Abstract
The biomass and carotenoid productivities of a freshwater microalga, Ettlia sp. YC001 (Chlorophyta, Chlamydomonadales), were investigated in continuous culture systems irradiated with various LEDs. Green light was effectively used by Ettlia sp. for its biomass growth compared to blue and red LED light. This effective use of green light was assumed to be beneficial for Ettlia sp., especially in competition with other microalgae. Thus, in a competition between Ettlia sp. and Chlorella vulgaris, Ettlia sp. out-competed C. vulgaris without losing any overall biomass productivity when green and white LEDs were used simultaneously. However, since Ettlia sp. also showed relatively low oxygen production under green light, further studies are needed on its photosynthesis and biomass production mechanisms. Notwithstanding, LEDs are potentially useful for identifying strain-specific photosynthetic characteristics of microalgae, thereby increasing their biomass productivity and controlling contamination from other microalgae in open culture systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microalgae are regarded as ecofriendly and renewable feedstocks for biodiesel production as they grow fast and commonly have high lipid content (Chisti 2007). Despite the proven potential of microalgae, their economic viability as a biofuel feedstock still largely depends on their growth and productivity (Griffiths and Harrison 2009; Scott et al. 2010; Ritchie and Larkum 2012). The growth of microalgae is a holistic response to various abiotic and biotic environmental factors. Thus, disentangling the response of microalgae to environmental factors is of critical importance to optimize their biomass productivity. It has already been reported that abiotic environmental factors, such as light, nutrient composition, carbon dioxide, pH, and temperature, affect the growth of microalgae (Atta et al. 2013; Bartley et al. 2014; Yu et al. 2018). Among these, light is one of the critical factors as the main energy source for the photoautotrophic cultivation. Accordingly, many studies have investigated the improvement of cell growth using various light sources, intensities, and penetrations (Huang et al. 2010; Atta et al. 2013; Yan et al. 2013; Szwaja et al. 2016). Ritchie and Larkum (2012) showed that photoinhibition at high irradiances was a major factor in limiting primary productivity in ponds. They developed a model for estimating photosynthesis in shallow ponds and to establish the optimum production for a given depth rather than achieving the maximum production.
The effects of light on microalgal growth essentially can be interpreted based on the main components of light, the wavelength, and intensity. Microalgae, as well as most photosynthetic microorganisms, have adapted to sunlight, which is a continuum of various wavelengths. However, it is also evident that, even at the strain level, microalgae have divergently evolved to use specific wavelengths and intensities of sunlight available in their specified niche (Rocap et al. 2003). Thus, investigating how microalgae respond to light in the context of its wavelength and intensity can provide a more detailed understanding of the responses and consequently growth optimization of microalgae.
A light emitting diode (LED) is a suitable light source for investigating the responses of microalgae to a specified wavelength and intensity. An LED provides 10–30 nm of wavelength bands in various spectral regions, including red, green, blue, yellow, and purple. It has already been reported that the use of specific narrow wavelengths from an LED promotes microalgae growth and provides several advantages. For example, microalgae can achieve maximum growth with a specific narrow wavelength within the visible color spectrum (Brown et al. 1995). Saha et al. (2013) increased the biomass productivity of Haematococcus pluvialis when various LEDs were used as a stress-regulatory factor.
Ettlia sp., isolated from a fresh water pond in Korea, has been reported as a promising algal strain with a high biomass productivity and high lipid and carotenoid content. Yoo et al. (2013) reported that Ettlia sp. can achieve high lipid (40% of dry cell weight) and carotenoid contents under 5–10% CO2 concentration. Seo et al. (2017) obtained high biomass productivity (1.48 g L−1 day−1) and lipid productivity (291.4 mg L−1 day−1) for Ettlia sp. through optimization of continuous culture condition and showed that the lipid productivity of Ettlia sp. was much higher compared to other microalgae such as Nannochloropsis salina or Chlorella pyrenoidosa. Thus, the optimization of Ettlia sp. growth is of critical importance for its full-scale application as a biofuel feedstock and carotenoid resource.
Accordingly, since Ettlia sp. can be expected to exhibit a strain-specific response to certain light wavelengths and intensities, this study investigated the responses of Ettlia sp. to various colored LEDs in a chemostat culture system. The characteristics of the responses can then provide strategies for Ettlia sp. growth optimization and its maintenance as the dominant population in field-scale cultivation, for example, race-way ponds.
Materials and methods
Microalgal strain and inoculum preparation
Ettlia sp. was obtained from the Korean Collection for Type Cultures (KCTC 12109BP) at the Korea Research Institute of Bioscience and Biotechnology (KRIBB). The Ettlia sp. was cultivated in a 4-L photobioreactor (PBR) and used as the inoculum. The working volume of the photobioreactor was set at 3 L and continuously fed with a fresh BG-11 medium (Stanier et al. 1971). The dilution rate of the photobioreactor was set at 0.125 day−1. The photobioreactor was also continuously illuminated using a three-wavelength fluorescent lamp with peaks at 436, 546, and 611 nm (Cool daylight, Dulux L, 36 W, OSRAM, Germany) of 240 μmol photons m−2 s−l and the temperature maintained at 23 ± 1 °C using a water bath (JSRC-13C, JS Research Inc., Korea). Chlorella vulgaris (KCTC AG10006), a domestic isolate, was also obtained from the KCTC.
Cultivation of Ettlia sp. in continuous mode under various LEDs
The Ettlia sp. was cultivated in continuous culture mode (Supplementary Fig. 1) to minimize the influence of other factors such as nutrient deficiency, except light. Twelve continuous culture systems were established. The volume of each PBR was 1 L and the working volume was set at 700 mL. The dilution rate of each PBR was set at 0.25 day−1. BG-11 medium was continuously supplied to each reactor through a 16-channel peristaltic pump (200 series multi-channel cassette pump, Watson-Marlow, UK). The agitation speed was maintained at 300 rpm using magnetic stirrers (Color Squid, IKA, Japan). Air was sparged into each PBR through a sterilized air filter. The aeration rate was controlled at 0.1 vvm using an air pump (DK 9000, Dae-Kwang Inc., Korea) and flow meter (Series RM, Dwyer Instruments, USA). The cultivation temperature was maintained at 23 ± 1 °C in the constant temperature room.
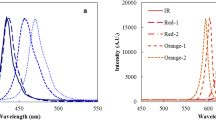
Panels of LEDs (Photron, Korea) including white, blue, green, red, and white + green were used as the light sources and installed in parallel on the right side of the PBRs. The peak wavelength of each LED panel was 457 (558 as a minor peak), 457, 516, and 634 nm for the white, blue, green, and red light, respectively (Fig. 1a). Phosphor-converted white LEDs were used resulting in a single, compact, white light source having a broad spectral power distribution. External cooling-fans (NMB-fan motor, Minebea Co., Japan) were installed on the opposite side of the LED panels to circulate the air and thereby minimizing the temperature gradient established by the LED panels. The light intensity was measured at a distance of 30 and 50 cm in front of each LED panel using an optical spectrum analyzer (70417, KRISS, Korea) at the Korea Research Institute of Standards and Science (KRISS). As all the light intensities asymptotically approached zero at a distance of 80 cm in front of each LED panel, the PBRs were located at distances of 20, 40, and 60 cm from each LED panel to establish various light intensity conditions. The light intensities of each LED panel at distances of 20, 40, and 60 cm are shown in Supplementary Fig. 2. The light-dark (L/D) cycle was set at 16:8 h. Also, 1 mL of the Ettlia sp. culture was used to measure the absorption spectrum using a UV/Vis spectrophotometer (wavelength range of 400–700 nm; UV-2450, Shimadzu, Japan; Fig. 1b).
Measurement of biomass, chlorophyll-a, and total carotenoid
The biomass and total carotenoid contents of each PBR were measured every 2 days for 20 days, once all the PBR cultures had entered a steady state. Five milliliters of the culture was sampled at 9 a.m. (an end-time of dark cycle) from each PBR using a syringe and filtered using a GF/C glass-fiber filter (1.2-μm pore size, 25-mm diameter, Whatman, USA). Each filter was dried overnight at 105 °C to measure the dry cell weight (DCW). The DCW of each sample was measured in triplicate.
One milliliter of the sample from each PBR was then used for the chlorophyll-a and total carotenoid measurement. The samples were filtered using GF/C glass-fiber filters, each filter transferred to a 15-mL conical tube, and then 10 mL of methanol immediately added to the conical tube. Thereafter, the solution with methanol and the filter was sonicated for 30 s at 750 W using an ultra-sonicator (Vibra cell, Sonic & Materials Inc., USA) for 2 min to extract the chlorophyll-a and total carotenoids. Each sonicated sample was centrifuged for 5 min at 2700 rcf, and the supernatant then was filtrated using a 0.45-μm syringe filter (Whatman, USA). The absorbance of the supernatant was measured at 650 and 665 nm for chlorophyll-a measurement and 470, 653, and 666 nm for total carotenoid measurement using a UV/Vis spectrophotometer (UV-2450, Shimadzu, Japan) in triplicate. Finally, the chlorophyll-a and total carotenoid concentration of each sample were calculated using the following equations (Hipkins and Baker 1986; Wellburn 1994):
where Ai indicates the absorbance of the sample at the wavelength of i nm.
Photosynthetic characteristics of Ettlia sp.
The photosynthetic oxygen production profiles of Ettlia sp. were measured. One milliliter of the sample of Ettlia sp. cultured in different light quality conditions was measured under different light quality and intensity conditions for 1 min using a Clark-type O2 electrode (Oxygraph Plus, Hansatech, UK). Custom-made circular LED panels were used to investigate the photosynthetic characteristics, and each LED panel had LED of four different light qualities (white, green, blue, and red LEDs). The light intensity of the LED panels ranged from 0 to 380 μmol photons m−2 s−1. All the measurements were conducted in triplicate.
Competition between Ettlia sp. and C. vulgaris
Ettlia sp. and C. vulgaris were co-cultivated to assess their relative growth capability under the irradiation of various LEDs. Chlorella vulgaris was selected to compete with Ettlia sp. since it is one of the prevailing and dominant strains in Korean domestic environments and has also been reported to show a high cell growth rate in various culture conditions (Liang et al. 2009).
Chlorella vulgaris was cultivated under the same culture conditions of Ettlia sp. to obtain the specific and corresponding growth rates, which were then used to determine the initial mixing ratio of Ettlia sp. and C. vulgaris. The distance of the PBRs from the LED panels was also determined for co-cultivation. White, green, and white + green (1:1) LEDs were used as the light sources with an L/D cycle of 16:8 h.
The cell number ratio of Ettlia sp. and C. vulgaris in the co-cultivation PBRs was monitored using a microscope (Eclipse 80i, Nikon, Japan) and fluorescence-activated cell sorter (FACSAria II cytometer, Becton-Dickinson, USA). The sorting gates were established for each microalgal strain to minimize the overlap in the dot plot spanned by the forward scatter (FSC) and CP-cy5 (695 nm) fluorescence. The cell event numbers for each gate were recorded for 60 s, and the cell counting conducted every 2 days during the 8-day experimental period.
Statistical analysis
The influence of the light quality on the biomass and carotenoid productivity was estimated using a one-way ANOVA and Pearson correlation analysis (Excel 2013, Microsoft, USA). A significant difference was considered at a p value < 0.01.
Results and discussion
Cell growth of Ettlia sp. under various LEDs
Ettlia sp. showed significantly different cell densities and biomass productivities under the various wavelengths and intensities of the LEDs (Fig. 2, Supplementary Fig. 3). The biomass productivity of each PBR asymptotically increased to a steady-state value, termed as BPasy. In Fig. 2a, the biomass productivity at day 20, termed as BPd20, is presented as bar, while the BPasy is denoted as black circle. At high and intermediate light intensity (20 and 40 cm, respectively), white LED has the highest BPd20 showing a statistically significant difference (p < 0.01) compared to other wavelengths. At low light intensity (at a distance of 60 cm), blue LED showed a higher biomass production compared to others (p < 0.01, Fig. 2a). Moreover, significant correlation was observed between BPd20 and BPasy (p < 0.001, r = 0.962) by the simple correlation analysis (SPSS ver. 12.0). Based on the BPasy, Ettlia sp. showed maximum biomass productivities of 111.6 mg L−1 day−1 at 40 cm, 66.9 mg L−1 day−1 at 40 cm, 77.6 mg L−1 day−1 at 20 cm, and 73.4 mg L−1 day−1 at 20 cm with the white, blue, green, and red LED, respectively (Fig. 2a). The photosynthetic capability of Ettlia sp. appeared to be inhibited at a shorter distance of 20 cm with the white and blue LEDs, as the optimal biomass productivities were achieved at a distance of 40 cm. Thus, based on the maximum BPasy for each LED, the suitability of LED light for the biomass production of Ettlia sp. was in the descending order of white, green, red, and blue.
Biomass productivities of Ettlia sp. under various light irradiation conditions. Bars represent biomass productivity on day 20 (BPd20) of continuous cultivation, while black dots denote maximum biomass productivities (BPasy) estimated using curve fitting of DCW data. “W,” “B,” “G,” and “R” denote white (white-bars), blue (diagonal line-bars), green (gray-bars), and red (dot-bars) LED, respectively. “H,” “M,” and “L” denote high, middle, and low light intensities, representing distance of 20, 40, and 60 cm from LED panels, respectively. a Actual biomass productivity in DCW. b, c Normalized biomass productivities according to light energy and intensity based on quantum meter, respectively
The biomass productivity of Ettlia sp. under the various LEDs was also evaluated on the basis of the energy flux or quantum yield, which is a function of the light intensity irradiated from the LEDs. As such, a much higher energy was irradiated from the blue and red LEDs when compared with that from the white and green LEDs. For example, the energy fluxes from the blue and red LEDs at a distance of 30 cm from the panels were 0.09 and 0.10 J s−1 cm−2, respectively, while that from the white and green LEDs was only 0.03 J s−1 cm−2. As shown in Fig. 2b, the normalized biomass productivities according to the energy flux did not show a statistically significant correlation to the biomass productivities based on DCW (p = 0.424, r = 0.255). Based on the normalized values, the maximum biomass productivity of Ettlia sp. according to the energy flux was in the descending order of white, green, blue, and red (Fig. 2b) and this pattern was similar to that of chlorophyll-a content (Supplementary Fig. 4). However, when the biomass productivities were normalized according to the light intensity measured using a quantum meter, the normalized biomass productivities showed a low and slightly negative correlation with the values based on DCW (Fig. 2c, p = 0.418, r = − 0.258). The discrepancies in the biomass productivity patterns between the DCW based ones and the light-intensity normalized ones may have arisen from the characteristics of the quantum meter and LEDs used in this study. A quantum meter is specifically designed to measure the light in the photosynthetically active radiation (PAR) region, corresponding to 400–700 nm. However, the spectra of the blue and red LEDs used in this study were somewhat overlapping with the PAR, according to the manufacturer (data not shown). Therefore, this overlap may have caused an underestimation of the light intensity for the blue and red spectrum. Thus, when evaluating the biomass productivity of Ettlia sp. from the PBR cultures, the biomass productivity patterns normalized according to the light energy were considered more realistic than those normalized according to the light intensity. Consequently, the biomass productivity and its normalization according to the light energy indicated that the green light was effectively used by Ettlia sp. for its biomass growth compared to blue and red LED.
Most photosynthetic organisms, including microalgae and even plants, have evolved a sensitivity to use the blue and red spectrum of the PAR (Chen et al. 2011). For example, microalgae such as Chlamydomonas reinhardtii and C. vulgaris show a higher cell growth under a red or blue LED (Atta et al. 2013; Szwaja et al. 2016). At the molecular level, the efficiency of the photosystem I and II of microalgae can be enhanced using a red and blue wavelength (You and Barnett 2004; Ravelonandro et al. 2008). Green light has previously been reported to be ineffective for microalgae growth. In a study by Yan et al. (2013), a green LED produced very low growth when compared with other LED colors, such as white, purple, blue, yellow, and red. In another study, Vadiveloo et al. (2015) reported no net growth of Nannochloropsis sp., when grown under green light. In contrast, some studies have reported that several green algae or plants with large amount of chlorophyll can utilize green light for photosynthesis. Mohsenpour and Willoughby (2013) obtained high biomass concentration of C. vulgaris under green light condition compared to other light qualities. Sun et al. (1998) reported that thick leaves with a higher concentration of chlorophyll can absorb green light. Moreover, high intensity (300 μmol photons m−2 s−1) and short-wavelength (510 nm) of green LED light promoted growth in Lactuca sativa (Johkan et al. 2012). These results indicated that utilization of light wavelengths is species-specific or chlorophyll concentration and/or light intensity-dependent. Thus, the relatively higher growth of Ettlia sp. under green light is an intriguing characteristic that requires further investigation.
Photosynthetic characteristics of Ettlia sp.
The relatively active growth of Ettlia sp. using green light suggests that the photosynthetic characteristics of Ettlia sp. are unique. Thus, to understand the photosynthetic characteristics of Ettlia sp. further, its photosynthetic oxygen production rates were investigated under various LEDs. Ettlia sp. samples were taken from the PBRs with the maximum biomass productivity under specific LEDs. The O2 evolution profiles of Ettlia sp. under the LEDs sharply decreased after a peak, suggesting strong photo-inhibition by the LEDs at a higher intensity after the peak value (data not shown). Thus, the parameters related to the oxygenic photosynthetic activities were obtained by fitting each photosynthesis-irradiance curve (P-Icurve) using the following equation (Jasby and Platt 1976).
where P is the photosynthetic rate, Pmax is the maximum potential photosynthetic rate, α is the initial linear slope at a low irradiance, and β is the negative slope at a high irradiance.
The photosynthetic parameters are summarized in Table 1. When the Ettlia sp. was cultivated and adapted under mixed light, i.e., white LED, it actively produced oxygen using the irradiation of the various LEDs. Plus, the relatively higher values of α and β suggest that the Ettlia sp. sharply increased its photosynthetic rate with a low light intensity, whereas the rate was sharply inhibited with a higher light intensity. However, when the Ettlia sp. was cultivated and adapted under the LEDs with narrow wavelengths, it only showed a high oxygen production under the white LED, and little oxygen was produced under the other LEDs. This pattern was most distinctive when the Ettlia sp. was adapted to the green light (Table 1), as the green-light-adapted Ettlia sp. showed the lowest Pmax and α with blue, red, and even green light irradiation. Thus, the low oxygen production with the narrow wavelength LEDs, especially the green LED, suggests that Ettlia sp. might be utilizing the green light spectrum more efficiently at higher cell densities due to its deeper penetration to increase the biomass. Previously, it has been reported that under strong white light, any additional green light absorbed by the lower chloroplasts would increase leaf photosynthesis to a greater extent than would additional red or blue light (Terashima et al. 2009). It was argued that for green light, loss in the efficiency of absorbance by the sieve effect is small, while gain in absorbance by the détour effect is large. Consequently, green leaves absorb much green light (Terashima et al. 2009). Action spectra of oxygen production and chlorophyll-a for Nannochloropsis revealed the wavelength dependence of photosynthesis (Tamburic et al. 2014). Here, blue light was shown to be absorbed and directed to photosystem II more effectively than red light. In the current study, oxygen evolution (Table 1) and biomass productivity (Fig. 2), however, could be used as the indicator of the photosynthetic activity of the Ettlia sp. under irradiation of various LEDs. In addition, the spectra of the blue (400–525 nm) and green light (510–600 nm) show some overlapping, which might affect the growth of Ettlia sp. A relatively lower Pmax value measured by oxygen production was observed under red light compared to that of other light qualities (Table 1), while the biomass productivities were not significantly lower except when compared with that of white light (p > 0.01, Fig. 2a). In this study, red light LED (634 nm) can be more specifically defined as an orange-red, and the orange-red LED is known to have poor photosynthetic efficiency than deep-red (670/680 nm) LED (de Mooij et al. 2016). Although C. reinhardtii cultured under deep-red light (661 nm) had higher Fv/Fm value, the biomass productivity was lower which could be attributed to the oversaturation of the photosystems (de Mooij et al. 2016).
Elucidation of the detailed mechanisms of Ettlia sp. in terms of utilizing green light will need further intensive studies. The high carotenoid content of Ettlia sp. may provide a first clue, as the carotenoid productivities from the PBR experiments showed a similar pattern to that of the biomass productivities (Fig. 3) and a strong correlation (Fig. 4, p < 0.001, r = 0.968). Carotenoids are known to act as light-harvesting pigments and transfer excitation energy to chlorophyll in photosynthesis (Young and Frank 1996). These fat-soluble pigments are shown to capture blue and green light and pass energy on to chlorophylls in photosynthesis (Ostroumov et al. 2013). Tamburic et al. (2014) reported the more efficient absorption and use of blue light by Nannochloropsis under the photosaturation irradiance that resulted in higher photosynthetic rate compared to the red light. Some green algae such as Codium fragile and Ulva pertusa, inhabiting the deepest part of the green algae zonation, have been reported to contain a keto-carotenoid, siphonaxanthin, which absorbs green light and transfers energy to chlorophylls (Kageyama et al. 1977; Akimoto et al. 2007). It was suggested that carotenoid siphonaxanthin absorbs green light when the peak of available photosynthetically active photon flux density shifts toward blue with depth of sea-water. Thus, the high correlation between the carotenoids and the biomass productivity under the various LEDs suggest that carotenoid production may at least contribute to enhancing the biomass productivity of Ettlia sp. Moreover, the absorption spectrum of Ettlia sp. was measured to identify the mechanisms of green light harvesting (Fig. 1b). Better penetration capacity of green as compared to the blue and red light at higher cell densities could contribute in biomass productivity. Earlier, we have shown the variations in the absorption spectra of Ettlia sp. with different cell concentrations (Sarrafzadeh et al. 2015). As the cell concentration increased, the spectra especially in the green wavelength region became less variant indicating the difference in the absorption of green wavelength with varying cell concentrations. Earlier also, Mattos et al. (2015) reported that as Scenedesmus bijuga cell density increased, weakly absorbed green light was able to penetrate deeper into high-density samples and became photosynthetically more effective than strongly absorbed red and blue lights. High-density culture (2.19 g L−1) of S. bijuga cultivated under green light showed higher biomass production rate with 8.43% biomass growth in a 6-day period compared to the same quantum flux of red light that induced 4.35% biomass growth in the same period.
Total carotenoid productivities of Ettlia sp. under various LED irradiances. Carotenoid productivities are normalized according to incident light energy from LEDs. White-bar, diagonal line-bars, gray-bars, and dot-bars denote white, blue, green, and red LED, respectively. “H,” “M,” and “L” denote high, middle, and low light intensities, representing distance of 20, 40, and 60 cm from LED panels, respectively
Application of photosynthetic features of Ettlia sp.
The relatively higher biomass and carotenoid productivity of Ettlia sp. under green light was hypothesized to be beneficial for the prevention of invasion by other microalgae strains, especially in mass cultivation. Thus, to test this hypothesis, a competition experiment was conducted using Ettlia sp. and C. vulgaris as the model competitor.
The selected C. vulgaris showed a comparable growth rate to Ettlia when it was grown under the same chemostat culture conditions (Fig. 5). The diameter ratio of C. vulgaris to Ettlia sp. ranged from 0.6 to 0.8. Thus, when assuming the same densities for both strains and that both microalgae are spheres, the relative growth rate of C. vulgaris in numbers can be obtained from their biomass productivity using the following equation:
where NP is the cell number productivity, ρi is the density, ri is the radius, c is the C. vulgaris, and e is the Ettlia sp.
Comparison of the cell number productivity of Ettlia sp. (white) and C. vulgaris (gray) based on their biomass productivities under the same continuous culture conditions. C. vulgaris shows the highest biomass productivity under white LED at the distance of 20 cm, and this value is used as the reference (100%) for the relative cell number productivity calculation. The cell number productivity is calculated assuming a size ratio of 0.8 (C. vulgaris/Ettlia sp.). The black circles denote the relative cell number productivity ratio between C. vulgaris and Ettlia sp. under the various continuous culture conditions
Based on the differences in biomass productivity, the initial mixing ratio of Ettlia sp. and C. vulgaris was selected as 1:9 on a DCW basis. This ratio guaranteed a higher number and larger volume of C. vulgaris over Ettlia sp., thereby favoring the photosynthesis of C. vulgaris. After 8 days of mixed and continuous cultivation, the population ratio of the two microalgae was then enumerated by FACS and a microscope when using white, green, and white + green LED irradiation conditions. Interestingly, Ettlia sp. proportion increased as compared to C. vulgaris under all the test conditions (Fig. 6). While the initial cell number ratio of C. vulgaris to Ettlia sp. under the white, green, and white + green LED irradiation was 8.8, 8.9, and 8.8, respectively, after 8 days of co-cultivation, these cell number ratios changed to 7.0, 6.2, and 3.6, respectively. In addition, the DCWs of the continuous culture systems under the white, green, and white + green LEDs were 0.233, 0.160, and 0.227 g L−1, respectively (Fig. 6). At similar cell density, although the absorption spectra of Ettlia sp. and C. vulgaris was similar (Supplementary Fig. 5), Ettlia sp. showed better competitive ability. Thus, under the white + green light, the Ettlia sp. showed a relatively stronger competitive ability without losing its overall biomass productivity.
Relative cell count ratio changes for Ettlia sp. and C. vulgaris after 8 days of competition in continuous cultivation under white, green, and combined white + green LEDs. Heights of gray (C. vulgaris) and white (Ettlia sp.) bars are proportional to relative number ratio measured using fluorescence-activated cell sorting (FACS) and light microscope. Open circles denote whole dry cell weight (DCW) of mixed culture of Ettlia sp. and C. vulgaris in PBRs after 8 days of competition experiments. All experiments were conducted in duplicate
The stronger competitive capability of Ettlia sp. especially under white + green LEDs also suggested that Ettlia sp. might be utilizing green spectrum in white light for driving the photosynthesis. Terashima et al. (2009) also reported that even with low photosynthetic efficiency, green light drove higher photosynthesis rate than red light in strong white light conditions once it is absorbed by the leaves. The competition between microalgae in certain environments is a very dynamic process affected by various parameters. However, the effective utilization of green light spectrum by Ettlia sp. could provide a useful strategy for the control of contamination by other microalgae in open ponds for the mass cultivation of Ettlia sp.
Green light is an available spectral band underneath the water surface based on direct penetration from the sun and reflection from other photosynthetic microorganisms. The relatively higher settling ability of Ettlia sp., compared to other microalgae (La et al. 2016), is one of its physiological traits that may have evolved in close association with its ability to use green light. Thus, use of green light may provide several advantages for the survival of Ettlia sp.
Conclusions
A relatively higher biomass productivity was achieved for Ettlia sp. under green light due to its better penetration compared to blue or red light. The biomass productivity of Ettlia sp. was strongly correlated with its carotenoid productivity, which may affect the photosynthetic characteristics of Ettlia sp. by transferring the energy to chlorophyll. In a competition experiment, Ettlia sp. out-competed C. vulgaris under white and green illumination, and especially under white + green LEDs. Consequently, irradiation with the green spectrum as supplementary light of white light could be an effective tool for preventing invasion by other microalgae in the mass cultivation of Ettlia sp.
References
Akimoto S, Tomo T, Naitoh Y, Otomo A, Murakami A, Mimuro M (2007) Identification of a new excited state responsible for the in vivo unique absorption band of siphonaxanthin in the green alga Codium fragile. J Phys Chem B 111:9179–9181
Atta M, Idris A, Bukhari A, Wahidin S (2013) Intensity of blue LED light: a potential stimulus for biomass and lipid content in fresh water microalgae Chlorella vulgaris. Bioresour Technol 148:373–378
Bartley ML, Boeing WJ, Dungan BN, Holguin FO, Schaub T (2014) pH effects on growth and lipid accumulation of the biofuel microalgae Nannochloropsis salina and invading organisms. J Appl Phycol 26:1431–1437
Brown CS, Schuerger AC, Sager JC (1995) Growth and photomorphogenesis of pepper plants under red light-emitting diodes with supplemental blue or far-red lighting. J Am Soc Hortic Sci 120:808–813
Chen CY, Yeh KL, Aisyah R, Lee DJ, Chang JS (2011) Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: a critical review. Bioresour Technol 102:71–81
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306
de Mooij T, de Vries G, Latsos C, Wijffels RH, Janssen M (2016) Impact of light color on photobioreactor productivity. Algal Res 15:32–42
Griffiths MJ, Harrison STL (2009) Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J Appl Phycol 21:493–507
Hipkins MF, Baker NR (1986) Photosynthesis, energy transduction. In: Hipkins MF, Baker NR (eds) Spectroscopy. IRL Press, Oxford, pp 51–101
Huang G, Chen F, Wei D, Zhang X, Chen G (2010) Biodiesel production by microalgal biotechnology. Appl Energ 87:38–46
Jasby AD, Platt T (1976) Mathematical formulation of the relationship between photosynthesis and light for phytoplankton. Limnol Oceanogr 21:540–547
Johkan M, Shoji K, Goto F, Hahida S, Yoshihara T (2012) Effect of green light wavelength and intensity on photomorphogenesis and photosynthesis in Lactuca sativa. Environ Exp Bot 75:128–133
Kageyama A, Yokohama Y, Shimura S, Ikawa T (1977) An efficient excitation energy transfer from a carotenoid, siphonaxanthin to chlorophyll a observed in a deep-water species of chlorophycean seaweed. Plant Cell Physiol 18:477–480
La HJ, Seo SH, Lee JY, Lee CS, Kim BH, Srivastava A, Han MS, Oh HM (2016) Improved mixing efficiency and biomass productivity of Ettlia sp. in co-cultivation system with loaches. Algal Res 17:300–307
Liang Y, Sarkany N, Cui Y (2009) Biomass and lipid productivities of Chlorella vulgaris under autotrophic, heterotrophic and mixotrophic growth conditions. Biotechnol Lett 31:1043–1049
Mattos ER, Singh M, Cabrera ML, Das KC (2015) Enhancement of biomass production in Scenedesmus bijuga high-density culture using weakly absorbed green light. Biomass Bioenergy 81:473–478
Mohsenpour SF, Willoughby N (2013) Luminescent photobioreactor design for improved algal growth and photosynthetic pigment production through spectral conversion of light. Bioresour Technol 142:147–153
Ostroumov EE, Mulvaney RM, Cogdell RJ, Scholes GD (2013) Broadband 2D electronic spectroscopy reveals a carotenoid dark state in purple bacteria. Science 340:52–56
Ravelonandro PH, Ratianarivo DH, Joannis-Cassan C, Isambert A, Raherimandimby M (2008) Influence of light quality and intensity in the cultivation of Spirulina platensis from Toliara (Madagascar) in a closed system. J Chem Technol Biotechnol 83:842–848
Ritchie RJ, Larkum AWD (2012) Modelling photosynthesis in shallow algal production ponds. Photosynthetica 50:481–500
Rocap G, Larimer FW, Lamerdin J, Malfatti S, Chain P, Ahlgren NA, Arellano A, Coleman M, Hauser L, Hess WR, Johnson ZI, Land M, Lindell D, Post AF, Regala W, Shah M, Shaw SL, Steglich C, Sullivan MB, Ting CS, Tolonen A, Webb EA, Zinser ER, Chisholm SW (2003) Genome divergence in two Prochlorococcus ecotypes reflects oceanic niche differentiation. Nature 424:1042–1047
Saha SK, McHugh E, Hayes J, Moane S, Walsh D, Murray P (2013) Effect of various stress-regulatory factors on biomass and lipid production in microalga Haematococcus pluvialis. Bioresour Technol 128:118–124
Sarrafzadeh MH, La HJ, Seo SH, Asgharnejad H, Oh HM (2015) Evaluation of various techniques for microalgal biomass quantification. J Biotechnol 216:90–97
Scott SA, Davey MP, Dennis JS, Horst I, Howe CJ, Lea-Smith DJ, Smith AG (2010) Biodiesel from algae: challenges and prospects. Curr Opin Biotechnol 21:277–286
Seo SH, Ha JS, Yoo C, Srivastava A, Ahn CY, Cho DH, La HJ, Han MS, Oh HM (2017) Light intensity as major factor to maximize biomass and lipid productivity of Ettlia sp. in CO2-controlled photoautotrophic chemostat. Bioresour Technol 244:621–628
Stanier RY, Kunisawa R, Mandel M, Cohen-Bazire G (1971) Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol Rev 35:171–205
Sun J, Nishio JN, Vogelmann TC (1998) Green light drives CO2 fixation deep within leaves. Plant Cell Physiol 39:1020–1026
Szwaja S, Dębowski M, Zieliński M, Kisielewska M, Stańczyk-Mazanek E, Sikorska M (2016) Influence of a light source on microalgae growth and subsequent anaerobic digestion of harvested biomass. Biomass Bioenergy 91:243–249
Tamburic B, Szabó M, Tran NAT, Larkum AW, Suggett DJ, Ralph PJ (2014) Action spectra of oxygen production and chlorophyll fluorescence in the green microalga Nannochloropsis oculata. Bioresour Technol 169:320–327
Terashima I, Fujita T, Inoue T, Chow WS, Oguchi R (2009) Green light drives leaf photosynthesis more efficiently than red light in strong white light: revisiting the enigmatic question of why leaves are green. Plant Cell Physiol 50:684–697
Vadiveloo A, Moheimani NR, Cosgrove JJ, Bahri PA, Parlevliet D (2015) Effect of different light spectra on the growth and productivity of acclimated Nannochloropsis sp. (Eustigmatophyceae). Algal Res 8:121–127
Wellburn AR (1994) The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol 144:307–313
Yan C, Zhang L, Luo X, Zheng Z (2013) Effects of various LED light wavelengths and intensities on the performance of purifying synthetic domestic sewage by microalgae at different influent C/N ratios. Ecol Eng 51:24–32
Yoo C, Choi GG, Kim SC, Oh HM (2013) Ettlia sp. YC001 showing high growth rate and lipid content under high CO2. Bioresour Technol 127:482–488
You T, Barnett SM (2004) Effect of light quality on production of extracellular polysaccharides and growth rate of Porphyridium cruentum. Biochem Eng J 19:251–258
Young AJ, Frank HA (1996) Energy transfer reactions involving carotenoids: quenching of chlorophyll fluorescence. J Photochem Photobiol B 36:3–15
Yu J, Wang P, Wang Y, Chang J, Deng S, Wei W (2018) Thermal constraints on growth, stoichiometry and lipid content of different groups of microalgae with bioenergy potential. J Appl Phycol 30:1503–1512
Acknowledgements
We would like to offer special thanks to Dr. HJ La, who, although no longer with us, was a warm and creative scientist and continues to inspire by his example.
Funding
This research was supported by a grant from the Advanced Biomass R&D Center (ABC), a Global Frontier Program funded by the Korean Ministry of Science and ICT.
Author information
Authors and Affiliations
Contributions
Jae-Yon Lee and Hyun-Joon La made the primary conception and design of the study, experiments, analysis and interpretation of data, and drafting the article; Seong-Hyun Seo and Ankita Srivastava revised it critically; Chi-Yong Ahn, Chang Soo Lee, and Kwang-Guk An suggested important intellectual content; and Hee-Mock Oh has done final approval of the version to be submitted.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 451 kb)
Rights and permissions
About this article
Cite this article
Lee, JY., Seo, SH., Ahn, CY. et al. Green light as supplementary light for enhancing biomass production of Ettlia sp. and preventing population invasion from other microalgae. J Appl Phycol 31, 2207–2215 (2019). https://doi.org/10.1007/s10811-019-1737-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-019-1737-x