Abstract
Microalgae have been highlighted in the production of biocompounds to apply in the food, pharmaceutical, and chemical sectors. Strategies in microalgae culture conditions, such as two-stage cultivation, can improve the synthesis of macromolecules of interest allied to increasing biomass productivity. The objective of this work was to evaluate the production of macromolecules [proteins, carbohydrates, polyhydroxybutyrate (PHB), and lipids] by Spirulina sp. LEB 18 cultivated in two-stage strategy. In the first stage of culture, modified Zarrouk medium was used, containing 100% of nitrogen (NaNO3, 2.5 g L−1) and 50% of carbon (NaHCO3, 8.4 g L−1). In the second stage, the culture medium was replaced by a medium containing nitrogen reduction (NaNO3, 0.25 g L−1) on the 5th, 10th, or 15th day. The nitrogen reduction the 5th day reached the highest lipid content (37.0%). To reach 58.1% of carbohydrates, cultivations must be conducted in a single stage for 20 days, using 10% of nitrogen source. The highest protein content was obtained with 100% nitrogen, on the 10th day of the first stage of cultivation (73.8%). The two-stage cultivation with the second stage on day 10 provides proteins (57.4%) on day 5, lipids (34.6%) on day 15, and PHB (29.6%) and carbohydrates (15.8%) on day 20, enabling operating microalgal biomass in a biorefinery system. Thus, two-stage strategy contributes to determining cultivation conditions that involve the optimization of macromolecules by Spirulina sp. LEB 18.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Commercial production of microalgae biomass began in 1960 with the cultivation of Chlorella in Japan. In 1970, there was the first commercial Spirulina (Arthrospira platensis) production plant (Mexico). Chlorella and Spirulina comprise more than 90% of the total production of microalgal biomass. Production is mainly intended (~ 90%) for the human food and nutrition (nutraceuticals) market. Globally, commercial autotrophic microalgae biomass production is around 20,000 tons/year, dry. Bulk sales prices for Spirulina and Chlorella range from around $10,000 to $30,000/ton. The market is about half a billion dollars annually and is increasing tenfold at the consumer level (Benemann et al. 2018).
Many countries have been producing algae-based products (Chew et al. 2021). Compared to other microalgae species, Spirulina is successfully marketed in China for the production of human feed and aquaculture (Show 2022). Spirulina is widely known for its protein content (50–70%) (Lafarga et al. 2021). Moreover, this microalga also contains carbohydrates (~ 20%), lipids (~ 10%) (Moreira et al. 2016), as well as vitamins, minerals (Lafarga et al. 2021), and biopolymers such as polyhydroxybutyrate (PHB) (Moreira et al. 2016). More than 60 Spirulina plants with 7,500,000 m2 produce 9600 tons of dry biomass/year in China. The yearly retail value is over US$650 million (Li et al. 2015; Show 2022). According to Show (2022), this would summarize the biomass productivity of 13 ha/year, where around 70 kg of biomass is being sold to consumers (Show 2022).
On the other hand, the microalgae industries in Japan and the European Union focus on biofuel production (Murata et al. 2021). The size of the microalgae biofuel market in Japan is worth about 128 billion yen (USD 1170 million). In addition, there is ongoing investment in research and development to expand into other markets such as food, chemicals, and supplements. According to Mintel's Global New Products Database (GNPD), Europe accounted for the highest number of Spirulina-containing products, with 70% of global market launches. Next, Asia (14%), North America (10%), East Middle and Africa (5%), and Latin America (1%) are highlighted. In this sense, the biorefinery concept emerges to promote the economic viability of microalgae to replace fossil fuels and other bioproducts (Show 2022).
Biorefineries have emerged as a new concept in the conversion of biomass into products with high added value, biofuels, and/or energy in an integrated manner, which reduces environmental impacts and maximizes the potential of raw materials (Chew et al. 2017; Costa et al. 2017). Microalgae biomass has several biomolecules, such as proteins, lipids, carbohydrates, polymers, vitamins, pigments, and antioxidants (Hornes et al. 2010). In the context of a biorefinery, proteins obtained from microalgal biomass can be used to increase the nutritional value of food. Long-chain fatty acids can be used as a food supplement. Carbohydrates can be applied as a carbon source in the fermentation industries for ethanol production, while lipids can be used as raw material for biodiesel production (Chew et al. 2017). Degradable polymers synthesized by microalgae can act as a substitute for petrochemical plastics (Ansari and Fatma 2016).
The two-stage cultivation strategy can positively influence macromolecular synthesis by the microalgae and reduce the time of cultivation. The first stage involves optimal conditions for growth, resulting in increased biomass productivity. In the second stage, there is a reduction of a specific nutrient in the culture medium, such as nitrogen, which can stimulate the accumulation of lipids, carbohydrates, or biopolymers by microalgae. Thus, the main advantage of using two-stage cultivation is to obtain high productivity in biomass simultaneously with the high concentration of macromolecules (Monshupanee et al. 2016).
Synthesis of proteins, carbohydrates, lipids, and PHB depends on the supply of nitrogen. Moreover, the nitrogen compound is one of the most important components in microalgae cultivation, as it directly interferes with cellular metabolism. Nitrogen limitation diverts the microalgae's metabolic pathway to produce carbohydrates and/or lipids. The production of carbohydrates occurs during the carbon dioxide (CO2) biofixation metabolism of the microalgae through photosynthesis (Braga et al. 2019). The PHB polymer is a lipid component and has its synthesis initiated through the condensation of two molecules of acetyl-CoA, in which β-ketothiolase produces acetoacetyl-CoA. The demand for acetyl-CoA becomes a limiting factor in the production of PHB, as it inhibits the action of the β-ketothiolase enzyme. When the CoA molecule is no longer produced intracellularly by the microorganism (due to the nutritional limitations of the culture medium), the metabolic pathways converge toward the production of PHB (Costa et al. 2018a, b).
The two-stage cultivation with nitrogen limitation is a pioneer in defining the best period to start altering the nutritional supply of microalgae, and the total cultivation time to maximize the production of proteins, carbohydrates, lipids, and PHB. In this context, the objective of this work was to evaluate the production of macromolecules by Spirulina sp. LEB 18 cultivated in two-stage strategy.
Material and methods
Microorganism and culture conditions
The microalga Spirulina sp. LEB 18, belonging to the strain bank of the Laboratory of Biochemical Engineering, was isolated from Lagoa Mangueira (latitude 33°31′08″ S and longitude 53°22′05″ W) located in the extreme south of Brazil (Morais et al. 2008). The inoculum was kept in a 6 L Erlenmeyer flask with modified Zarrouk medium containing 50% of the carbon source (NaHCO3, 8.4 g L−1) for 10 days.
The two-stage experiments were performed in tubular photobioreactors of 2 L with a working volume of 1.6 L, containing 0.5 g L−1 of initial biomass concentration. Cultivations were performed at 30º C in a thermostatic camera, 12 h light/dark photoperiod and 41.6 µmol m−2 s−1 luminosity provided by 40 W fluorescent lamps. Continuous agitation was applied through the injection of sterile air, and the cultivations were carried out in triplicate for 20 days. The microalgae Spirulina sp. LEB 18 was grown in Zarrouk medium (Zarrouk 1966) modified with 50% of carbon (Martins et al. 2014).
The two-stage cultures were performed with medium containing the original concentration of Zarrouk medium, including the nitrogen source (NaNO3, 2.5 g L−1) in the first stage of cultivation. The second stage started at times 5, 10 or 15 days, in which the medium was replaced with nitrogen reduction (NaNO3, 0.25 g L−1). The control experiments were carried out in a single stage containing 10% (0.25 g L−1) and 100% (2.5 g L−1) of the original nitrogen source (NaNO3) of the culture medium.
For the change of the culture medium, corresponding to the second stage, the cultures were subjected to centrifugation (HITACHI himac CR-GIII, Japan) at 15,200g, 20 °C for 30 min. The cells were washed twice with sterile distilled water to remove salts from the medium by centrifugation at 15,200g, 20 °C for 10 min. Thus, the Zarrouk medium was added with a 90% reduction in the nitrogen source (NaNO3, 0.25 g L−1), maintaining the same cell concentration. The volume of the experiments was kept constant with the daily addition of sterile water to supply the evaporation losses.
Analytical determinations and kinetic parameters
Daily aliquots were taken from the cultivations to determine the biomass concentration by an optical density at 670 nm in spectrophotometer (UVmini-1240, Shimadzu, Japan) (Costa et al. 2002). Daily monitoring of pH was performed using a digital pH meter (Satra, PHS-3E, Brazil).
The maximum specific growth rate (μmax, day−1) and maximum productivity (Pmax, g L−1 day−1) were determined from the cell concentration values. The maximum cell concentration (Xmax, g L−1) was the maximum concentration value obtained during cultivation, and the generation time (tg, d) was determined in the exponential growth phase of the microalgae (Bailey and Ollis 1986), according to Eq. (1). Productivity was obtained according to Eq. (2), with Xt being the cell concentration (g L−1) at time t (days) and X0 being the cell concentration (g L−1) at time t0 (days). The maximum value of productivity found during the cultivation corresponded to Pmax.
μmax was calculated by exponential regression applied to the logarithmic growth phase, according to Eq. (3).
Nitrogen analysis
The nitrogen content was determined at 3-day intervals by adding 0.8 mL of sulfuric acid (H2SO4) with 5% (w v−1) salicylic acid (C7H6O3), and 19 mL of 2 M sodium hydroxide (NaOH) to the samples. The spectrophotometer reading was performed at 410 nm and the absorbance values were correlated with the standard curve of NaNO3 (Cataldo et al. 1975).
Proximal composition of microalgal biomass
Samples were taken from each tubular reactor every 5 days, and then the biomass of Spirulina sp. LEB 18 was separated from the liquid medium by centrifugation (HITACHI himac CR-GIII, Japan) at 4600g for 30 min. Subsequently, the biomass was resuspended in distilled water and again centrifuged for two more times, for 10 min, to remove the salts from the culture medium. The concentrated biomass was frozen at − 80 °C for 48 h and then was lyophilized for 48 h. Therefore, the lyophilized sample was stored at − 18° C for later use in analyzes (Rosa et al. 2016). The lyophilized biomass was pre-treated by an ultrasonic probe. Thus, 10 mL of distilled H2O was added to 5 mg of microalgae biomass and sonicated for 10 min in 59 s cycles (59 s on and 50 s off). Finally, using magnetic, the homogenized extract was ready for further characterization (Moreira et al. 2016).
The lipid concentration was determined by the gravity method of Marsh and Weinstein (1966), using a standard curve of tripalmitin. The concentration of carbohydrates in the biomass was evaluated according to the phenol–sulfuric method of Dubois et al. (1956), using a standard curve of glucose. The protein concentration was determined by the colorimetric method of Lowry et al. (1951), using the standard curve of albumin.
Quantification and yield of PHB
Gas chromatography was performed to determine the PHB concentration in the biomass at times 0, 5, 10, 15, and 20 days. The biomass was lyophilized and subjected to pigment extraction by adding 99.8% methyl alcohol (v v−1) according to the method described by Lichtenthaler (1987). Thus, it was dried in an oven at 105 °C for 24 h, and then methanolysis was performed in 1 ml of chloroform, 0.85 ml of methanol and 0.15 ml of sulfuric acid at 100 °C for 3.5 h (Brandl et al. 1988). The gas chromatograph (G-C2014, Shimadzu, Japan), equipped with a Restek Rtx-5 silica capillary column (30 m, 0.25 mm, 0.25 μm) and a flame ionization detector (FID), was used to analyze the methyl ester groups. The operation was performed at 250 °C for injector and detector. To separate the methyl esters, 60 °C programming was used for 2 min, with an increase rate of 25 °C min−1 until reaching 180 °C. For the construction of the calibration curve, the poly(3-hydroxybutyrate-co-hydroxyvalerate) was used as standard 88 mol% HB and 12 mol% HV (Sigma-Aldrich) (Brandl et al. 1988; Zhang et al. 2015). The yield (ɣ) of polyhydroxybutyrate (PHB) was determined using Eq. (4).
Statistical analysis
The experiments were performed in triplicate, and the averages of the results were subjected to analysis of variance (ANOVA) followed by the Tukey test with a 95% confidence level.
Results and discussion
Microalgal growth and kinetic parameters
All experiments showed absence of the latency phase (Fig. 1) since the inoculum was previously adapted to the modified Zarrouk medium containing 50% of the carbon source (NaHCO3, 8.4 g L−1). The adaptation of the inoculum contributed to eliminating the time of the latency phase (Schmidell et al. 2001). Therefore, the microalga culture showed the exponential phase at the beginning of cultivation.
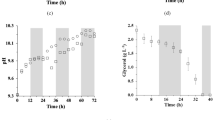
Growth curves of Spirulina sp. LEB 18 cultivated with 2.5 g L−1 of NaNO3 in a single stage (open circle), 0.25 g L−1 of NaNO3 in a single stage (open square), and two-stage with the first stage using 2.5 g L−1 of NaNO3 and the second stage using 0.25 g L−1 of NaNO3 on 5 days (filled circle), 10 days (filled square) or 15 days (filled triangle)
The control assays carried out with 10 and 100% of the original nitrogen source showed cell growth up to the 10th day and 18th day, respectively. In the experiment performed with 100% of NaNO3, the supply of nitrogen was not limited. Thus, this nutrient was used by the cell to produce compounds essential to growth, such as proteins, nucleic acids, and photosynthetic pigments. According to Castro et al. (2015), nitrate salts promote a higher concentration of biomass when compared to other sources of nitrogen, especially NaNO3, which is the reason for its application in the Zarrouk medium. Studies show that this nitrogen source can be reduced by approximately 25% in the culture media without reducing the microalgae cell concentration (Colla et al. 2007). Therefore, the absence of this nutrient prevents or reduces cell growth.
The two-stage cultivations showed cell multiplication until the moment of substitution of the initial culture medium for the nitrogen-limited medium. After this replacement, the cells went through an adaptation period, which varied from 1 to 2 days. This adaptation in growth may be related to the stress caused to the cells by the centrifugation performed to separate the initial culture medium, leaving the cells injured. Subsequently, the microalgae again presented a linear growth phase, for a period of 7 days for the experiment with nitrogen reduction on the 5th day, 5 days for the experiment with nitrogen reduction on the 10th day, and 5 days for the experiment with nitrogen reduction nitrogen at the 15th day, which was finished at 20 days of cultivation.
The two-stage assays and the control assay with 100% of nitrogen did not present a stationary phase, with cell decline immediately after the linear growth phase. The cell death phase of the assays with the start of the second stage at 5 and 10 days was on the 15th and 17th day of cultivation, respectively. The control assay with 10% of nitrogen showed this phase on the 11th day. Braga et al. (2019) cultivated Spirulina sp. LEB 18 in a nitrogen-limited medium (0.25 g L−1 of NaNO3), and they found the cell death phase on the 13th day of the culture. The control assay with 100% of nitrogen showed cellular a decline on the 19th day. The assay with the beginning of the second stage at 15 days did not show a cell death phase, as there was cell productivity until the 20th day of the culture.
The control assay with 10% of the nitrogen source obtained a shorter cell multiplication time, starting the decline phase on the 11th day of cultivation. However, the reduction of nitrogen, at times 5, 10, and 15 days of experiment, did not prevent cell growth. In these conditions, possibly the nitrogen supplied was used by the cell to produce the compounds necessary for growth, and stored for later use. According to Nayak et al. (2019), nitrogen is responsible for the synthesis of amino acids and proteins, contributing to the production of biomass.
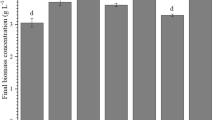
All two-stage cultivations showed a higher maximum cell concentration after nitrogen reduction. The supply of a new nitrogen-reducing culture medium has provided important nutrients, such as phosphorus and carbon that contribute to cell growth. The control experiment with 10% of nitrogen obtained the lowest maximum cell concentration when compared to the two-stage experiments. Therefore, it is possible to prove the advantage of the two-stage cultivation strategy to achieve greater biomass productivity.
The nitrogen reduction assay on the 15th day obtained the highest cell concentration when compared to the other assays (Table 1). This greater growth of Spirulina sp. LEB 18 is related to nitrogen being available throughout the exponential phase, ensuring better cell multiplication. According to Martín-Juárez et al. (2017), nitrogen is the second most abundant component of biomass.
The lowest cell concentration of the two-stage assays was obtained with the reduction of nitrogen in the 5th day of cultivation. Nevertheless, this value is higher when compared to the results obtained by Braga et al. (2019), who also cultivated Spirulina sp. LEB 18 with 10% of the nitrogen source (0.25 g L−1 of NaNO3). The authors reached a value of 0.56 g L−1 as the highest Xmax using CO2 injection every 1 min and 120 ppm of fly ashes. In this context, it is possible to affirm that the accomplishment of the two-stage cultivation contributes to microalgae growth, because in the first 5 days of cultivation the microalga had nitrogen for the production of essential macromolecules, and there was also growth in the following 7 days after the limitation of this nutrient.
The maximum specific growth rate was higher in the first 5 days of cultivation (exponential phase), both for the control and two-stage experiments. This behavior may have occurred due to the incidence of uniform luminosity to the microalga cells in this period of the culture since the cell concentration is lower. According to Caprio et al. (2016), microalgae grow using light, but the increase in cell concentration prevents light from penetrating the suspension, resulting in a reduction in cell multiplication. Consequently, there is a reduction in the maximum specific growth rate.
The increase in the maximum specific growth rate causes a reduction in generation time. According to Braga et al. (2019), microalgae can duplicate cell concentration on 1–7 days. The experiment conducted in a two-stage with nitrogen reduction on the 5th day conferred the highest maximum specific growth rate in the first stage of cultivation, which resulted in the shortest generation time.
All two-stage cultivations showed a decrease in productivity on 2–3 days after the start of the second stage, due to adaptation to the new nutritional conditions. After this adaptation, there was a sudden increase in productivity, probably due to the supply of nutrients (Fig. 2a). From the 15th day of cultivation, the assays with nitrogen limitation in the 5th and 10th day exhibited a reduction in productivity. The control experiment with 10% of nitrogen showed higher productivity in the first two days of cultivation, remaining constant until the 10th day. From the 11th day, there was a reduction in cell productivity reaching zero in the 16th day, possibly due to the scarcity of nitrogen, since this nutrient is essential for cell development (Fig. 2c). Thus, it can be inferred that the control with 100% nitrogen showed an increase in productivity on the first 3 days of the experiment, remaining constant throughout the cultivation, with a decrease only in the last 2 days.
Biomass productivity (a), pH (b) and nitrogen concentration (c) curves of Spirulina sp. LEB 18 cultivated with 2.5 g L−1 of NaNO3 in a single stage (open circle), 0.25 g L−1 of NaNO3 in a single stage (open square), and two-stage with the first stage using 2.5 g L−1 of NaNO3 and the second stage using 0.25 g L−1 of NaNO3 on 5 days (filled circle),10 days (filled square) or 15 days (filled traingle)
The highest maximum productivity was observed after replacing the medium in the 15th day. On the other hand, the lowest maximum productivity was observed in the assay with culture medium replacement on the 5th day. This fact occurred because the nitrogen reduction was carried out after the beginning of the cultivation. Thus, most of the exponential phase occurred without the presence of this nutrient. Therefore, it can be inferred that the closer to the beginning of the cell death phase is the replacement of the culture medium, the greater the maximum productivity. Since all nutrients, including nitrogen, were available for 15 days, the two-stage strategy provided a higher concentration of microalgal biomass.
Moraes et al. (2018) evaluated the effect of NaNO3 concentration on the growth kinetics of the microalgae Spirulina sp. LEB 18. The experiments were carried out in fed-batch, in a single stage. The authors obtained Xmax = 1.60 g L−1, Pmax = 0.109 g L−1 day−1 and μmax = 0.208 day−1 when using 1.25 g L−1 of NaNO3. Comparing these results with those obtained in two-stage assays, it is possible to note the advantage of applying this cultivation strategy for microalgal growth and biomass production. Two-stage cultivations of Spirulina sp. LEB 18 provided greater productivity in all experimental conditions when compared to the study developed by Moraes et al. (2018), even though the nitrogen reduction was greater in our experiments (0.25 g L−1 of NaNO3).
The pH of Spirulina sp. LEB 18 cultures varied according to the microalgae growth (Fig. 2b). The ideal pH for the cultivation of this microalga is between 8.3 and 11.0. In this range, there is greater availability of carbonate and bicarbonate, which provides optimal growth conditions (Costa et al. 2002). The fixation of CO2 during the respiration of microalgae causes greater dissociation of carbonate and bicarbonate, promoting the release of hydroxyl ions (OH−), which generates an increase in pH (LI et al. 2011). Martins et al. (2014) performed the cultivation of Spirulina sp. LEB 18 using sodium bicarbonate, glucose, and sodium acetate as a carbon source. The highest concentration of biomass found by these authors was obtained with sodium bicarbonate, due to the microalga having photosynthetic enzymes active in the assimilation of carbon.
Control and two-stage experiments showed an increase in pH according to the use of bicarbonate by microalgae for its development. However, the replacement of the culture medium led to a decrease in the cellular concentration of the microalga by 2 days. From this period, there was a decrease in pH, which confirms the adaptation phase of Spirulina sp. LEB 18 with the new culture medium. During the cultivation, the pH increased as the growth of microalga.
The distribution of chemical carbon species is determined by pH (Mangan et al. 2016). When the microalgal culture has a pH below 6, carbonic acid is present in greater quantity in the medium, whereas at pH 6 to 10.5, bicarbonate is predominant. In cultivation with a pH higher than 10.5, carbonate is found in greater concentration. Thus, the higher the pH of the medium, the greater is the possibility of the available CO2 being converted to carbonate (Hille et al. 2014). The pH of single and two-stage cultivations remained within the optimum pH range (9.6 to 10.9) for this microalga, which promoted cell growth.
Production of macromolecules
The two-stage cultivation of Spirulina sp. LEB 18 no present significant difference (p > 0.05) in the concentration of proteins, carbohydrates, and lipids at times 0 and 5 days when compared to the control experiment with 100% of nitrogen (Table 2), since the microalga was exposed to the same nutritional conditions.
Nitrogen makes up about 10% of the dry weight of microalgae, and it is a fundamental component used by cells for the synthesis of amino acids, proteins, nucleic acids, chlorophyll, and other accessory pigments, such as phycobilins. The concentration of nitrogen present in the culture medium is related to the protein content produced by the cell. In this way, the less there is nitrogen available in the culture medium, the lower the protein synthesis by the microalgae.
Nitrogen is capable of limiting microbiological growth when supplied in small concentration, both in natural and artificial environments. Microalgae assimilate the nutrients in the cultivation and incorporate them into its biomass, resulting in a nutritionally rich product that can be used as a food supplement. The composition of micronutrients and macronutrients affect the composition of macromolecules, such as lipids, proteins and carbohydrates (Hornes et al. 2010; Matos et al. 2015).
According to Hinai et al. (2019), Spirulina platensis has 50 to 75% protein in its composition. In this way, it can be said that all the results obtained for the control and two-stage experiments, at times 0 and 5 days of the cultivations, remained within the expected protein content range. However, due to nitrogen limitation, all two-stage cultivations showed a decrease in protein concentration immediately after the nutrient limitation. The two-stage cultivation with nitrogen reduction on the 5th day decreased the protein content from the 10th day of culture up to 21%. The assay with a nitrogen reduction on the 10th day decreased the protein content by up to ~ 20% from the 15th day. The experiment with a nitrogen reduction on the 15th day showed a decline in the protein concentration (~ 12%) on the 20th day.
In the second stage, the cultivation with nitrogen reduction in the 10th and 15th day did not show a significant difference in protein content. On the other hand, the experiment with nitrogen reduction on the 5th day had higher protein content when compared to the control with 10% of nitrogen. Therefore, for protein production, it is important that the first stage of cultivation is performed with 100% of the nitrogen source. The control cultivation with 10% of nitrogen showed the lowest concentration of proteins (20%) since there was not an essential element (nitrogen) available for protein synthesis. The assays with nitrogen reduction in the 10th and 15th day and the control with 100% nitrogen showed increasing protein concentration up to the 10th day. From this time of cultivation, if the nitrogen is not reduced, the protein content remains. However, if this nutrient is limited, the content of the macromolecule reduces.
The control experiment with 100% nitrogen presented the highest protein content at the end of the cultivation, as it received a nitrogen concentration from the Zarrouk medium without any limitation of this nutrient. In this experiment, there was no significant difference between the protein content synthesized in the 10th and 20th day of the culture. Therefore, the ideal would be to reduce the cultivation time to decrease costs. It also can be inferred that the first stage without nitrogen reduction stimulates the protein content. Thus, to improve protein production, the best cultivation time for microalgae harvest is on the 10th day, which found 73.8% of this macromolecule.
Microalgae need nitrogen for growth, however, when they do not receive this supply, cell multiplication reduces or ceases, and it begins to accumulate reserve compounds. Thus, when the microalgae are subjected to stress conditions, as nitrogen limitation for long periods, it can produce higher concentrations of lipids (Sun et al. 2018). This statement was observed in the experiment with nitrogen reduction on the 5th day (Table 2), because during the 15 days of the second stage the culture medium was limited by nitrogen, requiring a reserve of energy that would be consumed long-term.
The concentration of lipids obtained in the assay with nitrogen limitation on the 5th day was higher than that found in the assays with nitrogen reduction on the 10th and 15th day. The time difference in which the cultivations were maintained after nitrogen limitation can justify these results. The cultivation that underwent nitrogen reduction on the 5th day remained for longer with the scarcity of this nutrient (Fig. 2c). The experiments with nitrogen reduction on the 10th and 15th days remained with the limited nitrogen source for 10 and 5 days, respectively, which is a shorter time interval when compared to the nitrogen reduction test on the 5th day.
Nayak et al. (2019) evaluated the two-stage as a cultivation strategy to maximize the productivity of lipids in Chlorella sp. HS2. Urea was used as a nitrogen source by the authors, being deficient in the second stage of cultivation. The authors reached a higher lipid content of 36.7%, a result close to that found in our experiment with Spirulina sp. LEB 18 subjected to nitrogen reduction on the 5th day of cultivation (37.0%). Moreover, the second stage of cultivation showed the highest concentration of lipids, which is the best condition to produce this macromolecule. There was no significant difference between the concentration of lipids on the 15th (34.6%) and 20th day (37.0%) of cultivation. Thus, the best cultivation time to decrease costs and produce a maximum concentration of lipids is 15 days. In this condition, the N-NO3 was reduced to zero from the 12th day of cultivation (Fig. 2c), totaling three days without remaining nitrogen until the period of maximum lipid concentration.
The experiment with nitrogen reduction on the 15th day showed lipid concentration in the first stage of cultivation (0, 5, 10, and 15 days) without significant difference from the values obtained for the control experiment with 100% of nitrogen. The lipid contents found in these experiments were around 15%. This fact occurred because microalgae were subjected to the same nutrient conditions. However, the lipid content in this two-stage experiment was 5.5% higher in the second stage compared to the control cultivation with 100% of nitrogen due to the nutrient limitation on the 15th day.
Caprio et al. (2016) described a metabolic change observed in the cells when the metabolizable carbohydrates available in the culture medium decrease as they are used by the cells. Therefore, carbohydrates are stored intracellularly and then converted to lipids. This statement can be seen in the control experiment with 10% of nitrogen that exhibited the highest carbohydrate content, and the lowest concentration of lipids at the end of the cultivation. Moreover, the cultivation with nitrogen reduction on the 5th day produced the highest concentration of lipids and the lowest concentration of carbohydrates at the end of cultivation.
When the cell needs a reserve of energy to be used in the short term, it follows the metabolic route of carbohydrate production. In this process, every carbohydrate produced is quickly consumed by the cell as an energy source (Sun et al. 2018). On the 10th day of cultivation, the microalga was in the linear phase, a period in which cell growth occurs. Therefore, the reduction of nitrogen at this time made the microalgae need an energy source for consumption to keep the cell multiplication. As a result, there was an improvement in carbohydrate content (up to 10%) compared to experiments with nitrogen reduction on the 5th and 15th days (Table 2).
According to Becker (2007), Spirulina platensis has from 8 to 14% of carbohydrates in its composition, which corroborates with the results found in time 0 and 5 days of the cultures in single and two-stage. However, studies claim that this microalga can accumulate higher levels of carbohydrates due to the limitation of nutrients, such as nitrogen. Salla et al. (2016) cultivated the microalgae Spirulina platensis in diluted Zarrouk medium (20%) with the addition of residues containing whey protein. The authors reached a carbohydrate content of 58.0%. Braga et al. (2019) found that the reduction of nitrogen (10% NaNO3) together with the injection of CO2 and the addition of fly ashes to the cultivation of Spirulina sp. LEB 18 provided macromolecule accumulation in up to 63.3%. The results achieved by these authors were similar to that found at the end of the control experiment with 10% of nitrogen (58.1%).
The experiment with nitrogen reduction on the 15th day showed no significant difference in the concentration of carbohydrates in the first stage of cultivation when compared to the control with 100% of nitrogen. This fact occurred due to the microalga being submitted to the same nutrient conditions in both. However, due to the nutrient limitation on the 15th day, the carbohydrate content was higher in the second stage when compared to the control with 100% of nitrogen.
The cultivation with nitrogen reduction on the 5th day did not show a significant difference in carbohydrates at the end of the cultivation concerning the control with 100% of nitrogen, because in the two-stage experiment the microalga presented a metabolic route deviation for the production of lipids. In all assays, the carbohydrate content was higher in the 20th day of cultivation, reaching a maximum of 58.1%. Moreover, this statement implies that it is possible to increase the carbohydrates concentration if the cultivation is maintained for a longer time. Thus, from this study, it can be affirmed that to obtain a higher carbohydrate content by Spirulina sp. LEB 18, the best condition is cultivation with 10% of the nitrogen source for 20 days. Furthermore, it can be seen that in this experiment condition, the N-NO3 was reduced to zero from the 6th day of cultivation (Fig. 2c). Thus, it totaled 14 days without remainder nitrogen until the 20th day, when the maximum concentration of carbohydrates was obtained.
Nitrogen-dependent macromolecules, such as proteins, were synthesized faster (10 days) than lipids and carbohydrates, which take longer to be produced (15 and 20 days). This statement can be justified considering that carbohydrates and lipids are reserve compounds accumulated at the end of the cultivation when there is stress due to a shortage of nutrients. Although the experiment with nitrogen reduction on the 15th day has reached greater biomass production, there was no PHB synthesis. In this experimental condition, the cells multiplied throughout the cultivation period, indicating that all nutrients necessary for microalgal growth were available in the culture medium. On the other hand, the experiment with nitrogen reduction on the 5th day produced PHB, probably because it remained in cultivation for a long time after the nutrient limitation. The cultivation with nitrogen limitation on the 5th day of the cultivation showed cell death in 4 days before the period found in the control experiment with 100% of nitrogen. This statement corroborates with the production of PHB since the scarcity of nitrogen promotes a drop in the concentration of biomass with consequent accumulation of PHB as a carbon and energy reserve.
From the chromatograms obtained for PHB quantification, it was possible to observe the occurrence of the 3-hydroxybutyrate (3HB) polymer and 3-hydroxyvalerate (3HV) copolymer, since these monomers have a retention time equal to that found 3.9 and 5.1, respectively. The accumulation of PHB (29.6%) was reached on the 20th day of the two-stage cultivation of the microalga Spirulina sp. LEB 18 with nitrogen reduction on the 5th day, which can be confirmed by the high lipid concentration found at this time of the experiment. The production of this macromolecule occurred due to the microalgae having been in a shortage of nitrogen for 15 days. In this way, it produced lipids as an energy reserve for long-term use. Besides, it can be seen that in the condition of maximum PHB production, the N-NO3 was reduced to zero from the 12th day of cultivation, totaling 8 days without remaining nitrogen until the period of maximum concentration of this macromolecule.
According to Schmidell et al. (2001), the levels of free coenzyme A (CoA) are high when all the nutrients necessary for microbial growth are available. This fact occurs due to the high demand for acetyl groups for the production of carbon skeletons and the generation of energy by the Krebs cycle. When there is a limitation of some nutrients, the demand for acetyl decreases, thus the levels of free CoA are reduced, also decreasing the inhibition on β-ketothiolase, which triggers PHB synthesis.
The limitation of nitrogen in the cultivations corresponds with the accumulation of PHB in the cells because as the nitrogen concentration reaches minimum values, the production of PHB increases. PHB synthesis process by the microalgae Spirulina sp. LEB 18 cultivated in two-stage occurs in two moments, in which the first consists of the presence of nutrients that are a sufficient quantity for cell multiplication, and in the second, due to the exhaustion of the essential nutrient (nitrogen), the microalgae accumulate this biodegradable polymer as an energy reserve. Moreover, the low concentration of nitrogen changes the color of the cells, since this nutrient is part of the pigment composition, and there is probably a reduction in the chlorophyll content. In this way, there is a change in the metabolic route for the accumulation of lipid compounds, such as PHB, and consequently, the cultivation takes on a slightly yellowish color.
Martins et al. (2017) evaluated the potential of microalgae for the production of biopolymers. The authors observed that Spirulina sp. LEB 18 was grown for 15 days in the Zarrouk medium and reached 20.6% of biopolymers. Costa et al. (2018a, b) also evaluated the nitrogen limitation in the cultivation of Spirulina sp. LEB 18 for the production of polymer. The authors obtained an accumulation of 12% PHA on the 15th day of the cultivation, limiting 70% of nitrogen from the Zarrouk medium. Therefore, it is possible to state that two-stage cultivation contributes to the greater production of polymers by microalga. Furthermore, this cultivation strategy allows first to reach a higher cellular concentration, and then to accumulate the bioproduct of interest.
Two-stage cultures with nitrogen reduction on the 10th and 15th day did not show a peak in the chromatogram in the first stage. The characteristic peak of PHB was exhibited only after nitrogen limitation. However, in these cultivations, the production of PHB was below 1%. The control assay with 10% nitrogen showed, at culture times 0, 5, 10 and 15 days, PHB content less than 1%, and did not show a peak of the polymer on the 20th day. The control assay with 100% of nitrogen did not synthesize PHB until the 15th day of the experiment, obtaining 0.03% of the polymer only on the 20th day of cultivation. This production may have been influenced by the lack of nitrogen present at the end of the cultivation. Similar results were found for microalga Spirulina platensis by Campbell et al. (1982), who obtained 0.06% of PHB in cultivation with CO2 injection of 5%, and by Jau et al. (2005) who reached 0.03% of PHB in mixotrophic conditions with nitrogen exhaustion in the culture medium, 9 g L−1 of NaHCO3 and 5% of CO2.
Conclusions
In two-stage cultivation of Spirulina sp. LEB 18, it was found that the first stage of cultivation is the best condition to obtain high productivity of biomass (0.19 g L−1 day−1) and protein content (73.8%). This productivity remained high and constant until the 10th day, which corresponds to the maximum protein concentration. Thus, the 10th day is ideal for the beginning of the second stage with nitrogen limitation. The maximum production of lipids (34.6%), carbohydrates (58.1%), and PHB (29.6%) occurred in the second stage after the nitrogen has been y consumed at 3, 14, and 8 days, respectively. Moreover, in a microalgal biorefinery system, the two-stage cultivation condition with the second stage on day 10 makes it possible to extract proteins, lipids, PHB, and carbohydrates on days 5, 15, 20, and 20 with a yield of approximately 60, 35, 30 and 15%, respectively.
References
Ansari S, Fatma T (2016) Cyanobacterial polyhydroxybutyrate (PHB): screening, optimization and characterization. PLoS ONE 11:1–20. https://doi.org/10.1371/journal.pone.0158168
Bailey JE, Ollis DF (1986) Biochemical engineering fundamentals, 2nd edn. McGraw-Hill, Singapore
Becker EW (2007) Micro-algae as a source of protein. Biotechnol Adv 25:207–210. https://doi.org/10.1016/j.biotechadv.2006.11.002
Benemann JR, Woertz I, Lundquist T (2018) Autotrophic microalgae biomass production: from Niche markets to commodities. Ind Biotechnol 14(1):3–10. https://doi.org/10.1089/ind.2018.29118.jrb
Braga VS, Moreira JB, Costa JAV, Morais MG (2019) Enhancement of the carbohydrate content in Spirulina by applying CO2, thermoelectric fly ashes and reduced nitrogen supply. Int J Biol Macromol 123:1241–1247. https://doi.org/10.1016/j.ijbiomac.2018.12.037
Brandl H, Gross RA, Lenz RW, Fuller RC (1988) Pseudomonas oleovorans as a source of poly(β-hydroxyalkanoates) for potential applications as biodegradable polyesters. Appl Environ Microbiol 54:1977–1982. https://doi.org/10.1128/AEM.54.8.1977-1982.1988
Campbell J, Stevens E Jr, Balkwill DL (1982) Accumultion of poli-β-hydroxybutyrate in Spirulina platensis. J Bacteriol 149:361–363. https://doi.org/10.1128/JB.149.1.361-363.1982
Caprio F, Visca A, Altimari P, Toro L, Masciocchi B, Iaquaniello G, Pagnanelli F (2016) Two stage process of microalgae cultivation for starch and carotenoid production. Chem Eng Trans 49:415–420. https://doi.org/10.3303/CET1649070
Castro GFPS, Rizzo RF, Passos TS, Santos BNC, Dias DS, Domingues JR, Araújo KGL (2015) Biomass production by Arthrospira platensis under different culture conditions. Food Sci Technol 35:18–24. https://doi.org/10.1590/1678-457X.6421
Cataldo DA, Haroon M, Schrader LE, Young VL (1975) Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun Soil Sci Plant Anal 6:71–80. https://doi.org/10.1080/00103627509366547
Chew K, Yap JY, Mostrar PL, Suan NH, Juan JC, Ling TC, Lee D, Chang J (2017) Microalgae biorefinery: high value products perspectives. Bioresour Technol 229:53–62. https://doi.org/10.1016/j.biortech.2017.01.006
Chew KW, Khoo KS, Foo HT, Chia SR, Walvekar R, Lim SS (2021) Algae utilization and its role in the development of green cities. Chemosphere 268:129322. https://doi.org/10.1016/j.chemosphere.2020.129322
Colla LM, Reinehr CO, Reichert C, Costa JAV (2007) Production of biomass and nutraceutical compounds by Spirulina platensis under different temperature and nitrogen regimes. Bioresour Technol 98:1489–1493. https://doi.org/10.1016/j.biortech.2005.09.030
Costa JAV, Colla LM, Duarte Filho P, Kabke K, Weber A (2002) Modelling of Spirulina platensis growth in fresh water using response surface methodology. World J Microbiol Biotechnol 18:603–607. https://doi.org/10.1023/a:1016822717583
Costa JAV, Moraes L, Moreira JB, Rosa GM, Henrard ASA, Morais MG (2017) Microalgae-based biorefineries as a promising approach to biofuel production. In: Tripathi B, Kumar D (eds) Prospects and challenges in algal biotechnology. Springer, Singapore
Costa AS, Miranda AL, Andrade BB, Assis DJ, Souza CO, Morais MG, Costa JAV, Druzian JI (2018a) Influence of nitrogen on growth, biomass composition, production, and properties of polyhydroxyalkanoates (PHAs) by microalgae. Int J Biol Macromol 116:552–562. https://doi.org/10.1016/j.ijbiomac.2018.05.064
Costa JAV, Moreira JB, Lucas BF, Braga VS, Cassuriaga APA, Morais MG (2018b) Recent advances and future perspectives of PHB production by cyanobacteria. Ind Biotechnol 14(5):249–256. https://doi.org/10.1089/ind.2018.0017
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356. https://doi.org/10.1021/ac60111a017
Hille RV, Fagan M, Bromfield L, Pott R (2014) A modified pH drift assay for inorganic carbon accumulation and external carbonic anhydrase activity in microalgae. J Appl Phycol 26:377–385. https://doi.org/10.1007/s10811-013-0076-6
Hinai MA, Kalbani AA, Rubkhi BA, Kalbani UA, Walke S (2019) Protein extraction from Spirulina platensis. Int J Innov Technol Explor Eng 8:1524–1530. https://doi.org/10.35940/ijitee.L3110.1081219
Hornes M, Silva AG, Mitterer ML, Queiroz MI (2010) Influence of nitrogen compounds on the protein concentration of the cyanobacterium Aphanothece microscopica Nägeli. Ciênc Tecnol Aliment 30:364–371. https://doi.org/10.1590/S0101-20612010000200012
Jau MH, Yew SP, Toh PSY, Chong ASC, Chu WL, Phang SM, Najimudin N, Sudesh K (2005) Biosynthesis and mobilization of poly(3-hydroxybutyrate) [P(3HB)] by Spirulina platensis. Int J Biol Macromol 36:144–151. https://doi.org/10.1016/j.ijbiomac.2005.05.002
Lafarga T, Sánchez-Zurano A, Villaró S, Morillas-España A, Acién G (2021) Industrial production of spirulina as a protein source for bioactive peptide generation. Trends Food Sci Technol 116:176–185. https://doi.org/10.1016/j.tifs.2021.07.018
Li Y, Chen YF, Chen P, Min M, Zhou W, Martinez B, Zhu J, Ruan R (2011) Characterization of a microalga Chlorella sp. well adapted to highly concentrated municipal wastewater for nutrient removal and biodiesel production. Bioresour Technol 102:5138–5144. https://doi.org/10.1016/j.biortech.2011.01.091
Li J, Liu Y, Cheng JJ, Mos M, Daroch M (2015) Biological potential of microalgae in China for biorefinery-based production of biofuels and high value compounds. New Biotechnol 32(6):588–596. https://doi.org/10.1016/j.nbt.2015.02.001
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382. https://doi.org/10.1016/0076-6879(87)48036-1
Lowry O, Rosenbrough N, Farr A, Randall R (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–272
Mangan NM, Flamholz A, Hood RD, Milo R, Savage DF (2016) pH determines the energetic efficiency of the cyanobacterial CO2 concentrating mechanism. Proc Natl Acad Sci USA 113:5354–5362. https://doi.org/10.1073/pnas.1525145113
Marsh JB, Weinstein DB (1966) Simple charring method for determination of lipids. J Lipid Res 7:574–576. https://doi.org/10.1016/S0022-2275(20)39274-9
Martín-Juárez J, Markou G, Muylaert K, Lorenzo-Hernando A, Bolado S (2017) Breakthroughs in bioalcohol production from microalgae: solving the hurdles. Microalgae-Based Biofuels Bioprod 8:183–207. https://doi.org/10.1016/B978-0-08-101023-5.00008-X
Martins RG, Gonçalves IS, Costa JAV, Morais MG (2014) Bioprocess engineering aspects of biopolymer production by the cyanobacterium Spirulina strain LEB 18. Int J Polym Sci 2014:1–6. https://doi.org/10.1155/2014/895237
Martins RG, Gonçalves IS, Morais MG, Costa JAV (2017) New technologies from the bioworld: selection of biopolymer-producing microalgae. Polym J 27:285–289. https://doi.org/10.1590/0104-1428.2375
Matos AP, Morioka LRI, Sant’anna ES, França KB (2015) Protein and lipid contents from Chlorella sp. cultivated in residual concentrated desalination. Cienc Rural 45:364–370. https://doi.org/10.1590/0103-8478cr20121104
Monshupanee T, Nimdach P, Incharoensakdi A (2016) Two-stage (photoautotrophy and heterotrophy) cultivation enables efficient production of bioplastic poly-3-hydroxybutyrate in auto-sedimenting cyanobacterium. Sci Rep 6:1–9. https://doi.org/10.1038/srep37121
Moraes L, Rosa GM, Souza MRAZ, Costa JAV (2018) Carbon dioxide biofixation and production of Spirulina sp. LEB 18 biomass with different concentrations of NaNO3 and NaCl. Braz Arch Biol Technol 61:1–10. https://doi.org/10.1590/1678-4324-2018150711
Morais MG, Reichert CC, Dalcanton F, Durante AJ, Marins LF, Costa JAV (2008) Isolation and characterization of a new Arthrospira strain. Z Naturforsch 63:144–150. https://doi.org/10.1515/znc-2008-1-226
Moreira JB, Terra ALM, Costa JAV, Morais MG (2016) Utilization of CO2 in semi-continuous cultivation of Spirulina sp. and Chlorella fusca and evaluation of biomass composition. Braz J Chem Eng 33(3):691–698. https://doi.org/10.1590/0104-6632.20160333s20150135
Murata MM, Ito Morioka LR, Da Silva Marques JB, Bosso A, Suguimoto HH (2021) What do patents tell us about microalgae in agriculture? AMB Express 11(1):1–12. https://doi.org/10.1186/s13568-021-01315-4
Nayak M, Suh WI, Chang YK, Lee B (2019) Exploration of two-stage cultivation strategies using nitrogen starvation to maximize the lipid productivity in Chlorella sp. HS2. Bioresour Technol 276:110–118. https://doi.org/10.1016/j.biortech.2018.12.111
Rosa GM, Moraes L, Souza MRAZ, Costa JAV (2016) Spirulina cultivation with a CO2 absorbent: influence on growth parameters and macromolecule production. Bioresour Technol 200:528–534. https://doi.org/10.1016/j.biortech.2015.10.025
Salla ACV, Margarites AC, Seibel FI, Holz LC, Brião VB, Bertolin TE, Colla ML, Costa JAV (2016) Increase in the carbohydrate content of the microalgae Spirulina in culture by nutrient starvation and the addition of residues of whey protein concentrate. Bioresour Technol 209:133–141. https://doi.org/10.1016/j.biortech.2016.02.069
Schmidell W, Lima AU, Aquarone E, Borzani W (2001) Biotecnologia industrial. Edgard Blücher LTDA, São Paulo
Show PL (2022) Global market and economic analysis of microalgae technology: status and perspectives. Bioresour Technol 357:127329. https://doi.org/10.1016/j.biortech.2022.127329
Sun XM, Ren LJ, Zhao QY, Ji XJ, Huang H (2018) Microalgae for the production of lipid and carotenoids: a review with focus on stress regulation and adaptation. Biotechnol Biofuels 11:1–16. https://doi.org/10.1186/s13068-018-1275-9
Zarrouk C (1966) Contribution à l’étude d’une cyanophycée. Influence de diveurs facteurs physiques et chimiques sur la croissance et photosynthese de Spirulina maxima Geitler. Thesis, University of Paris, Paris
Zhang YZ, Liu GM, Weng WQ, Ding JY, Liu SJ (2015) Engineering of Ralstonia eutropha for the production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) from glucose. J Biotechnol 195:82–88. https://doi.org/10.1016/j.jbiotec.2014.12.014
Acknowledgements
This research was developed within the scope of the Capes-PrInt Program (Process # 88887.310848/2018-00). This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001. The authors also acknowledge CGTEE (Company of Thermal Generation of Electric Power) and MCTIC (Ministry of Science Technology, Innovations and Communications).
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001.
Author information
Authors and Affiliations
Contributions
MGM and JAVC provided the Laboratory of Microbiology and Biochemistry, and the Laboratory of Biochemical Engineering, respectively, to perform this project. MGM and JBM designed this research. JAVC and MGM contributed with reagents and equipment. RCK conducted the experiments and analyzes. RCK, MGM and JBM analyzed the data. RCK and JBM wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kosinski, R.d., Moreira, J.B., Costa, J.A.V. et al. Two-stage cultivation of Spirulina sp. LEB 18: a strategy to increase biomass productivity and synthesis of macromolecules. Braz. J. Chem. Eng. 40, 673–683 (2023). https://doi.org/10.1007/s43153-022-00279-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43153-022-00279-7