Abstract
Light (quantity and quality) is the main growth-limiting factor of photoautotrophic microalgae. The integration of selective permeable photovoltaic filters above microalgae cultivation systems has been proposed previously to improve both production efficiencies and economics. In order to optimize such system, we evaluated the growth and photosynthesis of two spectrally acclimated strains of Nannochloropsis sp. (MUR 266 and MUR 267) grown semi-continuously under different light spectra in this study. No significant differences in biomass productivity were observed between cultures acclimated under full blue (BL, 400–525 nm) and narrow blue (LEDB, 430–490 nm) light when compared to the positive control of white light (WL, 400–700 nm), while lower values were recorded under red (RL, 600–700 nm) and pink light (PL, 400–525, 600–700 nm) for both species. When compared to WL, the photosynthetic performance (Fq′/Fm′, αETR, ETRmax) of both species was higher under both BL and LEDB except for the Fq′/Fm′ of MUR 267 under LEDB. Chlorophyll a content was highest in cultures acclimated to RL while values tended higher under LEDB, RL and PL for MUR 267. Total lipid yield of both MUR 266 and MUR 267 was higher under BL and PL than WL. Based on the results of this study, theoretical modelling of the proposed photovoltaic-microalgae system indicate approximately 150–210 W m−2 of electricity could be potentially generated if only blue wavelengths (BL and LEDB) are selectively filtered from sunlight while converting the remaining unused spectrum of sunlight into electricity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Among many growth parameters, the availability of light (quality and quantity) is by far the main limiting factor affecting the growth and productivity of any nutrient replete microalgae culture (Mandalam and Palsson 1998; Simionato et al. 2011). Incoming sunlight is composed of different wavelength bands such as ultraviolet (10–400 nm), visible light (400–700 nm) and infrared (700–1000 nm). However, only the photosynthetic active radiation (PAR region, 400–700 nm), constituting 43.8% of the entire solar energy, is absorbed and utilized by autotrophs (Haxo and Blinks 1950; Moheimani and Parlevliet 2013).
Although, in theory, all incident photons of PAR can be used for photosynthesis, not all these photons are equally absorbed and utilized by microalgae. Photosynthetic pigments directly govern the ability of algal cells in absorbing and utilizing specific photons (Kirk 1994). Therefore, algal light absorption and conversion efficiency is species specific and can significantly vary due to inherent properties such as the pigment profile, cellular architecture and chloroplast arrangement (Moheimani and Parlevliet 2013).
As illustrated by Keeling (2013), based on the evolutionary history of microalgal pigments, blue (≈420–470 nm) and red (≈660) light are seen to be the most preferred spectral choice for the growth of microalgae when compared to other spectra such as green and yellow. In addition, remaining underutilized wavelengths such as infrared (IR) and ultraviolet (UV) can be detrimental to algal cells, either by increasing culture temperatures (Xue et al. 2005) or by causing irreversible damage to photosynthetic proteins (Zeebe et al. 1996)
Therefore, in order to significantly improve the viability and efficiency of commercial-scale microalgae production facilities, substantial research efforts are still required for growth optimization. Moheimani and Parlevliet (2013) proposed the integration of spectrally selective photovoltaic (PV) filters above mass microalgae cultivation units to improve both biomass yield and solar conversion efficiencies. Through such a system, wavelengths of sunlight most efficient for the growth and photosynthesis of the selected microalgae would be transmitted to the culture through the photovoltaic-filter apparatus while the remaining unused wavelengths, including IR and UV, would be captured and converted to electricity (Vadiveloo et al. 2016a).
The biomass productivity and photosynthesis of spectrally acclimated Nannochloropsis sp. (MUR 266) was found to be enhanced under blue (400–525 nm) and pink light spectra (400–525 nm and 600–700 nm), respectively (Vadiveloo et al. 2015, 2016b). However, these studies were performed and standardized based on the concept of filtering incoming sunlight as required by the PV-microalgae system where there were changes in both radiant flux (light irradiance) and spectral quality. Variations in spectral quality and irradiance were seen to affect the results of our previous studies. Therefore, in order to only clarify the effect of spectral quality as well as optimizing the proposed PV-microalgae system (Moheimani and Parlevliet 2013), there is a need to study the effect of various light spectra without changing the irradiance. The current study was carried out to determine the effects of different light spectra including narrow wavebands of blue light with the same amount of radiant flux on the growth, photosynthesis and biomass productivity of two strains of spectrally acclimated Nannochloropsis sp. (MUR 266 and 267). The objective of this study was to identify the most productive light spectra for both of these strains of Nannochloropsis sp. for future application in the PV-microalgae system.
The microalgal genus of Nannochloropsis is widely sought after due to its ability in synthesizing large amounts of lipid bodies and also for its high biomass productivity, making it an ideal choice for the production of food, feed, nutraceuticals and also biofuel (Gouveia and Oliveira 2009). However, significant variation in pigment expression has been reported between different species/strains of Nannochloropsis resulting in changes of optical properties and wavelength-specific absorption of cells (Millie et al. 2002; Tamburic et al. 2014). Thus, two different strains of Nannochloropsis with dissimilar pigment concentration were used in this study to identify if physiological differences between strains may cause discrepancy in light spectra absorption resulting in changes in growth and productivity.
Materials and methods
Microalgae strain and culture condition
The marine eustigmatophyceae, Nannochloropsis MUR 266 (3.5 ± 0.5 μm in diameter), was isolated from Rottenest Island, Western Australia (32.0062o S, 115.5123o E) while MUR 267 (3.5 ± 0.4 μm in diameter) was isolated from the Swan-Canning Estuary, Western Australia, (32.0001o S, −115.8300o E). Both strains were obtained from the culture collection of the Algae R&D Centre, Murdoch University. Both strains were grown in 500 mL Erlenmeyer flask with a working volume of 250 mL and a start cell density of 1.5 × 107 cells mL−1. The inoculum cultures were maintained at 12:12 light/dark cycle and at a controlled temperature of 25 ± 1 °C by the culture room air conditioner unit. Nannochloropis sp. MUR 267 grown under white light (100 μmol photons m−2 s−1) was found to have significantly higher chlorophyll a and accessory pigment content when compared to Nannochloropsis sp. MUR 266 (Vadiveloo et al. 2016b). Both strains of Nannochloropsis sp. were cultivated in charcoal-filtered (50 μm) and autoclaved natural seawater (Hillary’s Beach, Perth, Western Australia) at 33 g L−1 NaCl salinity. The seawater was enriched using F/2-Si medium nutrients as formulated by Guillard (1975).
Cultivation setup
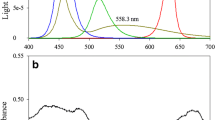
Illumination with the different light spectra was achieved by positioning culture flasks inside customized light boxes with their front panels either covered by coloured acetate filters or subjected to the direct radiation of LED lamps (Vadiveloo et al. 2015). To prevent any light reflection, internal walls of each light box were dark (black). The selected filters (LEE Filters) were LEE 026 Bright Red (RL), Lee 363 Medium Blue (BL) and LEE 128 Bright Pink (PL), and these filters were illuminated by a Verbatim PAR 38 Cool White LED lamp (Verbatim LED Lighting). The narrow band of blue light (LEDB) was supplied using a Plusrite LED Floodlight (AU01-FL50 W/G/RGB, Plusrite Australia Pty Ltd). To provide experimental control for the treatments, both strains of Nannochloropsis were also grown under the full PAR spectrum of white light illuminated by the Verbatim PAR 38 Cool White LED lamp (positive control).
Sufficient open space was provided on top of each light box for ventilation (temperature control), sample extraction and media renewal. Cultures were grown at 25 ± 1 °C controlled by the temperature unit of the culture room. The temperature inside of the light boxes was regularly measured and no difference was found between the temperature inside the light boxes and the culture room. The mixing of cultures was ensured by using an 80-mm magnetic stirrer with a mixing speed of 150 rpm. The light/dark cycle was standardized as 12 h/12 h. Three independent replicates were carried out for each treatment and the standard error of mean was calculated.
The incident photon-flux density of PAR on the surface centre point of each culture flask was standardized to 100 ± 10 μmol photons m−2 s−1 using a Li-185B quantum meter equipped with a PAR quantum sensor, Li-190SB (LI-COR, USA) to ensure all cultures received the same input (radiant flux) of light energy at its surface level. The spectral distribution of each light condition was measured using a BLACK-Comet CXR-SR-50 Spectrometer (StellarNet, USA) and is illustrated in Fig. 1.
Growth measurement
All cultures were maintained in semicontinuous growth mode and acclimated to each light spectrum for at least 22 days prior to any measurements (2 weeks batch and 2 weeks semicontinuous). Cell concentration was measured daily using a haemocytometer and was used to calculate the specific growth rates of cultures in each condition. During the semicontinuous growth, 50% of cultures (125 mL) were harvested and renewed with fresh medium every time the cultures reached 90% of maximum cell density (≈ 6.5 ± 0.54 × 107 cells mL−1). Harvested samples were immediately used for biomass and photosynthetic measurements. The specific growth rates (μ) and biomass productivity using the ash-free dry weight procedure (i.e. gram of organic weight (AFDW) L−1 day−1) under each light spectrum were calculated according to methods previously described by Moheimani et al. (2013).
Chlorophyll and total lipid determination
Chlorophyll a concentration was determined according to the methods of Jeffrey and Humphrey while total lipid yield during each harvest was measured according to the protocol by Bligh and Dyer as detailed in Moheimani et al. (2013).
Saturation pulse based chlorophyll a fluorescence measurements
Chlorophyll fluorescence measurements were performed using a WATER-PAM fluorometer (Walz GmbH, Germany), consisting of a PAM-CONTROL unit and a WATER-ED (emitter/detector) unit. The WATER-ED unit consisted of LED’s which provided the non- actinic measuring light (spectral peak at 650 nm), actinic light/saturation pulse (spectra peak at 660 nm) and far-red light (spectra peak at 730 nm). The maximum quantum yield in light (Fq′/Fm′) of harvested samples was evaluated and calculated using the saturation light method (≈3500 μmol photons m−2 s−1) as described in Cosgrove and Borowitzka (2006). The rapid light curves (RLCs) were performed with a 10-s actinic exposure duration to each of eight pre-set incremental irradiances (43, 651 96, 147, 218, 333, 499 and 709 μmol photons m−2 s−1) followed by a saturation pulse that lasted for 0.8 s. Samples harvested from each light condition were rapidly transferred to the ED unit, followed by immediate measurements. A fresh sample was used for each replicate measurement (minimum of 3).
Absolute electron transport rates (ETR) were calculated using the quantum yield values of PSII (ΦPSII) derived from the RLCs and the numbers of absorbed photon per chlorophyll a per second according to the equation ETR = ΦPSII × Q phar × 0.5 (Wagner et al. 2006) where Q phar is the amount of photosynthetic usable radiation (PUR) absorbed by the microalgae while 0.5 represents an assumption that radiant energy is divided equally between PSI and PSII (Gilbert et al. 2000). Q phar was calculated as described in Wagner et al. (2006) using the incident spectra of each light treatment and the chlorophyll a-specific in vivo absorption coefficients of corresponding samples measured using a StellarNet Inc. IC2 integrating sphere attached to the StellarNet Spectrometer, Model BLACK-Comet CXR-SR-50. These ETR values obtained were then plotted against calculated Q phar values to represent photosynthesis-irradiance curve. The plotted curves were subsequently modelled and fitted with a best fit curve through the waiting-in-line model function as suggested in Ritchie (2008). The model assisted in deriving fitting parameters such as ETRmax and αETR which were then used to estimate the photosynthetic efficiency of samples. Changes in the curve-based parameters (between treatments) were considered significant if the 95% confidence intervals were not overlapping (Wagner et al. 2006).
Statistical analysis
All experiments were carried out with a minimum of three replicates. The results are expressed as arithmetic means ± standard error (SE). Significant differences in specific growth rates, biomass productivity (organic dry weight), photosynthetic parameters, lipid and chlorophyll a content were compared using one-way repeated measure (RM) analysis of variance (ANOVA) followed by the post hoc test of Holm-Sidak. Significance was based on P < 0.05. All statistical analysis was performed using SigmaPlot version 12.5 for Windows.
Results
Specific growth rate and biomass productivity.
As illustrated in Fig. 2, no significant differences were observed in the specific growth rate (μ) of both MUR 266 and MUR 267 when grown semi-continuously and acclimatized under the different light spectra. Although growth rates are important in evaluating the progress of a culture, the most relevant parameter used to assess the potential of any commercial-scale cultivation system is the biomass productivity. No differences in biomass productivity were observed between cultures grown under full blue light (BL), narrow blue light (LEDB) and white light (WL) for both Nannochloropsis, MUR 266 and 267 (Fig. 2). However, lower values were recorded for both strains acclimatized under pink (PL) and red light (RL) when compared to the positive control of WL (Fig. 2).
Specific growth rate (μ) and biomass productivity (g AFDW L−1 day−1) values for both species of Nannochloropsis grown semicontinuously and acclimated under the different light spectra (n = 5 ± standard error). The same letter above each column indicates no significant differences (one-way repeated measures ANOVA P > 0.05)
Chlorophyll a and total lipid yield
Chlorophyll a content of MUR 266 during each harvest was found to be higher under RL than the other treatments while values tended to be higher for cultures acclimated under PL, RL and LEDB for MUR 267 with no significant differences observed among them (Fig. 3). Total lipid yield (based on similar cell density during each harvest) was higher in cultures grown under BL and PL for MUR 266 and MUR 267 respectively when compared to the positive control of WL (Fig. 3).
Maximum quantum yield in light
Figure 4 illustrates the maximum quantum yield in light (Fq′/Fm′) of both MUR 266 and MUR 267 when acclimatized under the different light spectra. Fq′/Fm′ values were found to be higher under BL and LEDB than the other treatments for MUR 266 while values tended to be higher under WL, BL and RL for MUR 267 with no significant differences noted among them.
Maximum quantum yield in light (Fq′/Fm′) and the curve fitting parameters (α and ETRmax) derived from the chlorophyll fluorescence P–I curves of both MUR 266 and MUR 267 grown and acclimated under the different light spectra (n = 5 ± standard error). The same letter above each column indicates no significant differences (one way repeated measures ANOVA P > 0.05)
Fluorescence-based ETR curves
The initial slope (α) recorded from the plotted ETR against Q phar curves for both MUR 266 and MUR 267 tended to be higher under BL, LEDB and RL when compared to the control of WL and also PL (Fig. 4). The photosynthetic capacity (ETRmax) of cultures was found to be highest under LEDB followed by BL for both MUR 266 and MUR 267 (Fig. 4).
Discussion
Biomass productivity
Although many recent studies have looked into the response of various microalgae to different light spectra, almost none of these studies have been performed on cultures acclimated to the incident spectral conditions or on cultures maintained in continuous growth mode in order to eliminate the effects of constantly changing parameters such as cell density, growth rate, light penetration and also nutrient content (Das et al. 2011; Teo et al. 2014; Ra et al. 2016). As highlighted earlier, the main aim of this applied study was to determine the growth response of two strains of Nannochloropsis acclimated and grown semi-continuously under different light spectra when supplied irradiance was kept constant. The objective of this study was to identify the most efficient light quality which is able to enhance biomass productivity, biochemical synthesis and photosynthetic performance of both the selected Nannochloropsis sp. in comparison to the full spectrum of white light for further application in the proposed PV-microalgae integrated system. This study does not aim to compare the outcome of both strains of Nannochloropsis in respect to each other. It is also important to note here that although the white light treatment (WL) used in this study was found to have visible lower emission peaks in the blue-green region (490 nm, Fig. 1), it still compromised of the entire PAR region (400–700 nm) and was only used as a proxy to incident sunlight.
The higher biomass productivity of Nannochloropsis recorded under BL and LEDB for both MUR 266 and 267 when compared to RL and PL were in accordance with many previous studies (Das et al. 2011; Teo et al. 2014; Vadiveloo et al. 2015). In any photoautrophic culture of microalgae, variation in biomass productivity between cultures under different illumination conditions is directly governed by the efficient capture of light energy and its correlation with carbon fixation (Atta et al. 2013). The increase in biomass productivity recorded under both treatments of blue light (BL and LEDB) in this study correlates well with the ability of Nannochloropsis in absorbing wavelengths of blue light more efficiently than other wavelengths of light such as red (Tamburic et al. 2014). The added ability of blue light in stimulating gene transcription while also enhancing the regulation of various activated enzymes and the synthesis of DNA and RNA in microalgae cells might have also attributed to the increase in biomass productivity (Ruyters 1980; Wallen and Geen 1971).
Chlorophyll a and total lipid yield
Acclimation to different light spectra has been well observed to bring forward changes in the photosynthetic apparatus of microalgae such as the distribution and concentration of chlorophyll a and other photosynthetic accessory pigments (Sánchez-Saavedra and Voltolina 1996, 2002, Vesk and Jeffrey 1977). The higher chlorophyll a content recorded under RL for Nannochloropsis MUR 266 and under RL, LEDB and PL for Nannochloropsis MUR 267 is believed to be a response of chromatic photoacclimation in which the concentration of major light-harvesting pigments of Nannochloropsis such as chlorophyll a and accessory pigments such as violaxanthin and vaucheriaxanthin with complementary absorption patterns to that of the incident light spectra is regulated (Lubián et al. 2000; Mouget et al. 2004,). Although chromatic photoacclimation is well reported in cyanobacteria, this distinct phenomenon has also observed to occur in other microalgae groups such as Chlorophyta and to a lower extent in some diatoms in which the response of these groups are seen to vary and are taxon-specific (Wallen and Geen 1971; Humphrey 1983; Mouget et al. 2004).
The in vivo absorption peaks of Nannochloropsis pigments (chlorophyll a, violaxanthin and vaucheriaxanthin) are found to be mostly at the blue (440 nm for chlorophyll a and 490 nm for violaxanthin) and red (630 and 675 nm for chlorophyll a) portions of PAR (Gitelson et al. 2000; Owens et al. 1987). Further, it is also important to note that changes in pigment concentration is species are strain-specific and can vary due to chromatic photoacclimation, explaining some of the discrepancy in the outcome observed between in Nannochloropsis MUR 266 and MUR 267 tested in our studies (Falkowski 1980; Prezelin and Matlick 1980).
The enzymes carbonic anhydrase and ribulose biophosphate carboxylase/oxygenase (Rubisco) are vital for the regulation of carbon dioxide in microalgae (Teo et al. 2014). These requisite enzymes which are also responsible for the production of triglycerides have been observed to be under the direct control of blue light (Roscher and Zetsche 1986). Thus, an increase in these enzyme activities stimulated by the illumination of monochromatic blue light correlates well with the higher total lipid yield of MUR 266 (Roscher and Zetsche 1986).
The difference in lipid yield between both strains of Nannochloropsis (MUR 266 and MUR267) can be possibly related to variation in morphological and physiological conditions (Wahidin et al. 2013). Various factors such as light spectra, irradiance level and the nature of microalgal photochemical machinery affect the photosynthetic response and the accumulation of organic matter in various microalgae (Sánchez-Saavedra et al. 2016). These variables govern the biomass production and overall biochemical concentration of algal cells (Sánchez-Saavedra et al. 2016; Muller-Feuga et al. 2003).
Photosynthesis
The changes in the maximum quantum yield of PSII in light-adapted samples (Fq′/Fm′) is a valuable indicator of the instant physiological condition of a particular organism and represents the effectiveness of the open PSII reaction centres in capturing excitation energy (Genty et al. 1989). The higher Fq′/Fm′ values recorded under both BL and LEDB when compared to the other treatments is believed to be an outcome of multiple factors. Previously, Das et al. (2011) reported that photons of shorter wavelengths such as blue light have greater possibility in striking the light-harvesting complex (LHC) of microalgae such as Nannochloropsis resulting in higher photosynthetic efficiency. Building up on this, Tamburic et al. (2014) successfully measured the action spectrum of Nannochloropsis sp., illustrating the photosynthetic response of the microalga to different wavelengths of light. They concluded that shorter wavelengths of blue light (414 nm) were absorbed more efficiently and transferred more effectively to PS II when compared to red light (679 nm) at light intensities below the saturation threshold, resulting in higher photosynthetic rates under monochromic blue light (Tamburic et al. 2014). In addition, the acclimation of various microalgae such as diatoms to monochromatic blue light has also been reported to increase the photoprotective potential of cells (Costa et al. 2013; Brunet et al. 2014). This outcome is deduced by the ability of blue light in enhancing the capacity of non-photochemical quenching in algal cells while also bringing forward an increase in the pool size of xanthophyll cycle pigments when compared to red and white light of the same irradiance (Costa et al. 2013; Brunet et al. 2014).
Rapid light curves represent a non-destructive evaluation of the light response of PSII activity by providing important information regarding the saturation characteristics of electron transport and the overall photosynthetic performance including the photoacclimation ability of an organism (Ralph and Gademann 2005; Serôdio et al. 2006). The higher initial slope (α) recorded from the ETR curve of MUR 267 under LEDB, BL and RL for both species of Nannochloropsis is due to by an increase in the efficiency of both light absorption and energy conversion under these treatments (Henley 1993). On the other hand, the remarkable in-crease in photosynthetic capacity (ETRmax) under blue wavelengths (BL and LEDB) can be associated with the overall content and activity of the Calvin cycle enzyme, Rubisco. An increase in Rubisco activity coupled together with the stimulation of the photosynthetic electron transfer chain reactions by blue wavelengths have been previously credited for an increase in the photosynthetic capacity of microalgae (Voskresenskaya 1972).
Significance of this study
Although no statistical differences were observed in the biomass productivity between WL, BL and LEDB, all photosynthetic parameters measured (except for the Fq′/Fm′ of MUR 267) inclined to be higher under BL and LEDB when compared to WL for both species of Nannochloropsis. In addition, this study also demonstrated visible changes in the biochemical composition of Nannochloropsis such as the chlorophyll a content and total lipid yield when acclimated under different light spectra. Blue light (BL) was found to significantly enhance the lipid yield of Nannochloropsis MUR 266 On the other hand, no differences were observed in lipid yield of Nannochloropsis MUR 267 when grown using PL, RL, LEDB and BL. In terms of chlorophyll a content, RL was found to be the most appropriate wavelength for Nannochloropsis MUR 266 while LEDB, RL and PL were seen to bring forward the same outcome for MUR 267.
Overall, except for the chlorophyll a content, it can be safely summarized that both blue light treatments (BL and LEDB) irrespective of their wavelength band were as efficient as the multichromatic WL and certainly more efficient than the other spectra such as RL and PL in enhancing the growth, lipid yield and photosynthesis parameters of both strains of Nannochloropis sp. when radiant flux is kept constant.
Building on this outcome, we successfully modelled and calculated the amount of electricity that could be potentially produced through the proposed PV-microalgae system according to the blackbox filter model as proposed by Parlevliet and Moheimani (2014). Considering no spectrally selective PV cell is currently available for the purpose of calculating the potential electricity co-generation, LEE filters were used as proxy black box filters for system evaluation. This theoretical model used was customized to determine the maximum amount of electricity that could be generated if only blue wavelengths (BL and LEDB) of incoming sunlight were filtered and channelled to the microalgae culture while converting the remaining of the solar spectrum into electricity with minimal energy loss. These modelling figures were calculated using the spectral photoresponse and light conversion ability of highly efficient crystalline silicon solar cells when they are provided with a narrow spectrum of incident light.
In this case, they were modelled to be receiving the light that was not transmitted to the microalgae by both the BL and LEDB treatments. On average, a total of 431 W m−2 of solar energy in the PAR region is directly available to microalgae cultures in the locality of Western Australia if no PV/filter is placed above the algae pond (Parlevliet and Moheimani 2014). However, in an outdoor scenario, if incoming sunlight was to be filtered and only selective parts of the light spectrum such as BL of and LEDB used in this study were to be supplied to the microalgae culture using the proposed PV/filter device, a maximum of 151 Wm−2 (for BL) and 210 Wm−2 (for LEDB) of electrical energy could be potentially generated by the modelled PV system from the remaining unused portions of sunlight (i.e. green, red and yellow spectrum of sunlight).
The amount of electricity generated by the PV apparatus is more than sufficient for the operating mechanism of the microalgae cultivation system (e.g. pumps and paddlewheels) while also providing a viable option for powering extra artificial lighting (i.e. blue or/and red LEDs) if required by the microalgae. This study also demonstrates that by narrowing the incident spectra of light supplied to the microalgae, an increase in the amount of electricity co-generated from the modelled system is achieved without any compromise to the growth and yield of the microalgae culture.
Although the exact system for dividing the incident light spectrum for both purposes is not fully determined yet, several existing options can be potentially optimized to match the spectral distribution of BL and LEDB used in this study while channelling the remaining unused wavelengths into electricity. Among them include the use of specifically tailored semitransparent thin film PV, luminescent solar concentrators which allow selective incoming photons with insufficient excitation energy to pass through while capturing and converting portions of the remaining photons with sufficient excitation energy into electricity and, most interestingly, the use of spectrally selective glass panels composed of insulated glazing units that can transmit arbitrary wavelengths of visible light while capturing and converting portions of IR and UV into electricity (Debije and Verbunt 2012; Moheimani and Parlevliet 2013; Rosenberg et al. 2014). In addition, the recent development of high-performance PV cells which can successfully perform spectrum splitting on a small scale using dichroic mirrors and optics is of great value (McCambridge et al. 2011). A similar, albeit expensive system, could be built to match the spectral distribution of blue wavelengths used in this study or approach the ideal system through selective thin fill deposition (McCambridge et al. 2011).
Moreover, the results of this study would be of great use in aquaculture facilities where the nutritional content (i.e. lipid) of microalgae such as Nannochloropsis could be enhanced using specific light spectra (i.e. blue light) for the feed of rotifers and fish/prawn larvae. Although lipids are important elements required as part of the diet of microalgae, the fatty acid composition and concentration among many others actually determines the nutritional value of microalgae in the aquaculture industry. Therefore, further in depth studies looking into the effects of different light spectra on the fatty acid composition of spectrally acclimated Nannochloropsis would be of great value and interest. As observed with this study, variation in light spectra can bring forward significant physiological changes affecting growth, biochemical composition and the nutritional value of algal cells (Sánchez-Saavedra et al. 2016).
Conclusion
This study evaluated the growth, biomass productivity; biochemical composition and photosynthetic performance of two strains of Nannochloropsis sp. (MUR 266 and MUR 267) when grown under semi-continuous mode and acclimated under different light spectra. No differences were observed in the biomass productivity of strains of Nannochloropsis sp. when grown under blue light irrespective of the wavelength band (BL and LEDB) and white light. In addition, blue light was also found to be most efficient for the photosynthetic performance of both strains of Nannochloropsis sp. when compared to the other light spectra. Lipid yield and chlorophyll a content was found to be enhanced under blue light and red light respectively for MUR 266 while no significant differences were observed under pink, red and blue light for MUR 267 for both parameters. By only providing blue light to the microalgae culture, between 151 and 210 W m−2 of electrical energy could be potentially generated from the remaining unused wavelengths of light. By narrowing the spectrum of light received by the microalgae (i.e. LEDB), a larger proportional of incident sunlight can be diverted for other purposes such as electricity production without negatively affecting the growth, biomass productivity and photosynthetic performance of the cultures of Nannochloropsis sp.
References
Atta M, Idris A, Bukhari A, Wahidin S (2013) Intensity of blue LED light: a potential stimulus for biomass and lipid content in fresh water microalgae Chlorella vulgaris. Bioresour Technol 148:373–378
Brunet C, Chandrasekaran R, Barra L, Giovagnetti V, Corato F, Ruban AV (2014) Spectral radiation dependent photoprotective mechanism in the diatom Pseudo-nitzschia multistriata. PLoS One 9(1):e87015
Cosgrove J, Borowitzka MA (2006) Applying pulse amplitude modulation (PAM) fluorometry to microalgae suspensions: stirring potentially impacts fluorescence. Photosynth Res 88:343–350
Costa BS, Jungandreas A, Jakob T, Weisheit W, Mittag M, Wilhelm C (2013) Blue light is essential for high light acclimation and photoprotection in the diatom Phaeodactylum tricornutum. J Exp Bot 64:483–493
Das P, Lei W, Aziz SS, Obbard JP (2011) Enhanced algae growth in both phototrophic and mixotrophic culture under blue light. Bioresour Technol 102:3883–3887
Debije MG, Verbunt PP (2012) Thirty years of luminescent solar concentrator research: solar energy for the built environment. Adv Energy Mater 2:12–35
Falkowski PG (1980) Light-shade adaptation in marine phytoplankton. In: Falkowski PG (ed) Primary productivity in the sea. Springer, NY, pp 99–119
Genty B, Briantais J-M, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta Gen Subjects 990:87–92
Gilbert M, Domin A, Becker A, Wilhelm C (2000) Estimation of primary productivity by chlorophyll a in vivo fluorescence in freshwater phytoplankton. Photosynthetica 38:111–126
Gitelson AA, Grits YA, Etzion D, Ning Z, Richmond A (2000) Optical properties of Nannochloropsis sp and their application to remote estimation of cell mass. Biotechnol Bioeng 69:516–525
Gouveia L, Oliveira AC (2009) Microalgae as a raw material for biofuels production. J Ind Microbiol Biotechnol 36:269–274
Guillard RRL (1975) Culture of phytoplankton for feeding marine invertebrates. In: Smith WL, Chanley HH (eds) Culture of marine invertebrate animals. Plenum Press, New York, pp 29–60
Haxo F, Blinks L (1950) Photosynthetic action spectra of marine algae. J Gen Physiol 33:389–422
Henley WJ (1993) Measurement and interpretation of photosynthetic light-response curves in algae in the context of photoinhibition and diel changes. J Phycol 29:729–739
Humphrey G (1983) The effect of the spectral composition of light on the growth, pigments, and photosynthetic rate of unicellular marine algae. J Exp Mar Biol Ecol 66:49–67
Keeling PJ (2013) The number, speed, and impact of plastid endosymbioses in eukaryotic evolution. Annu Rev Plant Biol 64:583–607
Kirk JTO (1994) Light and photosynthesis in aquatic ecosystems, 2nd edn. Cambridge University Press, Cambridge
Lubián LM, Montero O, Moreno-Garrido I, Huertas IE, Sobrino C, González-del Valle M, Parés G (2000) Nannochloropsis (Eustigmatophyceae) as source of commercially valuable pigments. J Appl Phycol 12:249–255
Mandalam RK, Palsson BØ (1998) Elemental balancing of biomass and medium composition enhances growth capacity in high-density Chlorella vulgaris cultures. Biotechnol Bioeng 59:605–611
McCambridge JD, Steiner MA, Unger BL, Emery KA, Christensen EL, Wanlass MW, Gray AL, Takacs L, Buelow R, McCollum TA (2011) Compact spectrum splitting photovoltaic module with high efficiency. Prog Photovolt Res Appl 19:352–360
Millie D, Schofield O, Kirkpatrick G, Johnsen G, Evens T (2002) Using absorbance and fluorescence spectra to discriminate microalgae. Eur J Phycol 37:313–322
Moheimani NR, Parlevliet D (2013) Sustainable solar energy conversion to chemical and electrical energy. Renew Sust Energ Rev 27:494–504
Moheimani N, Borowitzka M, Isdepsky A, Fon Sing S (2013) Standard methods for measuring growth of algae and their composition. In: Borowitzka MA, Moheimani NR (eds) Algae for biofuels and energy. Springer, Dordrecht, pp 265–284
Mouget JL, Rosa P, Tremblin G (2004) Acclimation of Haslea ostrearia to light of different spectral qualities—confirmation of ‘chromatic adaptation’ in diatoms. J Photochem Photobiol B 75:1–11
Muller-Feuga A, Moal J, Kass S (2003) The microalgae of aquaculture. In: Støttrup JG, McEvoy LA (eds) Live feeds in marine aquaculture. Blackwell, Oxford, pp 206–252
Owens TG, Gallagher JC, Alberte RS (1987) Photosynthetic light-harvesting function of violaxanthin in Nannochloropsis spp. (Eustigmatophyceae). J Phycol 23:79–85
Parlevliet D, Moheimani NR (2014) Efficient conversion of solar energy to biomass and electricity. Aquat Biosyst 10:1–9
Prezelin B, Matlick H (1980) Time-course of photoadaptation in the photosynthesis-irradiance relationship of a dinoflagellate exhibiting photosynthetic periodicity. Mar Biol 58:85–96
Ra C-H, Kang C-H, Jung J-H, Jeong G-T, Kim S-K (2016) Effects of light-emitting diodes (LEDs) on the accumulation of lipid content using a two-phase culture process with three microalgae. Bioresour Technol 212:254–261
Ralph PJ, Gademann R (2005) Rapid light curves: a powerful tool to assess photosynthetic activity. Aquat Bot 82:222–237
Ritchie R (2008) Fitting light saturation curves measured using modulated fluorometry. Photosynth Res 96:201–215
Roscher E, Zetsche K (1986) The effects of light quality and intensity on the synthesis of ribulose-1, 5-bisphosphate carboxylase and its mRNAs in the green alga Chlorogonium elongatum. Planta 167:582–586
Rosenberg V, Vasiliev M, Alameh K (2014) Spectrally selective panel. US Patent US20140182679 A1
Ruyters G (1980) Blue light-enhanced phosphoenolpyruvate carboxylase activity in a chlorophyll-free Chlorella mutant. Z Pflanzenphysiol 100:107–112
Sánchez-Saavedra MP, Voltolina D (1996) The effect of different photon fluence rates of blue-green light on the biomass quality of a coastal diatom in pilot scale semicontinuous cultures. Sci Mar 60:267–272
Sánchez-Saavedra MP, Voltolina D (2002) Effect of photon fluence rates of white and blue-green light on growth efficiency and pigment content of three diatom species in batch cultures. Cienc Mar 28:273–279
Sánchez-Saavedra MP, Maeda-Martínez AN, Acosta-Galindo S (2016) Effect of different light spectra on the growth and biochemical composition of Tisochrysis lutea. J Appl Phycol 28:839–847
Serôdio J, Vieira S, Cruz S, Coelho H (2006) Rapid light-response curves of chlorophyll fluorescence in microalgae: relationship to steady-state light curves and non-photochemical quenching in benthic diatom-dominated assemblages. Photosynth Res 90:29–43
Simionato D, Sforza E, Carpinelli EC, Bertucco A, Giacometti GM, Morosinotto T (2011) Acclimation of Nannochloropsis gaditana to different illumination regimes: effects on lipids accumulation. Bioresour Technol 102:6026–6032
Tamburic B, Szabó M, Tran N-AT, Larkum AW, Suggett DJ, Ralph PJ (2014) Action spectra of oxygen production and chlorophyll fluorescence in the green microalga Nannochloropsis oculata. Bioresour Technol 169:320–327
Teo CL, Atta M, Bukhari A, Taisir M, Yusuf AM, Idris A (2014) Enhancing growth and lipid production of marine microalgae for biodiesel production via the use of different LED wavelengths. Bioresour Technol 162:38–44
Vadiveloo A, Moheimani NR, Cosgrove JJ, Bahri PA, Parlevliet D (2015) Effect of different light spectra on the growth and productivity of acclimated Nannochloropsis sp.(Eustigmatophyceae). Algal Res 8:121–127
Vadiveloo A, Moheimani N, Alghamedi R, Cosgrove J, Alameh K, Parlevliet D (2016a) Sustainable cultivation of microalgae by an insulated glazed glass plate photobioreactor. Biotechnol J 11:363–374
Vadiveloo A, Moheimani NR, Kosterink NR, Cosgrove JJ, Parlevliet D, Gonzalez-Garcia C, Lubián LM (2016b) Photosynthetic performance of two Nannochloropsis spp. under different filtered light spectra. Algal Res 19:168–177
Vesk M, Jeffrey S (1977) Effect of blue-green light on photosynthetic pigments and chloroplast structure in unicellular marine algae from six classes. J Phycol 13:280–288
Voskresenskaya NP (1972) Blue light and carbon metabolism. Annu Rev Plant Physiol 23:219–234
Wagner H, Jakob T, Wilhelm C (2006) Balancing the energy flow from captured light to biomass under fluctuating light conditions. New Phytol 169:95–108
Wahidin S, Idris A, Shaleh SRM (2013) The influence of light intensity and photoperiod on the growth and lipid content of microalgae Nannochloropsis sp. Bioresour Technol 129:7–11
Wallen D, Geen G (1971) Light quality and concentration of proteins, RNA, DNA and photosynthetic pigments in two species of marine plankton algae. Mar Biol 10:44–51
Xue L, Zhang Y, Zhang T, An L, Wang X (2005) Effects of enhanced ultraviolet-B radiation on algae and cyanobacteria. Crit Rev Microbiol 31:79–89
Zeebe RE, Eicken H, Robinson DH, Wolf-Gladrow D, Dieckmann GS (1996) Modeling the heating and melting of sea ice through light absorption by microalgae. J Geophys Res Oceans 101:1163–1181
Acknowledgements
The authors would like to thank the Algae R&D Center and the School of Engineering & Information Technology, Murdoch University, for technical and financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vadiveloo, A., Moheimani, N.R., Cosgrove, J.J. et al. Effects of different light spectra on the growth, productivity and photosynthesis of two acclimated strains of Nannochloropsis sp.. J Appl Phycol 29, 1765–1774 (2017). https://doi.org/10.1007/s10811-017-1083-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-017-1083-9