phycobilisome structures, whereas dead cells can partially preserve their cellular structures and the ability to autofluorescence (Fig. 7b). Thus, it may be possible that photosynthetic pigments maintained their structure in the inactive cells, and by passing time, cells abled to recover their phycobilisome and photosystems structures.
Confocal laser scanning photomicrographs of Synechocystis sp. FS 78 under 5 µM Cr(VI) concentration after four hours of inoculation. (a) injured cells, (b) fluorescence spectra of injured cell, (c) viable cell, (d) fluorescence spectra of viable cells, (e) an overlap of injured and viable autofluorescences. The fluorescence spectra of individual cells depicted by circles and numbers is shown in Fig. b & d. scale bars: 10 μm
Discussion
Comparison of growth curves, specific growth rate and generation time shows that Synechocystis sp. FS 78 can significantly acclimatize to survive and grow at extreme alkaline conditions (pH 11) in laboratory conditions. Our experimental results of growth indicate that this is the first native paddy-fields strain can be considered as an extremely alkaliphilic cyanobacteria. Previous workers, Soltani et al. (2006), Safaie Katoli et al. (2015), Amirlatifi et al. (2018) and Abbasi et al 2019, reported pH 9 as the optimum pH for the growth of some of Stigonematalean and Nostocalean cyanobacteria. Increasing pH—generally—activates some cellular functions, such as growth rate and biosorption of heavy metals. Sen et al. (2017) reported that a consortium of cyanobacterium and diatom—Limnococcus limneticus and Leptolyngbya subtilis—isolated from East Kolkata Wetland (India) has high growth, lipid content and fractional removal at pH 9 compared with pH 11. Tang et al. (2020) indicated that pH was an important factor affecting Cr(VI) adsorption. El-Din (2017) demonstrated that the biosorption of algal biomass was dependent on heavy metal concentrations and pH. He observed that the increase in the uptake of Cu 2+ and Pb 2+ was associated with the increasing of pH value of the medium. The maximum uptake of lead was at pH 11 and copper at pH 10.5. Moreover, Krausfeldt et al. 2019 revealed a strong and positively correlated relationship between cyanobacterial abundance and pH, where the pH increased in parallel with cyanobacterial abundance. However, a few specialized groups of microbes may consider such an environment as optimal for growth and survival. The ability to adapt to extreme environmental conditions relies on the high genetic plasticity of most microorganisms (Boussiba et al. 2000).
Growth results reveal that lower Cr(VI) concentrations (0.5 µM) are desired for the nutrition of Synechocystis sp. FS 78 and lead to a significant increase in biomass production and function of different parts of photosynthesis machinery, the effect not being time-dependent. In contrast, elevated Cr(VI) concentrations (3.5 and 5 µM) show a greater inhibitory effect on growth that its time dependent. Decrease of chlorophyll fluorescence, as observed in elevated Cr(VI) concentration, may be due to the inhibition of physiological processes in the cells, which may reveal the toxicity effect at the initial time (Munagamage et al. 2020). The present results are in agreement with those obtained by El-Sheekh et al. (2003), who reported that growth and pigment content of unicellular green alga and the diatom slightly increased at low concentrations and inhibited by high Co2+ concentrations. Sen et al. 2018 showed that enhanced biomass recovery and lipid content by living cyanobacteria Limnococcus sp. was higher at Cr(VI)-contaminated culture media compared with pure culture media. In addition, El-Naggar et al. 1999 found that low Co2+ concentration increased both O2 evolution and dark respiration in two cyanobacterial species, Calothrix fusca and Nostoc muscorum, whereas higher concentrations were inhibitory. Corradi et al. (1995), Hörcsik (2006), Shanab and Essa (2007), Arunakumara and Zhang (2009) and El-Din (2017) showed that low concentrations of heavy metals stimulate growth of the cyanobacterium through enhancing growth rate and biomass, whereas the higher doses inhibited growth and whereas Li et al. (2019) found that the growth of Synechocystis sp. PCC 7806 was inhibited by Cr (III) pollution (under 3000 lx illumination) during day 0 to day 10, and the suppression phenomenon becomes more obvious with the increase of chromium concentration.
In alkaliphilic cyanobacteria, under severe deficiency of carbon dioxide, while most of the carbon source is in the form of bicarbonate ions (Boyd 2015), carbon dioxide concentration mechanism (CCM) is the key process that enables them to adapt to alkaline conditions (Klanchui et al. 2017) and requires a strong operation of photosynthesis—along with other needs—and naturally high efficiency of PSI, PSII and phycobilisomes (Mangan and Brenner 2014). Time-course studying of the effect of 0.5 μM Cr(VI) shows that the stimulation effect decreases with increasing time on photosystems and phycobilisomes (regular pattern). Furthermore, this low concentration leads to increased activity of PSII, PSI and PBS and also coordination in the production and transfer of energy in photosynthesis machinery, which is not disturbed by time. In contrast, in other chromium concentrations cause irregular and unpredictable behaviours in coordination between energy production and transfer sectors under the influence of time. For example, concentration of 2 μM Cr(VI) causes high activity of PSII and PBS after 1 h but, in contrast, has a significant decreasing effect on PSI activity. Similarly, concentrations of 3.5–5 μM Cr(VI) activate one part of the photosynthetic apparatus and in contrast reduces the activity of the other part (parts) depending on the exposure time. Chromium stress can result in alterations of photosynthetic pigments such as chlorophyll (Pereira et al. 2013). Tiwari and Mchanty (1996) suggested that 10 mM CoCl2 to the normal growth medium of Synechocystis PCC 6803 caused multiple changes involving a small increase in PSII to PSI ratio. Further, El-sheekh et al. (2003) found that a low concentration of Co2+ stimulates electron transport at the donor side of PSII of Monoraphidium minutum and Nitzschia perminuta.
We observed the dynamism of the photosystems and phycobilisomes (as the main parts of energy and matter production) (Watanabe et al. 2014) depending on the Cr(VI) concentrations and time. The 0.5 µM Cr(VI) concentrations led to a noticeable increase in all parts of phycobilisome, chlorophyll and protein production and PSII activity from time zero to six—which caused a more stable structure and better coupling energy by PBS. In other words, low concentration Cr(VI) within 6 h increased the strength and flexibility of phycobilisomes at the extreme alkaline condition. In the presence of 3.5 and 5 μM Cr(VI) concentration, PBS activity gradually decrease by destroying the structure of the rode part of PBS after 2 h. These results are agreement with Wong and Chang (1991) that the chromate reduced growth, photosynthesis and chlorophyll synthesis in Chlorella pyrenoidosa. A significant correlation was observed in low Cr(VI) concentrations (0.5 μM), where PBS activity, biosynthesis of carotenoids and PSII increase from 2 to 6 h after inoculation, whereas high Cr(VI) concentrations (3.5 and 5 μM) lead to a significant decline. Tiwari et al. 2018 observed that Nostoc muscorum treated with Cr(VI) (100 and 150 μM) respect to control significantly reduced the photosynthetic process and PSII photochemistry after 96 h. Prasad et al. (1991) suggested that Cr(VI) declined the performance of PS II by interrupting electron flow at its oxidizing and reducing sides as well as by causing damage to energy transfer process within the phycobilisomes, the major light harvesting antenna pigments of PS II.
The viability and number of live cells are determined by factors within the tolerance range of the cyanobacteria which is still ambiguous (Reavie et al. 2010). Survivability of cyanobacteria under conditions of the stress and continuous environmental pollution are an important strategy to acclimate to unstable environmental conditions (Fogg 2001; Ramakrishnan et al. 2010; Shahid et al. 2020). Cell viability information is critical to identify the resistant strains in high stress conditions. To our knowledge, there are no previous reports on the short-time effect of chromium stress on survivability of cyanobacteria. To validate the fluorescence from the viable and dead cell, results were compared with OD 750 and absorption spectra (Schulze et al. 2011; Zhu and Xu 2013), which is a reliable parameter for monitoring viability in conditions even when biomass content is minimal (Johnson et al. 2016) and for evaluating toxicity tolerance and other environmental stresses (Singh et al. 2010). Our results indicated that cell survival was significantly influenced by low Cr(VI) concentration (0.5 µM), which remarkably increased the growth and photosynthetic pigment fluorescence in the first to 6 h after inoculation. In contrast, we observed a toxicity effect on Synechocystis sp. FS 78 in elevated Cr(VI) concentrations (3.5 and 5 µM) at the initial period (up to 4 h), while dead cells still have had the ability to growth and autofluorescence. Cyanobacterium may resist to elevated Cr concentration by reorienting its metabolite pathway for adaption to harsh condition. We observed the cells recover their structures and retain their ability to grow after 4 h. The detailed mechanism of metal toxicity to cyanobacteria is not yet known but is estimated to have an unfavourable effect on respiration, photosynthesis and other processes in sensitive cyanobacterial strains (Kiran et al. 2016). Millach et al. (2015) found that the live cells of Scenedesmus sp. DE2009 slightly decreased at 500 µM Cr (III) concentration after 9 days of inoculation. They confirmed that high level of viability of the strain have maintained at the highest concentration of chromium.
Conclusion
From the above study, we concluded that the combination of the three factors (extreme alkaline pH, different concentrations of Cr(VI) and times) can affect cyanobacterial behaviours individually or in combination. We observed that under different environmental factors, 0.5 µM Cr(VI)—not as toxic effect—can increase the operation of photosystems and phycobilisomes, survival and growth on Synechocystis sp. FS 78. Conversely, the behaviours of elevated Cr(VI) concentrations was time dependent and had toxic effect at initial time of exposed. Hence, it is clearly evident from the study that this strain (native) by growth at low concentrations of chromium, maintenance of survival at high concentrations of chromium and flexibility of the photosynthetic apparatus can be used as a determining factor for inoculation into paddy fields and agricultural lands as biofertilizer and the other biotechnological purposes.
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
References
Abbasi B, Shokravi Sh, Ah GM, Sateiee A, Kiaei E (2019) Effects of alkalinity, extremely low carbon dioxide concentration and irradiance on spectral properties, phycobilisome, photosynthesis, photosystems and functional groups of the native cyanobacterium Calothrix sp. ISC 65. Int J Algae 29:40–58
Ali H, Khan E, Ilahi I (2019) Environmental chemistry and ecotoxicology of hazardous heavy metals: Environmental persistence, toxicity, and bioaccumulation. J Chem https://doi.org/10.1155/2019/6730305
Amirlatifi HS, Shokravi S, Sateei A, Golsefidi MA, Mahmoudjanlo M (2018) Samples of cyanobacterium Calothrix sp. ISC 65 collected from oil polluted regions respond to combined effects of salinity, extremely low-carbon dioxide concentration and irradiance. Int J Algae 20:193–210
Anagnostidis K, Komárek J (1990) Modern approaches to the classification of cyanobacteria. 5-Stigonematales. Algol Stud/Arch Hydrobiol, Supp 59:1–73
Arunakumara KK, Zhang X (2009) Effects of heavy metals (Pb2+ and Cd2+) on the ultrastructure, growth and pigment contents of the unicellular cyanobacterium Synechocystis sp. PCC 6803. Chin J Oceanol Limnol 27:383
Boussiba S, Wu X, Zarka A (2000) Alkaliphilic cyanobacteria. In: Seckbach J (ed) Journey to Diverse Microbial Worlds. Springer, Dordrecht, pp 209–214
Boyd CE (2015) pH, carbon dioxide, and alkalinity. In: Water Quality. Springer, Boston pp 105–122
Cheng J, Yin W, Chang Z, Lundholm N, Jiang Z (2017) Biosorption capacity and kinetics of cadmium(II) on live and dead Chlorella vulgaris. J Appl Phycol 29:211–221
Corradi MG, Gorbi G, Ricci A, Torelli A, Bassi M (1995) Chromium-induced sexual reproduction gives rise to a Cr-tolerant progeny in Scenedesmus acutus. Ecotoxicol Environ Saf 32:12–18
Desikachary TV (1959) Cyanophyta. Indian Council of Agricultural Research, New Delhi
Dutta S, Bhadury P (2020) Effect of arsenic on exopolysaccharide production in a diazotrophic cyanobacterium. J Appl Phycol 32:2915–2926
El-Din SM (2017) Effect of copper and lead on growth and some metabolic activities of cyanobacterium Spirulina platensis (Nordstedt). Egypt J Bot 57:445–456
El-Naggar IM, Zakaria ES, Shady SA, Aly HF (1999) Diffusion mechanism and ion exchange equilibria of some heavy metal ions on Cerium(IV) antimonate as cation exchanger. Solid State Ionics 122:65–70
El-Sheekh MM, El-Naggar AH, Osman ME, El-Mazaly E (2003) Effect of Cobalt on growth, pigments and the photosynthetic electron transport in Monoraphidium minutum and Nitzchia perminuta. Braz J Plant Physiol 15:159–166
Fogg GE (2001) Algal adaptation to stress — Some general remarks. In: Rai L, Gaur J (eds) Algal Adaptation to Environmental Stresses. Springer Berlin, pp 1–19
Fraser JM, Tulk SE, Jeans JA, Campbell DA, Bibby TS, Cockshutt AM (2013) Photophysiological and photosynthetic complex changes during iron starvation in Synechocystis sp. PCC 6803 and Synechococcus elongatus PCC 7942. PLoS One 8:e59861
Grigoryeva N, Chistyakova L (2019) Confocal laser scanning microscopy for spectroscopic studies of living photosynthetic cells. Color Detection UK: Intechopen 2020:39–64
Hazarika J, Pakshirajan K, Sinharoy A, Syiem MB (2015) Bioremoval of Cu(II), Zn(II), Pb(II) and Cd(II) by Nostoc muscorum isolated from a coal mining site. J Appl Phycol 27:1525–1534
Hinners J, Hofmeister R, Hense I (2015) Modeling the role of pH on baltic sea cyanobacteria. Life 5:1204–1217
Hörcsik Z (2006) Effect of Chromium (VI) on growth, element and photosynthetic pigment composition of Chlorella pyrenoidosa. Acta Biologica Szegediensis 50:19–23
Hussein MH, Hamouda RA, Elhadary AM, Abuelmagd MA, Ali S, Rizwan M (2019) Characterization and chromium biosorption potential of extruded polymeric substances from Synechococcus mundulus induced by acute dose of gamma irradiation. Environ Sci Pollut Res 26:31998–32012
Ihnken S, Beardall J, Kromkamp JC, Serrano CG, Torres MA, Masojídek J, Malpartida I, Abdala R, Jerez CG, Malapascua JR, Navarro E (2014) Light acclimation and pH perturbations affect photosynthetic performance in Chlorella mass culture. Aquat Biol 22:95–110
Inoue-Kashino N, Kashino Y, Satoh K, Terashima I, Pakrasi HB (2005) PsbU provides a stable architecture for the oxygen-evolving system in cyanobacterial photosystem II. Biochemistry 44:12214–12228
John DM, Whitton BW, Brook AJ (2002) The freshwater algal flora of the British Isles. Cambridge University Press, Cambridge
Johnson et al (2016) Evaluating viable cell indicators for filamentous cyanobacteria and their application. J Microbiol, Biotechnol Food Sci 6:886–893
Kaushik BD (1987) Laboratory methods for blue-green algae. Associated Publishing Company, New Delhi
Kaushik BD (1994) Algalization of rice in salt-affected soils. Ann Agric Res 14:105–106
Kiran B, Rani N, Kaushik A (2016) Environmental toxicity: Exposure and impact of chromium on cyanobacterial species. J Environ Chem Eng 4:4137–4142
Klanchui A, Cheevadhanarak S, Prommeenate P, Meechai A (2017) Exploring components of the CO2-concentrating Mechanism in alkaliphilic cyanobacteria through genome-based analysis. Comput Struct Biotechnol J 15:340–350
Krausfeldt LE, Farmer AT, Castro Gonzalez HF, Zepernick BN, Campagna SR, Wilhelm SW (2019) Urea Is both a carbon and nitrogen source for Microcystis aeruginosa: Tracking 13C incorporation at bloom pH conditions. Front Microbiol 10:1064
Leganés F, Sánchez-Maeso E, Fernández-Valiente E (1987) Effect of indoleacetic acid on growth and dinitrogen fixation in cyanobacteria. Plant Cell Physiol 28:529–533
Li M, Ma C, Yin X, Zhang L, Tian X, Chen Q, Wang L (2019) Investigating trivalent chromium biosorption-driven extracellular polymeric substances changes of Synechocystis sp. PCC 7806 by parallel factor analysis (PARAFAC) analysis. Bioresource Technol Rep 7:100249
Mangan NM, Brenner MP (2014) Systems analysis of the CO2 concentrating mechanism in cyanobacteria. Elife 3:e02043
Millach L, Solé A, Esteve I (2015) Role of Geitlerinema sp. DE2011 and Scenedesmus sp. DE2009 as bioindicators and immobilizers of chromium in a contaminated natural environment. BioMed Res Int 2015:519769
Millach L, Obiol A, Solé A, Esteve I (2017) A novel method to analyse in vivo the physiological state and cell viability of phototrophic microorganisms by confocal laser scanning microscopy using a dual laser. J Microsc 268:53–65
Miranda J, Krishnakumar G, Gonsalves R (2012) Cr6+ bioremediation efficiency of Oscillatoria laete-virens (Crouan & Crouan) Gomont and Oscillatoria trichoides Szafer: Kinetics and equilibrium study. J Appl Phycol 24:1439–1454
Munagamage T, Rathnayake IV, Pathiratne A, Megharaj M (2020) Comparison of sensitivity of tropical freshwater microalgae to environmentally relevant concentrations of cadmium and hexavalent chromium in three types of growth media. Bull Environ Contam Toxicol 105:397–404
Pan X, Chen X, Zhang D, Wang J, Deng C, Mu G, Zhu H (2009) Effect of chromium(vi) on photosystem ii activity and heterogeneity of synechocystis sp. (cyanophyta): Studied with in vivo chlorophyll fluorescence tests. J Phycol 45:386–394
Pathak J, Maurya PK, Singh SP, Häder DP, Sinha RP (2018) Cyanobacterial farming for environment friendly sustainable agriculture practices: Innovations and perspectives. Front Environ Sci 6:7
Pawlik-Skowrońska B, Kaczorowska R, Skowroński T (1997) The impact of inorganic tin on the planktonic cyanobacterium Synechocystis aquatilis: The effect of pH and humic acid. Environ Pollut 97:65–69
Pereira M, Bartolomé MC, Sánchez-Fortún S (2013) Bioadsorption and bioaccumulation of chromium trivalent in Cr(III)-tolerant microalgae: A mechanisms for chromium resistance. Chemosphere 93:1057–1063
Poza-Carrión C, Fernández-Valiente E, Piñas FF, Leganés F (2001) Acclimation of photosynthetic pigments and photosynthesis of the cyanobacterium Nostoc sp. strain UAM206 to combined fluctuations of irradiance, pH, and inorganic carbon availability. J Plant Physiol 158:1455–1461
Prasad SM, Singh JB, Rai LC, Kumar HD (1991) Metal-induced inhibition of photosynthetic electron transport chain of the cyanobacterium Nostoc muscorum. FEMS Microbiol Lett 82:95–100
Qu M, Li W, Zhang C, Huang B, Zhao Y (2015) Assessing the pollution risk of soil Chromium based on loading capacity of paddy soil at a regional scale. Sci Rep 5:1–8
Quesada A, Fernández-Valiente E (1996) Relationship between abundance of N2-fixing cyanobacteria and environmental features of Spanish rice fields. Microb Ecol 32:59–71
Ramakrishnan B, Megharaj M, Venkateswarlu K, Naidu R, Sethunathan N (2010) The impacts of environmental pollutants on microalgae and cyanobacteria. Crit Rev Environ Sci Technol 40:699–821
Ramírez M, Hernández-Mariné M, Mateo P, Berrendero E, Roldán M (2011) Polyphasic approach and adaptative strategies of Nostoc cf. commune (Nostocales, Nostocaceae) growing on Mayan monument. Fottea 11:73–86
Reavie ED, Cangelosi AA, Allinger LE (2010) Assessing ballast water treatments: Evaluation of viability methods for ambient freshwater microplankton assemblages. J Great Lakes Res 36:540–547
Rocchetta I, Leonardi PI, Amado Filho GM, del Carmen Ríos de Molina M, Conforti V (2007) Ultrastructure and X-ray microanalysis of Euglena gracilis (Euglenophyta) under chromium stress. Phycologia 46:300-306
Roestorff MM, Chirwa EM (2019) Cr(VI) mediated hydrolysis of algae cell walls to release TOC for enhanced biotransformation of Cr(VI) by a culture of Cr(VI) reducing bacteria. J Appl Phycol 31:3637–3649
SafaieKatoli M, Nejad-Sattari T, Majd A, Shokravi Sh (2015) Physiological, morphological and ultrastructural responses of cyanobacterium Calothrix sp. ISC 65 to combination effects of extreme conditions. J Appl Environ Biol Sci 5:135–149
Schulze K, López DA, Tillich UM, Frohme M (2011) A simple viability analysis for unicellular cyanobacteria using a new autofluorescence assay, automated microscopy, and ImageJ. BMC Biotechnol 11:118
Sen S, Dutta S, Guhathakurata S, Chakrabarty J, Nandi S, Dutta A (2017) Removal of Cr (VI) using a cyanobacterial consortium and assessment of biofuel production. Int Biodeterior Biodegradation 119:211–224
Sen G, Sen S, Thakurta SG, Chakrabarty J, Dutta S (2018) Bioremediation of Cr (VI) using live cyanobacteria: experimentation and kinetic modeling. J Environ Eng 144:04018089
Shahid M, Zaidi A, Khan MS (2020) Modulations in growth, structure, cell viability and antioxidant enzyme of a nodule bacterium Mesorhizobium ciceri induced by pesticides. Environ Dev Sustain 23:4103–4119
Shanab SM, Essa AM (2007) Heavy Metal Tolerance, Biosorption And Bioaccumulation By Some Microalgae (Egyptian Isolates). New Egypt J Microbiol 17:76–88
Shokravi S, Soltani N (2011) Acclimation of the Hapalosiphon sp. (Cyanoprokaryota) to combination effects of dissolved inorganic carbon and pH at extremely limited irradiance. Int J Algae 13:379–391
Shokravi S, Amirlatifi HS, Pakzad A, Abbasi B, Soltani N (2014) Physiological and morphological responses of unexplored cyanoprokaryota Anabaena sp. FS 77 collected from oil polluted soils under a combination of extreme conditions. Int J Algae 16:164–180
Singh H, Fernandes T, Apte SK (2010) Unusual radioresistance of nitrogen-fixing cultures of Anabaena strains. J Biosci 35:427–434
Sirunyan AM, Tumasyan A, Adam W, Ambrogi F, Asilar E, Bergauer T, Brandstetter J, Dragicevic M, Erö J, Del Valle AE (2019) Flechl M (2019) Search for vector-like quarks in events with two oppositely charged leptons and jets in proton-proton collisions at √s = 13 TeV. Eur Phys J C 79:1–31
Śliwińska-Wilczewska S, Cieszyńska A, Konik M, Maculewicz J, Latała A (2019) Environmental drivers of bloom-forming cyanobacteria in the Baltic Sea: Effects of salinity, temperature, and irradiance. Estuar Coast Shelf Sci 219:139–150
Soltani N, Khavari RA, Tabatabaei MS, Fernández-Valiente SE (2006) Variation of nitrogenase activity, photosynthesis and pigmentation of the cyanobacterium Fischerella ambigua strain FS18 under different irradiance and pH values. World J Microbiol Biotechnol 22:571-6
Sugiura K, Itoh S (2012) Single-cell confocal spectrometry of a filamentous cyanobacterium nostoc at room and cryogenic temperature. diversity and differentiation of pigment systems in 311 cells. Plant Cell Physiol 53:1492–1506
Summerfield TC, Crawford TS, Young RD, Chua JP, Macdonald RL, Sherman LA, Eaton-Rye JJ (2013) Environmental pH affects photoautotrophic growth of Synechocystis sp. PCC 6803 strains carrying mutations in the lumenal proteins of PSII. Plant Cell Physiol 54:859–874
Tang EP, Vincent WF (1999) Strategies of thermal adaptation by high-latitude cyanobacteria. New Phytol 142:315–323
Tang Y, Liao X, Zhang X, Peng G, Gao J, Chen L (2020) Enhanced adsorption of hexavalent chromium and the microbial effect on quartz sand modified with Al-layered double hydroxides. Sci Total Environ 762:143094
Tiwari S, Mchanty P (1996) Cobalt induced changes in photosystem activity in Synechocystis PCC 6803: Alterations in energy distribution and stoichiometry. Photosynth Res 50:243–256
Tiwari S, Patel A, Prasad SM (2018) Kinetin alleviates chromium toxicity on growth and PS II photochemistry in Nostoc muscorum by regulating antioxidant system. Ecotoxicol Environ Saf 161:296–304
Tiwari S, Patel A, Prasad SM (2020) Phytohormone up-regulates the biochemical constituent, exopolysaccharide and nitrogen metabolism in paddy-field cyanobacteria exposed to chromium stress. BMC Microbiol 20:206
Touloupakis E, Cicchi B, Benavides AM, Torzillo G (2016) Effect of high pH on growth of Synechocystis sp. PCC 6803 cultures and their contamination by golden algae (Poterioochromonas sp.). Appl Microbiol Biotechnol 100:1333–1341
Vermaas WF, Timlin JA, Jones HD, Sinclair MB, Nieman LT, Hamad SW, Melgaard DK, Haaland DM (2008) In vivo hyperspectral confocal fluorescence imaging to determine pigment localization and distribution in cyanobacterial cells. Proc Natl Acad Sci 105:4050–4055
Watanabe M, Semchonok DA, Webber-Birungi MT, Ehira S, Kondo K, Narikawa R, Ohmori M, Boekema EJ, Ikeuchi M (2014) Attachment of phycobilisomes in an antenna-photosystem I supercomplex of cyanobacteria. Proc Natl Acad Sci 111:2512–2517
Wong PK, Chang L (1991) Effects of copper, chromium and nickel on growth, photosynthesis and chlorophyll a synthesis of Chlorella pyrenoidosa 251. Environ Pollut 72:127–139
Zabochnicka-Świątek M, Krzywonos M (2014) Potentials of biosorption and bioaccumulation processes for heavy metal removal. Polish J Environ Stud 23:551–561
Zhu T, Xu X (2013) Efficacy of a dual fluorescence method in detecting the viability of overwintering cyanobacteria. Lett Appl Microbiol 57:174–180
Zorz JK, Allanach JR, Murphy CD, Roodvoets MS, Campbell DA, Cockshutt AM (2015) The RUBISCO to photosystem II ratio limits the maximum photosynthetic rate in picocyanobacteria. Life 5:403–417
Acknowledgements
The authors would like to appreciate the kind collaboration of Professor Ester Boix Borrás (Autonomous University of Barcelona—UAB), Professor Neda Soltani (Shahid Beheshti University, Iran), Professor Douglas Campbell (Mount Allison University) and Professor Eduardo Fernandez Valiente (Autonomous University of Madrid—UAM).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shokravi, S., Bahavar, N. Effects of chromium (VI) at extreme alkaline condition (pH 11) on the survival, growth, photosystems and phycobilisome operation of the cyanobacterium Synechocystis sp. Strain FS 78. J Appl Phycol 33, 2909–2919 (2021). https://doi.org/10.1007/s10811-021-02521-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-021-02521-0