Abstract
β-Galactosidase is a commercially important enzyme widely used in the food industry for the manufacturing of lactose-hydrolyzed products and synthesis of probiotic food ingredients. It could also be used to reduce the environmental impacts of the dairy industry relative to lactose disposal. This enzyme has been isolated from different sources, with varying properties and potential for diverse applications. Several microalgae have been screened for β-galactosidase activity, among which the chlorophyte Tetradesmus obliquus showed significant levels. The production of enzymes from microalgae could emerge as a valuable avenue for the utilization of its biomass. In addition, this particular microalga can grow mixotrophically on lactose as an organic carbon source, offering additional possibilities for the utilization of lactose. This study investigates the production of β-galactosidase from T. obliquus under different trophic conditions and known media composition, in order to assess their influence on its productivity and selectivity. Results show that the photoautotrophic cultures provide a highest selectivity, while mixotrophic conditions provide higher productivities due to faster growth and higher biomass yields. Further studies on mixotrophic cultures using lactose revealed no significant differences on β-galactosidase production when varying the concentrations of organic carbon and nitrogen nutrients. The age of culture has a strong influence on the enzyme production, suggesting a dependence on the growth phase. Maximal enzyme productivities obtained in mixotrophic conditions on lactose reach about 12.35 U L−1 day−1 after 7 days, which is a realistic duration for producing the enzyme at larger scales in bioreactors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lactose, the predominant carbohydrate in milk, dairy products, and whey from dairy industries, is a disaccharide consisting of galactose bound to glucose. It makes up about 4.5–5.0% of the total solids in fluid milk and about 4.8–4.9% of the total solids in cheese whey (Kim and Rajagopal 2000). Its poor digestion may cause gastrointestinal problems in about 70% of the world adult population (Khabarova et al. 2011; Rosolen et al. 2015), and gastrointestinal symptoms may result as follows: diarrhea, bloating, flatulence, and abdominal discomfort (Shaukat et al. 2010). Another problem related to lactose is its low solubility, which often leads to its crystallization. This constitutes another problem because of the undesirable granular texture, which can occur in some dairy products, such as condensed milk, evaporated milk, dry milk, frozen milk, and ice cream, and confectionery products with a high content of milk (Zarate and Lopez-Leiva 1990). Moreover, lactose is largely responsible for the high biological oxygen demand (BOD) and chemical oxygen demand (COD) leading to the environmental pollution of the by-products of dairy industries (Shukla 1975; González Siso 1996). For example, a dairy farm processing 100 t of milk per day produces approximately the same quantity of organic products in its effluent as a town of 55,000 residents (Sienkiewicz and Riedel 1990). This sugar can be hydrolyzed by either acids or enzymes. However, in dairy products, enzymatic hydrolysis is preferred for its specificity, as their contents in proteins and other organic substances should not be impacted (Kim and Rajagopal 2000).
β-Galactosidase (EC 3.2.1.23) is widely distributed in nature and can be found in various microorganisms, plants, and animal organs (Nagy et al. 2001; Haider and Husain 2007). Depending on its origin, its properties (molecular weight, amino acid chain length, active site position, pH and thermal optimum and stability), specificity, and structure may differ significantly (Mlichova and Rosenberg 2006). A main characteristic for the selection of an enzyme in a particular application is its operational pH range: acidic pH enzymes derived from fungi are suitable for the treatment of acid whey and acid whey permeates whereas enzymes with neutral pH from yeasts and bacteria are suitable for the treatment of milk and sweet whey (Panesar et al. 2010). The main industrial productions are from microbial origin, as they present the highest productivity (Rosolen et al. 2015). For their part, microalgae as enzyme producers could have several advantages over other organisms, as their low nutritional requirements (CO2, cheap nitrogen sources, light from either natural or artificial sources, water, and salts) and synthesis of other valuable by-products might improve process profitability (Brasil et al. 2017).
The present work therefore addresses a first practical study for the quantification and the optimization of the β-galactosidase enzyme production with Tetradesmus obliquus (formerly known as Scenedesmus obliquus; Wynne and Hallan 2015). In our recent studies (Girard et al. 2013, 2014), cultures of this microalgae in the presence of lactose improved biomass productivity compared to autotrophic cultures on CO2. During growth a decrease in lactose concentration was observed over time, along with a concomitant increase in glucose and galactose concentrations, revealing β-galactosidase activity. However, this level of activity was not quantified at the time. The objectives of this work are to investigate and evaluate the β-galactosidase production by T. obliquus in different trophic modes under determined culture conditions. As this microalga can grow on lactose, there would be a particular interest for using this low-cost, largely available carbon source for the production of algal biomass in combination with the β-galactosidase enzyme. The paper is structured as follows: experimental methods including necessary adaptations to the standard approach for measuring β-galactosidase are first presented, followed by the experimental results. Influences of the trophic mode of culture, type of carbon source, nutrient concentrations, and production kinetics are investigated.
Materials and methods
Culture and medium
Tetradesmus obliquus was obtained from the Canadian Phycological Culture Center in Waterloo, Canada (CPCC 5). It was kept in autoclaved (121 °C, 15 min, 1–2 bar) modified Bold’s Basal medium (BBM), with an adjusted pH of 6.8 (Stein 1973). Seed cultures (for all experiments) were kept in exponential growth phase in a Multitron II incubator shaker (Infors-ht Switzerland) under continuous orbital agitation (120 rpm), constant temperature (21 °C), ambient air, and light intensity of 100 μmol photons m−2 s−1 for 15 days prior to inoculation.
The preparation of all samples was done under sterile conditions in a laminar flow biological hood and axenic culture conditions were confirmed at the beginning and the end of each culture using a cytometric flow analysis as described in Deschênes et al. (2015) and Tremblay et al. (2009).
Concentrated solutions of BBM medium and nitrate were prepared to constitute the medium for the experiments, allowing the necessary flexibility for varying the growth conditions. Concentrated solutions of pure D-(+)-glucose, pure D-(+)-galactose, and pure D-lactose monohydrate (all from Sigma-Aldrich, Canada) were also prepared (in nano-filtered water) for the heterotrophic and mixotrophic cultures. All solutions were autoclaved separately, then diluted to the desired concentration under sterile conditions.
Experimental design
All experiments were carried out in 250-mL Erlenmeyer flasks (75 mL operation volume) with an inoculation concentration of 1 × 106 cells mL−1 (day 0), using manual cell counts on a hemacytometer. The first experiment was conducted to verify whether the enzyme is produced in different trophic modes of growth (photoautotrophic, heterotrophic, and mixotrophic) and if so, how it affects the production of the enzyme. Thus, these three conditions were compared in triplicates using normal BBM concentrations (containing about 180 mg L−1 of nitrate as the sole nitrogen source) and a lactose concentration of 40 g L−1 for the heterotrophic and mixotrophic cultures. This concentration was selected from previous results (Girard et al. 2014), suggesting a certain optimum for cell growth.
The second experiment evaluated the influence of the dissolved organic carbon (DOC) source on the production of the enzyme in mixotrophic conditions. Glucose, galactose, and lactose, used as DOC sources, were tested at a concentration of 40 g L−1. All conditions were tested in triplicates, with the autotrophic condition as control.
In a third experiment the data were subjected to a 23 factorial analysis of variance to evaluate the influence of nitrogen availability (nitrate concentrations of 180 and 360 mg L−1), carbon availability (lactose concentrations of 20 and 40 g L−1), and age of culture (days 8 and 16) on the production of the β-galactosidase enzyme. All other ion concentrations were as in normal BBM composition.
A fourth experiment was finally carried out to study the kinetics of β-galactosidase production in mixotrophic conditions. Based on the results of the abovementioned experiments, a lactose concentration of 20 g L−1 was selected, with other ion concentrations as in standard BBM conditions. Due to sample volume restrictions the same condition was implemented simultaneously in five sets of triplicates, in which two measurements (at different days) were made for each set.
Measurements were taken at days 8 (growth phase) and 16 (expected stationary phase of culture) in the first three experiments to include the stage of culture in the comparisons. In the last (fourth) experiment, measurements were taken at days 3, 5, 7, 9, 11, 13, 15, 17, 19, and 21 of culture to grasp a more complete view of the enzyme production throughout the culture stages.
Statistical analyses were systematically conducted on the results using standard analysis of variance (ANOVA) tests for comparison of means with the Excel software. Mean values and standard deviation of measurements (n = 3) were calculated for each experiment and statistical significance was determined by the calculation of p values. A p value of less than 0.05 (p < 0.05) was considered to indicate significance. Results are expressed as means ± SD (the standard deviation).
Analytical methods
Samples were centrifuged (3400×g, 6 min) and then washed twice with distilled water to remove excess salts, sugars, or any component that could influence the measurement of the optical density. Cell enzymatic activity was analyzed based on Miller (1972) with a few adaptations for microalgae: a 0.5-mL concentrated cell solution was mixed with 0.5 mL of Z-buffer (0.43 g of 0.06 M Na2HPO4, 0.28 g of 0.04 M NaH2PO4.H2O, 0.04 g of 0.01 M KCl, 0.01 g of 0.001 M MgSO4.7H2O, and 0.135 mL of 0.05 M β-mercaptoethanol in 50 mL distilled water solution with pH adjustment to 7.0). The cell membrane was subsequently destroyed over 5 min using 100 μL of chloroform and 50 μL of 0.1% sodium dodecyl sulfate (SDS) to allow small molecules such as o-nitrophenyl-β-D-galactoside (ONPG) penetrate the cell walls. An enzymatic reaction was then started by adding 0.2 mL of ONPG (4 mg mL−1) to the mixture incubated at 28 °C. This reaction allows the ONPG molecules (colorless) to be hydrolyzed into galactose (colorless) and o-nitrophenol (yellow color) in the presence of the β-galactosidase enzyme. The addition of 0.5 mL of 1 M Na2CO3 after the development of a sufficient yellow color inactivates the enzyme and stops the reaction as the pH is increased to 11. The liberated o-nitrophenol was detected spectrophotometrically at 420 nm and was expressed in units (U) of enzyme activity: 1 U is equivalent to 1 nmol o-nitrophenol produced per minute.
Other adaptations of Miller’s method, initially developed for bacteria such as Escherichia coli, include a mandatory centrifugation step (3400×g, 6 min) after stopping the enzymatic reaction to remove all cell debris, improving the absorbance measurement (420 nm in a clear solution) for the yellow compound o-nitrophenol. Another measurement of absorbance at 420 nm was also included for a similar sample without ONPG addition to obtain a correction for the background color of the sample (mainly from alga pigmentation).
As the ONPG concentration is in excess, the result of the assay can be assumed to be linearly proportional to the amount of enzymes present (Miller 1972). We thus use the two following quantitative measurements for the enzyme produced: the specific enzymatic activity (SEA) and the (total) volumetric enzymatic activity (VEA). The first (Eq. 1) is defined as the amount of enzyme (U) per unit of algal biomass (g cells), which can be used as a measure of process selectivity (proportion of enzyme relative to the total biomass produced, from the same carbon source). The second (Eq. 2) is a measure of process volumetric production (total amount of enzyme produced per unit volume of culture).
where OD420 (with ONPG) and OD420 (without ONPG) are the absorbance measurements (discussed above), t is the reaction time (min), v is the volume of culture used in the assay (mL), DW is the actual dry weight (g L−1) of the sample, factor 0.0045 is the absorbance of 1 nmol of o-nitrophenol at 420 nm in a 10-mm light path, and factor 1000 is for conversion of units (mL L−1) after the product of v and DW.
Dry weight (DW) measurements were carried out for all experiments as follows (Zhu and Lee 1997): a culture sample of 10 mL was filtered through a glass-microfiber filter (VWR Grade 691, nominal pore size 1.2 μm) previously dried and weighted. The cells were washed with a 20-mL solution of 0.5% ammonium formate to ensure the removal of all dissolved compounds that could alter the dry weight measurement. The filtration technique was conducted as described in Strickland and Parsons (1968) and Zhu and Lee (1997). The filters were then dried at 95 °C until they reach a constant weight, cooled in a desiccator for 5 min, and then weighted.
Results
Influence of the culture mode
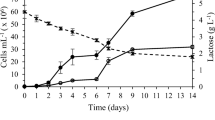
Experiments demonstrated that β-galactosidase activities were observed in all trophic modes of growth. A comparison of the enzymatic activities however revealed significant differences between the cultures. Indeed, the specific production of the enzyme was always higher in photoautotrophic mode (Fig. 1), whatever the time condition. Mixotrophic cultures then showed higher specific productivities than heterotrophic cultures at day 8 (F = 25.96, p = 0.007), though at day 16, the difference was not significant. All cultures showed an increase in SEA between days 8 and 16, whether the biomass concentration increased or not. The VEA, which accounts for the differences in biomass concentrations, was then higher in mixotrophic cultures at day 8, where the difference between photoautotrophic and heterotrophic cultures was not significant (F = 3.13, p = 0.15) (Fig. 1). After 16 days photoautotrophic cultures showed a higher production of the enzyme, followed by the mixotrophic and then the heterotrophic cultures.
A measure of process productivity (production of enzyme per unit of time) can be obtained by computing the ratio between the VEA and the age of culture. This overall daily-averaged quantity provides additional insights for scale-up perspectives and for determining an appropriate operation mode for an industrial setup. Experiments showed significant differences at this level between trophic modes: photoautotrophic cultures show much higher productivities over 16 days (9.4 U L−1 day−1) than over 8 days (3.7 U L−1 day−1), while mixotrophic cultures show better productivities over 8 days (7.3 U L−1 day−1) than over 16 days (6.6 U L−1 day−1). Heterotrophic cultures maintain a similar level of productivity (around 2.9 U L−1 day−1) over either 8 or 16 days.
Influence of the organic substrate
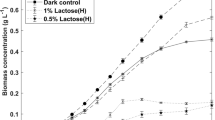
Mixotrophic cultures conducted with either pure glucose or pure galactose as the sole organic carbon source (the individual monosaccharides composing lactose) showed generally higher enzymatic activities compared to those with lactose (Fig. 2). Furthermore, similarly as in the previous experiment, VEAs were higher in mixotrophic cultures than in the photoautotrophic control at day 8 (due to higher biomass concentrations), whereas the SEAs remained higher in photoautotrophic cultures at days 8 and 16 (following a similar profile), suggesting repeatability. However, at this time, the VEA in the photoautotrophic cultures at day 16 was not higher than in the mixotrophic cultures at the same time condition. Also, comparing daily-averaged enzyme productivities, similar results were observed between photoautotrophic cultures over 16 days and mixotrophic cultures on lactose over 8 days: the latter produced about half the amount of the first in half the time. This suggests that long-term cultures are not necessarily a better option than short-term cultures for producing the enzyme.
Daily-averaged volumetric enzyme productivities showed the highest results with microalgae grown on glucose (17.7 U L−1 day−1) and galactose (15.6 U L−1 day−1) over 8 days; on lactose, a result of 9.8 U L−1 day−1 was obtained. All these (mixotrophic) cultures showed better productivities over 8 days than over 16 days (9.8 U L−1 day−1 for glucose, 12.7 U L−1 day−1 for galactose, and 8.8 U L−1 day−1 for lactose). As in the previous experiment (section “Influence of the culture mode”), photoautotrophic (control) cultures showed higher productivities over 16 days (9.7 U L−1 day−1) than over 8 days (4.9 U L−1 day−1), where its highest level is similar to that of cultures on lactose.
Influence of carbon and nitrogen concentrations
Under the conditions tested (lactose concentrations of 20 and 40 g L−1 and nitrate concentrations of 180 and 360 mg L−1), results showed similar SEA values for most cultures at either time condition (Fig. 3). The VEA appears to rise slightly as the nutrient concentrations are increased (Fig. 3), thought the only case of statistical significance is for a higher lactose concentration at a constant nitrate concentration of 360 mg L−1 at day 8 (F = 192.6, p < 0.01). Overall results of the statistical analyses about the influence of factors (age of culture, nitrate concentration, and lactose concentration) showed that only the age of culture has a consistent influence on the SEA (F = 48.02, p < 0.01) and the VEA (F = 8.90, p < 0.01). Biomass production is not being affected by increased nitrate concentrations at either day 8 or 16. However, increased lactose concentrations do positively influence the biomass concentration over the first 8 days of production, whether the nitrate concentration is 180 mg L−1 (F = 18.43, p = 0.013) or 360 mg L−1 (F = 31.56, p = 0.005).
Kinetics of enzyme production
As the age of culture has a significant influence on most results, characterizing the enzyme production through time was deemed of importance. Mixotrophic cultures on lactose (20 g L−1) were used for this study based on previous volumetric productivities over a rather short period of time (higher than in the autotrophic case) and low cost of this organic carbon source.
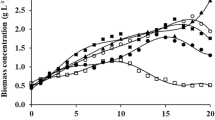
The SEA and VEA evolution through time showed a somewhat similar pattern (steady increase followed by a stationary phase), as does the biomass concentration (Fig. 4). At the end of the experiment (days 19 and 21), enzymatic activities tended to decrease (late stationary phase). To grasp a better view of the actual enzyme productivity, a daily-averaged volumetric enzyme productivity (VEA divided by culture time) was computed (Fig. 5): results show an optimum at around day 7 (12.35 U L−1 day−1), confirming the previous apprehensions in “Introduction,” “Materials and methods,” and “Results.”
Discussion
This study investigated β-galactosidase production by Tetradesmus obliquus across a range of DOC and nitrogen conditions. Results showed that the trophic mode has a significant influence on both the production of enzyme and biomass. The SEA was always higher in photoautotrophic mode, while the use of a DOC source reduced the specific enzyme production (both in heterotrophic and in mixotrophic modes). This observation contrasts with the case of bacteria E. coli, where lactose induces a higher specific enzyme production (Jacob and Monod 1961; Law et al. 2002). The use of a DOC source however significantly stimulated the growth of T. obliquus (as observed in Girard et al. 2014): in mixotrophic cultures, this resulted in higher volumetric enzyme productions after 8 days and overall higher enzyme productivities (U L−1 day−1). In heterotrophic cultures, however, the lowest enzyme productions were observed.
The highest enzyme productivities were observed in mixotrophic cultures over 8 days and in autotrophic cultures over 16 days. Since higher variability was observed in autotrophic cultures, mixotrophic conditions appear as a more viable alternative for producing the enzyme. Heterotrophic cultures presented the lowest enzyme production levels and therefore do not appear suitable for producing the enzyme.
The supplementation levels of carbon and nitrogen nutrients in β-galactosidase productions have not been previously studied to a great extent (Kazemi et al. 2016). The present study however showed no significant influence on the SEA in the range of tested concentrations (20 to 40 g L−1 lactose and 180 to 360 mg L−1 nitrate) in mixotrophic conditions. Most results on the VEA also suggested a similar pattern, though there some occurrences of small increases with increasing nutrient concentrations. These results suggested that further increasing these nutrient concentrations would not have a beneficial impact on enzyme production. In yeast Kluyveromyces marxianus, a generally positive influence was observed between the increasing nitrogen and lactose concentrations and the volumetric production of β-galactosidase (Perini et al. 2013): this study comprised a range of concentrations from near-zero to similar values as tested here with T. obliquus. Under the hypothesis that similar relations govern the β-galactosidase production with this microalga, further work should investigate lower concentrations and determine acceptable levels for improving nutrient utilization for the production of this enzyme.
The main variable of influence on the SEA was the age of culture (thus possibly the growth phase). At day 16, it was greater than at day 8 in most cultures (where the stationary growth phase is mainly attained), except for the mixotrophic culture on glucose. In the case of the latter, the difference might be explained by the fact that growth occurred so much faster than in other cultures that they have remained in stationary phase longer before day 16, and entered a different metabolic state. Photoautotrophic cultures at day 16 might still be under a slow growth process or in early stationary phase. Growth-phase dependency of β-galactosidase production has been observed in various bacteria (Miksch and Dobrowolski 1995): levels of expression remained high as long as logarithmic growth was maintained, but enzyme levels dropped by 12- to 30-fold when cells entered stationary phase (Dickson and Markin 1980). Our results might suggest that T. obliquus has a growth phase-dependent gene expression, as do other bacteria, though its maximum could be in the early stationary phase.
The kinetics of enzyme production in mixotrophic cultures grown on lactose showed that the SEA, VEA, and the biomass concentration evolved in a somewhat similar pattern (Fig. 4). Computing the enzyme productivity from these results (averaging the volumetric production per number of days of culture) suggested a maximum at around day 7, at 12.35 U L−1 day−1. Such culture duration would be reasonable for productions in bioreactors (and larger production scales) and gives a perspective for the development of a scaled-up enzyme production strategy. Future work entails the characterization of the β-galactosidase enzyme produced from microalga T. obliquus and the determination of its properties, specificity, and structure.
References
Brasil BSAF, De Siqueira FG, Salum TFC, Zanette CM, Spier MR (2017) Microalgae and cyanobacteria as enzyme biofactories. Algal Res 25:76–89
Deschênes J-S, Boudreau A, Tremblay R (2015) Mixotrophic production of microalgae in pilot-scale photobioreactors: practicability and process considerations. Algal Res 10:80–86
Dickson RC, Markin J (1980) Physiological studies of β-galactosidase induction in Kluyveromyces lactis. J Bacteriol 142:777–785
Girard J-M, Deschênes J-S, Tremblay R, Gagnon J (2013) FT-IR/ATR univariate and multivariate calibration models for in situ monitoring of sugars in complex microalgal culture media. Bioresour Technol 144:664–668
Girard J-M, Roy M-L, Ben Hafsa M, Gagnon J, Faucheux N, Heitz M, Tremblay R, Deschênes J-S (2014) Mixotrophic cultivation of green microalgae Scenedesmus obliquus on cheese whey permeate for biodiesel production. Algal Res 5:241–248
González Siso MI (1996) The biotechnological utilization of cheese whey: a review. Bioresour Technol 57:1–11
Haider T, Husain Q (2007) Preparation of lactose free milk by using ammonium sulphate fractionated proteins from almonds. J Sci Food Agric 87:1278–1283
Jacob F, Monod J (1961) Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol 3:318–356
Kazemi S, Khayati G, Faezi-Ghasemi M (2016) β-Galactosidase production by Aspergillus niger ATCC 9142 using inexpensive substrates in solid-state fermentation: optimization by orthogonal arrays design. Iran Biomed J 20.5:287–294
Khabarova Y, Tornianen S, Tuomisto S, Järvelä I, Karhunen P, Isokoski M, Mattila K (2011) Lactase non-persistent genotype influences milk consumption and gastrointestinal symptoms in Northern Russians. BMC Gastroenterol 11:124
Kim JW, Rajagopal SN (2000) Isolation and characterization of β-galactosidase from Lactobacillus crispatus. Folia Microbiol 45:29–34
Law J, Lee S, Tseng A, Tsui KW, Yu N (2002) The role of glycerol and isopropyl thiogalactoside in Escherichia coli growth and lactose induction of β-galactosidase. J Exp Microbiol Immunol 2:97–102
Miksch G, Dobrowolski P (1995) Growth phase-dependent induction of stationary-phase promoters of Escherichia coli in different gram-negative bacteria. J Bacteriol 177:5374–5378
Miller JH (1972) Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor
Mlichova Z, Rosenberg M (2006) Current trends of β-galactosidase application in food technology. J Food Nutr Res 45:47–54
Nagy Z, Keresztessy Z, Szentirmai A, Biro S (2001) Carbon source regulation of β-galactosidase biosynthesis in Penicillium chrysogenum. J Basic Microbiol 41:351–362
Panesar PS, Kumari S, Panesar R (2010) Potential applications of immobilized β-galactosidase in food processing industries. Enzyme Res 2010:473137
Perini BLB, Souza HCM, Kelbert M, Apati GP, Pezzin APT, Schneider ALS (2013) Production of β-galactosidase from cheese whey using Kluyveromyces marxianus CBS 6556. Chem Eng Trans 32:991–996
Rosolen MD, Gennari A, Volpato G, De Souza CFV (2015) Lactose hydrolysis in milk and dairy whey using microbial β-galactosidases. Enzyme Res 2015:806240
Shaukat A, Levitt MD, Taylor BC, MacDonald R, Shamliyan TA, Kane RL, Wilt TJ (2010) Systematic review: effective management strategies for lactose intolerance. Ann Intern Med 152:797–803
Shukla RT (1975) Beta-galactosidase technology: a solution to the lactose problem. CRC Crit Rev Food Technol 5:325–356
Sienkiewicz T, Riedel CL (1990) Whey and whey utilization: possibilities for utilization in agriculture and foodstuffs. Verlag Th., Mann, Gelsenkirchen-Buer
Stein JR (ed) (1973) Handbook of phycological methods: culture methods and growth measurements. Cambridge University Press, Cambridge
Strickland JDH, Parsons TR (1968) A practical handbook of seawater analysis. Fisheries Research Board of Canada, Ottawa, pp 181–184
Tremblay G, Belzile C, Gosselin M, Poulin M, Roy S, Tremblay J (2009) Late summer phytoplankton distribution along a 3500 km transect in Canadian Arctic waters: strong numerical dominance by picoeukaryotes. Aquat Microb Ecol 54.1:55–70
Wynne MJ, Hallan JK (2015) Reinstatement of Tetradesmus GM Smith (Sphaeropleales, Chlorophyta). Feddes Repert 126:83–86
Zarate S, Lopez-Leiva MH (1990) Oligosaccharide formation during enzymatic lactose hydrolysis: a literature review. J Food Prot 53:262–268
Zhu CJ, Lee YK (1997) Determination of biomass dry weight of marine microalgae. J Appl Phycol 9:189–194
Funding
This study received a financial support from NSERC (National Science and Engineering Research Council of Canada), INAF (Institute of Nutrition And functional Foods - Pilot projects program), FRQNT (Fonds de Recherche du Québec sur la Nature et les Technologies), and MAPAQ (Ministère de l’Agriculture, des Pêcheries et de l’Alimentation du Québec - Innov’Action program).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bentahar, J., Doyen, A., Beaulieu, L. et al. Investigation of β-galactosidase production by microalga Tetradesmus obliquus in determined growth conditions. J Appl Phycol 31, 301–308 (2019). https://doi.org/10.1007/s10811-018-1550-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-018-1550-y