Abstract
Light management methods are considered effective to enhance the quantum yield and photosynthetic efficiency and promote the biomass and nutrient production; however, light saturation and inhibition restrain further improvement. This work studies the effect of light mixing on algal light saturation/inhibition, growth kinetics, and biochemical profile. The green alga Chlamydomonas reinhardtii was cultivated with batch culture under an LED light panel with multiple spectra options. Different combinations of blue (B) and red-orange (RO) light intensities were tested with blue light ranging from 45 to 65 μmol photons m−2 s−1 and red-orange light ranging from 45 to 205 μmol photons m−2 s−1. Results reveal that the mixed blue and red-orange light significantly improved the growth kinetics and relieved the light saturation under blue light and the light inhibition under the red-orange light. The maximum specific growth rate, biomass concentration, and productivity increased by 22, 50, and 57%, respectively, compared with the results under the red-orange light. The lipid and protein synthesis were observed to be promoted under mixed light with relatively low red-orange light intensities (45 and 105 μmol photons m−2 s−1) and repressed at high red-orange light intensities (155 and 205 μmol photons m−2 s−1). The carbohydrate content did not change.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microalgae have a high growth rate and multiple nutrient components such as lipids, carbohydrates, proteins, and pigments. They have been used as food, feed, nutraceuticals, and pharmaceuticals (Wells et al. 2017) and are considered a potential feedstock of biofuel (Khan et al. 2018). They even have been considered for use as a part of the life support system for long-range space travel, to remove carbon dioxide, produce oxygen, and absorb the organic and inorganic nutrients to clean water in a closed or partially closed system (Matula and Nabity 2019). Enhancing algal biomass and nutrient production rate while decreasing the cost of algal products can increase its competitiveness in commercial markets and improve the potential for future uses in biofuel and long space travel (Khan et al. 2018; Matula and Nabity 2019).
Growing algae outdoors in open ponds or bioreactors under the natural sunlight has been widely accepted as the cheapest growth technique. However, there are several limitations: first, growing algae outdoors is subject to regional, diurnal, and seasonal variations of light intensity and temperature; second, harmful radiation, such as ultraviolet light, can cause damage to algae cells; and third, growing algae in open ponds is vulnerable to contamination and predators. Excess or insufficient light intensity or temperature delays the algal growth and results in low productivity. Light inhibition caused by high solar intensity is of special concern, as it significantly limits the improvement of the photosynthesis efficiency and algal productivity in open ponds or photobioreactors.
To enhance algal photosynthetic efficiency, light management methods such as photobioreactor design using spatial attenuation (Tan 2018), UV-filtration (Esperanza et al. 2019), or spectral conversion (Burak et al. 2019) of sunlight can be used, as well as genetic engineering to attenuate the volume of light harvest units and the amount of light energy absorption (Nwoba et al. 2019). Compared to the wild type, mutants with small antenna size did not show better performance (de Mooij et al. 2015, 2016). Sunlight processing with the methods mentioned above is feasible but currently expensive. As an alternative, growing algae indoors with artificial light sources under stringent culturing conditions could help to improve the algal photosynthetic efficiency and avoid growth delay caused by light inhibition under the natural sunlight.
For all light management methods, the optimal light spectra should be determined. However, several questions are still open: what are the metabolic responses of algae to different light conditions? What light spectra and their mixing intensities give higher biomass and nutrient production?
Light provides the energy through photosynthesis and activates photoreceptors for pathways of the regulation (Hegemann 2008). It has been reported that blue light enhances the function of photosystem I, and red light enhances the function of photosystem II (Baer et al. 2016; Ramanna et al. 2017). Thus, blue and red lights are considered the most appropriate for algal cultivation (Zhao and Su 2014; Choi et al. 2015; Ramanna et al. 2017). It has been reported that blue light promoted biomass productivity and lipid synthesis in Nannochloropsis sp. (MUR266) (Vadiveloo et al. 2015). Monochromatic red light prompted the production of Haematococcus pluvialis at 20 °C, while mixed red and blue light promoted astaxanthin accumulation at 30°C (Pereira and Otero 2020). In previous studies, it was found that, for the cultivation of Chlamydomonas reinhardtii, red-orange light promoted the growth kinetics and lipid and carbohydrate synthesis, while blue light promoted protein synthesis (Li et al. 2020, 2021).
Furthermore, photoreceptors in plants and algae such as phytochromes, cryptochromes, and phototropins interact with each other, i.e., the action of one pigment can be affected by the reaction of others (Casal 2000). Green algae and terrestrial plants have similar plastids, especially in regards to structure, metabolism, and biochemical composition. Therefore, the effect of the ratio of red to blue light in the growth spectra is expected to be similar to that in the terrestrial plants (Schulze et al. 2014). It has been reported that irradiation of blue light or far-red light enhanced the response of gherkin to red light (Meijer and Engelsma 1965), and red light enhanced the phototropic response to blue light (Casal 2000). Wavelengths of around 430 nm and 660 nm have an impact on algal biochemistry and growth, and a combination of both is recommended to improve biomass and nutrient production (Schulze et al. 2016). However, both positive and negative effects of the mixed blue-red light have been reported on the growth kinetics of crops (Ki-Ho and Myung-Min 2013; Kaiser et al. 2019) and algae (Kim et al. 2013; Yan and Zheng 2014; Sirisuk et al. 2018). The effect of mixed blue-red light on the algal biochemical profile is seldom studied.
In a previous study, we have measured the light saturation/inhibition of C. reinhardtii under blue and red-orange light (Li et al. 2020). It is interesting to see the effect of mixed blue and red-orange light on light saturation and inhibition, i.e., whether the combination of different light qualities will aggravate or relieve the light saturation and inhibition. In this study, different combinations of light intensities between blue and red-orange lights were tested to study the light saturation and inhibition, and the subsequent effects on the growth kinetics and the biochemical profile.
Materials and methods
Batch cultures were carried out in a constant-temperature incubator shaker. Culture samples were checked for the growth kinetics daily and the biochemical profile once during the growth cycle.
The light saturation and inhibition point (based on the maximum specific growth rate) of C. reinhardtii under the blue light we used was around 65 μmol photons m−2 s−1 and 205 μmol photons m−2 s−1; the light saturation/inhibition point under the red-orange light was around 155 μmol photons m−2 s−1 (Li et al. 2020). For microalgae growth, a blue to red light ratio of 1:1 to 1:3 has been reported to promote the biomass growth rate, pigment production, or biogas upgrading (Fu et al. 2013; Yan and Zheng 2014; Zhao et al. 2015; Yan et al. 2016; Lima et al. 2018). Thus, the blue light cannot be too high. To study the effect of mixed light on algal light saturation and inhibition, the blue light was set to 45 and 65 μmol photons m−2 s−1, which was lower or equal to the light saturation point, and the red-orange light intensity was set to 45, 65, 155, and 205 μmol photons m−2 s−1, which ranged from lower to higher than the light saturation/inhibition point. The combination of light intensities between blue and red-orange lights is shown in Table 1.
Algae strain and culturing method
The model organism Chlamydomonas reinhardtii WT CC-125 was from the Chlamydomonas Resource Center of the University of Minnesota, USA. Algae cultivation in TAP (Tris-acetate-phosphate) medium was carried out in batch cultures. The TAP medium consisted of Tris base, 20 mM; acetic acid, 17 mM; NH4Cl (Fisher A 649-500), 7.5 mM; CaCl2•2H2O (Sigma C-3881), 0.35 mM; MgSO4•7H2O (Sigma 230391), 0.4 mM; Na2HPO4 (Sigma S5136), 0.69 mM; KH2PO4 (Sigma P 0662), 0.45 mM; Na2EDTA•2H2O (Sigma ED255), 0.134 mM; ZnSO4•7H2O (Sigma Z 0251), 0.077 mM; H3BO3 (Baker 0084), 0.184 mM; MnCl2•4H2O (Baker 2540), 0.026 mM; FeSO4•7H2O, 0.018 mM; CoCl2•6H2O (Sigma C-3169), 0.007 mM; CuSO4•5H2O (MCIB3M11), 0.005 mM; and (NH4)6Mo7O24•4H2O (Mallinckrodt 3420), 0.0008 mM.
Alga inoculum for each culturing condition was acclimated for 5 days before being transferred to a new flask. For each flask (250-mL baffled flask, VWR Flask CLTR Baffled 250 mL CS6) of batch culture, 50 mL TAP medium and 1 mL acclimated algae slurry were added. Batch cultures (five to six cultures for each culturing condition) were cultivated under continuous light in a constant-temperature incubator shaker (3525-3CC, Lab-Line Instruments, Inc., USA). The temperature inside the shaker was controlled at 28 ± 0.3 °C. An LED light panel with blue and red-orange lights was installed in the shaker as a light source.
Light source and light quality
Compared with the conventional light sources examined for crops and algae cultivation such as fluorescent light (Fl), high-pressure sodium (HPS), metal halide (MH), and microwave, light-emitting diodes (LEDs) are safe, solid-state, robust, and long-lived with a small mass, volume, the lowest heat emission, and the highest conversion efficiency of electricity with desired wavelength (Massa et al. 2007; Nhut and Nam 2010; Mitchell et al. 2012). For growing algae, a mixing of narrow-spectrum LED lights (colored) with different wavelength bands was generally recommended to improve the production of biomass or specific nutrient components (Kommareddy and Anderson 2004; Mitchell et al. 2012; Olle and Viršile 2013; Schulze et al. 2016).
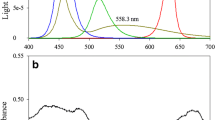
In this work, a dimmable LED light (VIPARSPECTRA Dimmable Reflector-Series 450W LED Grow Light, VIPARSPECTRA, China) with three dimmers and 12-band full spectrum was selected as the light source, with which the light quality (blue light and red-orange light) and light intensity can be controlled and adjusted. The emission spectra of the LEDs applied, as shown in Fig. 1, were measured with a spectrometer (FLAME-S-VIS-NIR-ES, Flame Ocean Optics Miniature Spectrometer, USA).
The red light includes wavelength bands peaking at 735 nm (4 IR lights), 656 nm (8 lights), and 630 nm (8 lights). The orange light has wavelength bands peaking at 580–594 nm (8 lights) and 604 nm (9 lights). The blue light has wavelength bands peaking at 433–447 nm (10 lights) and 458–470 nm (16 lights). The light intensity was calibrated with a quantum light meter (Light Scout Solar/Electric Quantum Meter 3415FSE, Spectrum Technologies, Inc., USA).
Measurement of growth kinetics
Six cultures were prepared and checked for each culturing condition. The optical density (OD) was checked twice a day from the beginning to the late exponential phase and then once a day in the stationary phase with the UV-vis spectrophotometer at 750 nm during the inoculation cycle (~144 h). The biomass concentration x (g L−1) was predicted with the OD – biomass concentration relationship, i.e., x = OD750/5.5 and used for growth kinetics analysis (Li et al. 2020, 2021).
For the growth kinetics evaluation of each culture condition, the maximum specific growth rate \( {\mu}_{\mathrm{max}}=\frac{\mathit{\ln}\left({x}_2\right)-\mathit{\ln}\left({x}_1\right)}{t_2-{t}_1} \) (day−1, where x1 and x2 are the biomass concentration at time t1 and t2), the maximum biomass concentration (g L−1), and the maximum biomass productivity (g L−1 day−1) were analyzed.
Measurement of biochemical profile
The biochemical profile of neutral lipid content, carbohydrate content, and protein content were checked with the Nile Red (NR) staining lipid assay (Kou et al. 2013; Velmurugan et al. 2013; Rumin et al. 2015), the Bradford protein assay (Guerlava et al. 1998), and the phenol-sulfuric acid carbohydrate assay, respectively (Chaplin and Kennedy 1994). The protein content is generally high in the late exponential growth phase and was checked on the 4th day at 68–72 h of growth. Lipid and carbohydrate contents were checked at the end of the growth cycle in the late stationary growth phase when both nutrient components accumulated due to nutrient starvation.
Statistical analysis
One-way analysis of variance (ANOVA) and Tukey test assuming equal variance (two-tailed) were used for the statistical analysis of the effect of different light intensity mixing between blue and red-orange lights on growth kinetics and nutrient composition. For studying the interaction of the B:RO ratio and total irradiance, a two-way ANOVA using regression (unbalanced factorial ANOVA) (Zaiontz 2020) was applied. A value of P = 0.05 was used as the cutoff to evaluate the significant difference.
Results
In this work, multiple wavelengths of blue and red-orange light LEDs were used to study the effect of wavelengths mixing on algal light saturation and inhibition. We also sought to determine the optimal light quality and intensity combination to enhance the biomass and nutrient production of C. reinhardtii.
Growth kinetics
Growth curves
Under the mixing of blue and red-orange lights, growth was higher in the exponential phase when the blue light fraction was low (B:RO = 45:155, 45:205, 65:155, and 65:205). This indicates that C. reinhardtii grew more rapidly with the decrease of blue light fraction (Fig. 2a and b). The only exception is that, under combination of B:RO = 65:205, the growth decreased compared with that of B:RO = 65:155 (Fig. 2b). This indicates the red-orange light inhibition. Additionally, the total biomass declined rapidly in the stationary phase (9–17% for B-45 μmol photons m−2 s−1 and 9–19% for B-65 μmol photons m−2 s−1) under mixed B-RO light.
Maximum specific growth rate, biomass concentration, and productivity
The maximum specific growth rate, biomass concentration, and biomass productivity are shown in Fig. 3. In Figs. 3, 4, 5, and 6, B refers to blue light, and RO refers to red-orange light; B0 means without blue light, i.e., blue light is 0 μmol photons m−2 s−1; B45 and B65 mean a blue light of 45 and 65 μmol photons m−2 s−1; and RO45 to RO205 means a red-orange light of 45 to 205 μmol photons m−2 s−1.
Growth kinetics of C. reinhardtii under the mixed blue and red-orange light (mean ± SD, n = 5–6), a maximum specific growth rate, b maximum biomass concentration, and c maximum biomass productivity. B—blue light; RO—red-orange light; B0—without blue light, i.e., blue light 0 μmol photons m−2 s−1; B45-blue light 45 μmol photons m−2 s−1; B65-blue light 65 μmol photons m−2 s−1; RO45–RO205—red-orange light 45–205 μmol·m−2 s−1. Statistical differences (P < 0.05) within the contents of B-0 μmol photons m−2 s−1 (blank bar), B-45 μmol photons m−2 s−1 (upward diagonal bar), and B-65 μmol photons m−2 s−1 (dark blue bar) are indicated by different letters
Lipid content of C. reinhardtii under the mixed blue and red-orange light (mean ± SD, n = 5–6), a lipid mass fraction, b lipid concentration (mean ± SD, n = 5–6). B—blue light; RO—red-orange light; B0—without blue light, i.e., blue light 0 μmol photons m−2 s−1; B45—blue light 45 μmol photons m−2 s−1; B65—blue light 65 μmol photons m−2 s−1; RO45–RO205—red-orange light 45–205 μmol photons m−2 s−1. Statistical differences (P < 0.05) within the contents of RO-0 μmol photons m−2 s−1 (blank bar), B-45 μmol photons m−2 s−1 (upward diagonal bar), and B-65 μmol photons m−2 s−1 (dark blue bar) are indicated by different letters
Carbohydrate content of C. reinhardtii under the mixed blue and red-orange light (mean ± SD, n = 5–6), a carbohydrate mass fraction, b carbohydrate concentration. B—blue light; RO—red-orange light; B0—without blue light, i.e., blue light 0 μmol photons m−2 s−1; B45—blue light μmol photons m−2 s−1; B65—blue light μmol photons m−2 s−1; RO45–RO205—red-orange light 45–205 μmol photons m−2 s−1. Statistical differences (P < 0.05) within the contents of RO-0 μmol photons m−2 s−1 (blank bar), B-45 μmol photons m−2 s−1 (diagonal bar), and B-65 μmol photons m−2 s−1 (blue bar) are indicated by different letters
Protein content of C. reinhardtii under the mixed blue and red-orange light (mean ± SD, n = 5–6), a protein mass fraction, b protein concentration. B—blue light; RO—red-orange light; B0—without blue light, i.e., blue light 0 μmol photons m−2 s−1; B45—blue light 45 μmol photons m−2 s−1; B65—blue light 65 μmol photons m−2 s−1; RO45–RO205—red-orange light 45–205 μmol photons m−2 s−1. Statistical differences (P < 0.05) within the contents of RO-0 μmol photons m−2 s−1 (blank bar), B-45 μmol photons m−2 s−1 (diagonal bar), and B-65 μmol photons m−2 s−1 (blue bar) are indicated by different letters
Based on a two-way ANOVA using regression, the effect of the B:RO ratio on the maximum specific growth rate and biomass productivity was dependent on the total radiance (P < 0.01), while independent for the biomass concentration (P = 0.3).
It has been reported that red-orange light stimulates the cell division of C. reinhardtii and results in a smaller average cell size, while blue light delays the cell division and results in a larger average cell size (Oldenhof et al. 2006). Thus, mixing the red and blue light could promote both the cell division rate and cell size growth at the same time and therefore enhance biomass production. In the previous study, it was shown that, under the red-orange light, C. reinhardtii obtained higher growth kinetics than that under the blue light (Li et al. 2020). Thus, in this study, the results under the mixed B-RO light were mainly compared with those under the red-orange light alone.
As can be seen in Fig. 3, the growth kinetics under the mixed blue and red-orange light was higher than under red-orange light alone under almost all mixed light conditions, especially under the lower blue light intensity, B-45 μmol photons m−2 s−1.
It is interesting that light mixing between the blue and red-orange lights relieved the light inhibition under red-orange light alone. As we measured in the previous study, when RO increased from 155 to 205 μmol photons m−2 s−1, the growth of C. reinhardtii was inhibited under the red-orange light alone (Li et al. 2020), i.e., the specific growth rate declined. While in this work, the maximum specific growth rate under the combination of B:RO = 45:205 was significantly higher than that of the red-orange light alone. This indicates that, with the addition of 45 μmol photons m−2 s−1 blue light, the inhibition caused by increasing the red-orange light to 205 μmol photons m−2 s−1 did not appear.
Also, as measured, under blue light alone, the light saturation appeared around a light intensity of 65 μmol photons m−2 s−1, and the highest specific growth rate reached was 1.703 day−1 (Li et al. 2020). While for all the light mixing conditions of red-orange light and B-65 μmol photons m−2 s−1, as shown in Fig. 3a, the maximum specific growth rate was significantly higher than that of blue light alone (P < 0.05 under B:RO = 65:45, P < 0.01 for the rest). Thus, B-RO light mixing relieved the light saturation caused by the blue light and promoted growth kinetics relative to blue light or red-orange light alone.
When comparing the two blue light intensities under mixing condition we can see for μmax and Pmax at RO-45 μmol photons m−2 s−1 and Cmax from RO-45 to 205 μmol photons m−2 s−1, when mixed with B-65 μmol photons m−2 s−1, that they were close to or higher than that mixed with B-45 μmol photons m−2 s−1. With an increase of RO light intensity to 105 μmol photons m−2 s−1 or higher, μmax and Pmax were lower at B-65 μmol photons m−2 s−1 than that at B-45 μmol photons m−2 s−1. This indicates an effect of light inhibition with the increase of blue light intensity.
Under B-RO mixing, the growth kinetics increased with the red-orange light intensity from 45 to 155 μmol photons m−2 s−1 and became saturated or inhibited at 205 μmol photons m−2 s−1. From RO-45 to 205 μmol photons m−2 s−1, the maximum specific growth rate increased from 1.726 to 2.059 day−1 at B-45 μmol photons m−2 s−1 and from 1.794 to 1.917 day−1 at B-65 μmol photons m−2 s−1; the biomass concentration increased from 0.693 to 0.784 g L−1 at B-45 μmol photons m−2 s−1 and from 0.730 to 0.812 g L−1 at RO-155 μmol photons m−2 s−1 and then declined to 0.788 g L−1 at RO-205 μmol photons m−2 s−1 when mixed with B-65 μmol photons m−2 s−1; and the biomass productivity increased from 0.241 to 0.363 g L−1 day−1 at B-45 μmol photons m−2 s−1 and from 0.246 to 0.340 g L−1 day−1 (RO-155 μmol photons m−2 s−1) and then declined to 0.310 g L−1 day−1 (RO-205 μmol photons m−2 s−1) at B-65 μmol photons m−2 s−1.
Lipid content
It has been reported that nutrient starvation, especially nitrogen limitation, can induce lipid accumulation in algae cells (Chen et al. 2011; Wang et al. 2019). Therefore, the lipid content was checked in the late stationary phase, as shown in Fig. 4.
According to the analysis results of two-way ANOVA using regression, the effect of B:RO ratio on the lipid mass fraction and concentration was dependent on the total radiance (P<0.01).
We can see from Fig. 4 that, when B-45 μmol photons m−2 s−1 was mixed with RO-45 μmol photons m−2 s−1 and RO-105 μmol photons m−2 s−1, lipid content was close to that under the red-orange light only. When B-65 μmol photons m−2 s−1 was mixed with RO-45 μmol photons m−2 s−1 and RO-105 μmol photons m−2 s−1, the lipid mass fraction and concentration were significantly higher. As RO light increased to 155 and 205 μmol photons m−2 s−1, mixed with both B-45 and 65 μmol photons m−2 s−1, lipid content under the mixed light was significantly lower than that under the red-orange light only.
When mixed with B-45 μmol photons m−2 s−1, increasing the RO light from 45 to 205 μmol photons m−2 s−1, the lipid mass fraction and concentration increased gradually from 4.6 to 6.4% w/w and from 27.6 to 42.3 mg L−1, respectively. When mixed with B-65 μmol photons m−2 s−1, from RO-45 to 105 μmol photons m−2 s−1, lipid mass fraction and concentration increased 5.3 to 6.1% w/w and from 34.8 to 42.5 mg L−1 then became almost stable with a further increase of RO light intensity.
Carbohydrate content
Similar to the lipid content, the carbohydrate content was checked in the late stationary phase for carbohydrate accumulation induced by nutrient starvation (Cheng et al. 2017). The results are shown in Fig. 5.
According to the analysis results of two-way ANOVA using regression, the effect of B:RO ratio on the carbohydrate mass fraction and concentration was dependent on the total radiance (P<0.01).
As shown in Fig. 5, the carbohydrate content variation pattern was different under the mixed light of B-45 and 65 μmol photons m−2 s−1. Under B-45 μmol photons m−2 s−1, the carbohydrate mass fraction and concentration reached the highest to 16.4% w/w and 97.8 mg L−1 at the low RO light intensity of 45 μmol photons m−2 s−1 and decreased to about 13.0% w/w and 84.4 mg L−1 at RO-105 μmol photons m−2 s−1 then increased gradually to 14.0% w/w and 92.2 mg L−1 at RO-205 μmol photons m−2 s−1. Under B-65 μmol photons m−2 s−1, carbohydrate mass fraction and concentration increased with the RO intensity from 15.0% w/w and 99.1 mg L−1 at RO-45 μmol photons m−2 s−1 to 20.3% w/w and 127.3 mg L−1 at RO-205 μmol photons m−2 s−1. Moreover, we can see under B-RO light mixing, except at RO-45 μmol photons m−2 s−1, when the blue light intensity increased from 45 to 65 μmol photons m−2 s−1, the carbohydrate content increased significantly.
Carbohydrate mass fraction and concentration under B:RO = 45:45 mixing was similar to that under the red-orange light only. Under B-65 μmol photons m−2 s−1, the carbohydrate mass fraction was significantly lower, while the carbohydrate concentration was significantly higher because of the higher total biomass concentration. As the RO light increased to 105 μmol photons m−2 s−1 or higher, under both B-45 and 65 μmol photons m−2 s−1, the carbohydrate mass fraction and concentration became significantly lower. It indicates that, when the red-orange light was mixed with the blue light, the carbohydrate accumulation was not improved by the combination of blue and red-orange lights.
Protein content
Unlike the lipid content and carbohydrate content which were checked in the late stationary phase, the protein content was checked at 68–72 h when the nutrient in the medium is enough that protein synthesis will not be inhibited (Gameiro and Struhl 2018). The results are shown in Fig. 6.
According to the analysis results of two-way ANOVA using regression, the effect of B:RO ratio on the protein concentration was dependent on the total radiance (P<0.05), while was independent on protein mass fraction (P = 0.07).
Under B-RO mixing, instead of increasing with the light intensity as under red-orange light alone, the protein mass fraction decreased with the red-orange light intensity. It decreased from 45.7 to 36.0% w/w under B-45 μmol photons m−2 s−1 and from 43.0 to 38.4% w/w under B-μmol photons m−2 s−1. The protein mass concentration also decreased with the RO light at B-45 μmol photons m−2 s−1 (from 319.4 to 286.3 mg L−1), while at B-65 μmol photons m−2 s−1, the protein concentration increased from 304.3 mg L−1 at RO-45 μmol photons m−2 s−1 to 321.6 mg L−1 at RO-105 μmol photons m−2 s−1 then declined to 302.5 mg L−1 at RO-205 μmol photons m−2 s−1.
Compared with red-orange light alone, the protein mass fraction and concentration were higher under the mixed light from RO-45 to 105 μmol photons m−2 s−1 under both B-45 and 65 μmol photons m−2 s−1, i.e., the protein synthesis is promoted at relatively low RO light intensity. As the red-orange light was increased to 155 μmol photons m−2 s−1, the protein mass fraction became lower than that under the red-orange light, while the protein concentration was still higher. When the red-orange light was increased to 205 μmol photons m−2 s−1, both the protein mass fraction and concentration were significantly lower than that under the red-orange light alone. Hence, the mixed light at relatively low RO intensity (45–105 μmol photons m−2 s−1) can improve the protein content.
Thus, mixed B-RO light did not improve the protein content significantly in C. reinhardtii relative to the red-orange light except under low RO light intensity.
Discussion
Mixed red and blue light has been shown to promote the biomass production of crops and microalgae compared with other light sources (Elmoraghy and Farag 2012; Fu et al. 2013; Yan and Zheng 2014; Choi et al. 2015; Schulze et al. 2016; Ra et al. 2018). It has been reported that using the mixed red-blue light improved the algal biomass production and nutrient removal rate for wastewater treatment (Kim et al. 2013). Mixed red-blue light improved the specific growth rate and biomass concentration of algae Phaeodactylum tricornutum and Isochrysis galbana and for Nannochloropsis salina both parameters under the mixed light were higher than that under the fluorescent light but lower than that under red light (Sirisuk et al. 2018). Blue- and red-mixed light (5:5) has been reported to provide the highest growth of microalgae P. tricornutum, I. galbana, N. salina, and Nannochloropsis oceanica among the red, blue, and fluorescent lights (Ra et al. 2018). Blue- and red-mixed light was also reported to induce higher biomass growth kinetics for Nannochloropsis oculata and Tetraselmis chui than non-optimal lamps such as LED CW or LED WW (Schulze et al. 2016). The combination of blue and red light has been reported to give the highest biomass of Chlorella sp. in terms of cell density (Severes et al. 2017).
There are also some studies showing no significant impact or even a lower growth induced by mixed red-blue light than by fluorescent light, or red and blue light alone (Lin et al. 2013; Leonardi et al. 2018; Pereira and Otero 2019).
Different optimal blue-red light ratios have been reported for the growth of crops and different algae species. A blue-red light ratio of 10–30% has been reported to result in the highest biomass production of crops (Nhut and Nam 2010), and a ratio of 1:1 has been reported to significantly promote the anthocyanin and proanthocyanidin content in strawberry fruits (Zhang et al. 2018). For microalgae, a blue to red light ratio of 1:3 has been reported to promote the biomass growth rate of Dunaliella salina (Fu et al. 2013), and a ratio of 3:7 has been reported optimal for biomass and pigment production of Arthrospira platensis (Lima et al. 2018). A blue-red light ratio of 5:5 has been reported for the growth of Chlorella sp., Scenedesmus obliquus, and Neochloris oleoabundans, and a ratio of 5:5 and 3:7 for biogas upgrading, and biogas fluid nutrient removal (Yan and Zheng 2014; Zhao et al. 2015; Yan et al. 2016). Changing the ratio between red and blue lights can affect the rate of Scenedesmus sp. biomass production and nutrient removal from the medium (Kim et al. 2013). However, it has also been reported that changing the red to blue light ratios resulted in no significant differences in the growth and morphology of tomato seedlings (Hernández and Kubota 2012). In this study, we can see from Fig. 3 that, with the decrease of blue to red-orange light ratio, the growth kinetics, including the maximum specific growth rate, biomass concentration, and productivity, increased. The best observed ratio is 45:155 (29%) and 45:205 (22%) (Fig. 3).
According to the results in this study, light mixing between blue and red-orange lights did work to relieve the light inhibition under red-orange light and also the adverse effect of light saturation under blue light. For the growth kinetics, as measured in our previous study, under the same red-orange light, C. reinhardtii showed light saturation and inhibition at 155 μmol photons m−2 s−1 (Li et al. 2020). The maximum specific growth rate decreased rapidly (light inhibition) with a further increase of red-orange light intensity. In this study, when red-orange light was mixed with blue light (45 and 65 μmol photons m−2 s−1), the maximum specific growth rate did not decrease with the light intensity from 155 to 205 μmol photons m−2 s−1 and still had a significant increase under the combination of B:RO = 45:205. This indicates that the effect of light inhibition under the red-orange light was relieved with the addition of blue light. Also, when red-orange light was mixed with blue light with an intensity of 65 μmol photons m−2 s−1, the maximum specific growth rate was significantly higher than the highest specific growth rate (appeared at the light saturation point B-65 μmol photons m−2 s−1) under blue light alone, i.e., the addition of red-orange light relieved the adverse effect of light saturation under the blue light. A similar result has been reported in the literature. With the addition of 42 μmol photons m−2 s−1 blue light to 128 μmol photons m−2 s−1 red light, the growth of D. salina was less inhibited than with a 170 μmol photons m−2 s−1 red light (Fu et al. 2013).
For lipid synthesis, the combination of blue and red light has been reported to promote lipid content in a few studies. For instance, mixed red-blue light improved the lipid production of alga P. tricornutum but showed no significant effect on the lipid production of Nocardiopsis salina or I. galbana (Sirisuk et al. 2018). Relative to the blue light alone, adding red light to blue light has been reported to improve the lipid content of Skeletonema marinoi (Orefice et al. 2016). Blue- and red-mixed light (5:5) has been reported to obtain higher lipid content than fluorescent light in microalgae P. tricornutum, I. galbana, N. salina, and N. oceanica (Ra et al. 2018). Fluorescent light with both red and blue peaks showed the highest lipid content for N. oculata and T. chuii among LED 405, LED 465, LED 630, LED 660, LED CW, and LED WW, but the authors did not compare the results of red-blue mixed light of HRLB, HBLR, HRmix, and HBmix like that for analysis of growth kinetics (Schulze et al. 2016). Nevertheless, some studies showed that the red-blue light combination did not improve the lipid content compared with other light sources such as red light (Severes et al. 2017). In this study, the improvement of lipid content under the mixed blue (65 μmol photons m−2 s−1) and red-orange light (45–105 μmol photons m−2 s−1) compared with that under the red-orange light only (45–105 μmol photons m−2 s−1) could be induced either by light mixing or by an increase of light intensity, since the mixed of blue (45 μmol photons m−2 s−1) and red-orange light (45–105 μmol photons m−2 s−1) did not show any improvement. When the red-orange light intensity increased to 155 and 205 μmol photons m−2 s−1, the lipid mass fraction and concentration were significantly lower than that of the red-orange light only. Hence, we can conclude that a combination of blue and red-orange lights might not be a good method to improve the algal lipid content, especially at high red-orange light intensities; the stimulating effect of red-orange light on lipid synthesis and accumulation was significantly hindered by being mixed with blue light.
For carbohydrate synthesis, red light promotes carbohydrate accumulation (Wynne and Rhee 1986), and blue light can enhance the synthesis of protein and amino acid by inducing the breakdown of the carbohydrate reserves in algae (Wynne and Rhee 1986; Kamiya and Saitoh 2002; Schulze et al. 2014). Light with wavelengths of 400–450 nm has been reported to have a negative effect on carbohydrate to protein ratios of algae (Schulze et al. 2016). Hence, the blue light and red light have an opposite impact on carbohydrate synthesis, and the addition of blue light hinders carbohydrate accumulation. Some studies have reported that the mixed red-blue light improved the carbohydrate content relative to blue light (Orefice et al. 2016), while some reported that the mixed blue-red light provides significantly lower soluble sugar content in lettuce leaves compared with the blue-red-white light and fluorescent light (Lin et al. 2013). Like crops, the carbohydrate accumulation and synthesis in C. reinhardtii in this study were delayed under the combination of blue and red-orange lights compared with that under red-orange light only.
For protein synthesis, it has been reported that mixed red-blue light provided higher protein content in lettuce leaves compared with the mixed blue-red-white light and fluorescent light, but the difference was not significant (Lin et al. 2013). For algae, the mixed red-blue light has also been reported to improve the protein content in S. marinoi relative to blue light only (Orefice et al. 2016).
It is difficult to explain why, under the B-RO mixing condition, the protein mass fraction decreased with the red-orange light intensity. One possible reason is that the blue and red lights have opposite effects on many algal metabolic processes, including protein synthesis. Like the examples mentioned above, blue light delays the cell division, and red light promotes cell division (Oldenhof et al. 2006), and blue light induces the protein and amino acid synthesis by consuming carbohydrate reserves, and red light promotes the carbohydrate synthesis (Wynne and Rhee 1986; Kamiya and Saitoh 2002; Schulze et al. 2014). Protein synthesis induced by blue light seems to be inhibited by the red light. It has been reported that blue light provided the highest levels of early light-inducible proteins (ELIPs) in pea plant leaves, while the addition of low-intensity red light (100–500 μmol photons m−2 s−1) significantly repressed the ELIPs synthesis (Adamska et al. 1992).
Conclusions
This study examined the effect of mixed blue and red-orange light on algal growth kinetics and biochemical profile. The adverse effect of light saturation under blue light and light inhibition under red-orange light on C. reinhardtii growth was significantly relieved by combining different light qualities and intensities. The growth kinetics increased significantly; protein and lipid mass fraction and/or concentration had a significant improvement with light intensity combination of B:RO = 45:45, 65:45, and 65:105 μmol photons m−2 s−1; and carbohydrate content had no apparent improvement. With the increase of red-orange light intensity in the mixed light, lipid, carbohydrate, and protein synthesis were repressed.
References
Adamska I, Ohad I, Kloppstech K (1992) Synthesis of the early light-inducible protein is controlled by blue light and related to light stress. Proc Nat Acad Sci U S A 89:2610–2613
Baer S, Heining M, Schwerna P, Buchholz R, Hübner H (2016) Optimization of spectral light quality for growth and product formation in different microalgae using a continuous photobioreactor. Algal Res 14:109–115
Burak H, Dunbar A, Gilmour DJ (2019) Enhancement of Dunaliella salina growth by using wavelength shifting dyes. J Appl Phycol 31:2791–2796
Casal JJ (2000) Phytochromes, cryptochromes, phototropin: photoreceptor interactions in plants. Photochem Photobiol 71:1–11
Chaplin MF, Kennedy JF (1994) Carbohydrate analysis: a practical approach. ILR Press, New York
Chen M, Tang H, Ma H, Holland TC, Ng KYS, Salley SO (2011) Effect of nutrients on growth and lipid accumulation in the green algae Dunaliella tertiolecta. Bioresour Technol 102:1649–1655
Cheng D, Li D, Yuan Y, Zhou L, Li X, Wu T, Wang L, Zhao Q, Wei W, Sun Y (2017) Improving carbohydrate and starch accumulation in Chlorella sp. AE10 by a novel two-stage process with cell dilution. Biotechnol Biofuels 10:75
Choi Y-K, Kumaran RS, Jeon HJ, Song H-J, Yang Y-H, Lee SH, Song K-G, Kim KJ, Singh V, Kim HJ (2015) LED light stress induced biomass and fatty acid production in microalgal biosystem, Acutodesmus obliquus. Spectrochim Acta A 145:245–253
de Mooij T, de Vries G, Latsos C, Wijffels RH, Janssen M (2016) Impact of light color on photobioreactor productivity. Algal Res 15:32–42
de Mooij T, Janssen M, Cerezo-Chinarro O, Mussgnug JH, Kruse O, Ballottari M, Bassi R, Bujaldon S, Wollman F-A, Wijffels RH (2015) Antenna size reduction as a strategy to increase biomass productivity: a great potential not yet realized. J Appl Phycol 27:1063–1077
Elmoraghy M, Farag IH (2012) Bio-jet fuel from microalgae: reducing water and energy requirements for algae growth. Int J Eng Sci 1:22–30
Esperanza M, Seoane M, Rioboo C, Herrero C, Cid Á (2019) Differential toxicity of the UV-filters BP-3 and BP-4 in Chlamydomonas reinhardtii: a flow cytometric approach. Sci Total Environ 669:412–420
Fu W, Guðmundsson Ó, Paglia G, Herjólfsson G, Andrésson ÓS, Palsson BØ, Brynjólfsson S (2013) Enhancement of carotenoid biosynthesis in the green microalga Dunaliella salina with light-emitting diodes and adaptive laboratory evolution. Appl Microbiol Biotechnol 97:2395–2403
Gameiro PA, Struhl K (2018) Nutrient deprivation elicits a transcriptional and translational inflammatory response coupled to decreased protein synthesis. Cell Rep 24:1415–1424
Guerlava P, Izac V, Tholozan JL (1998) Comparison of different methods of cell lysis and protein measurements in Clostridium perfringens: application to the cell volume determination. Curr Microbiol 36:131–135
Hegemann P (2008) Algal sensory photoreceptors. Annu Rev Plant Biol 59:167–189
Hernández R, Kubota C (2012) Tomato seedling growth and morphological responses to supplemental LED lighting red: blue ratios under varied daily solar light integrals. Acta Hortic 956:187–194
Kaiser E, Ouzounis T, Giday H, Schipper R, Heuvelink E, Marcelis LFM (2019) Adding blue to red supplemental light increases biomass and yield of greenhouse-grown tomatoes, but only to an optimum. Front Plant Sci 9:2002
Kamiya A, Saitoh T (2002) Blue-light-control of the uptake of amino acids and of ammonia in Chlorella mutants. Physiol Plant 116:248–254
Khan MI, Shin JH, Kim JD (2018) The promising future of microalgae: current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb Cell Factories 17:36–36
Ki-Ho S, Myung-Min O (2013) Leaf shape, growth, and antioxidant phenolic compounds of two lettuce cultivars grown under various combinations of blue and red light-emitting diodes. HortScience 48:988–995
Kim TH, Lee Y, Han SH, Hwang SJ (2013) The effects of wavelength and wavelength mixing ratios on microalgae growth and nitrogen, phosphorus removal using Scenedesmus sp. for wastewater treatment. Bioresour Technol 130:75–80
Kommareddy A, Anderson G (2004) Study of light requirements of a photobioreactor. North Central ASAE/CSAE Conference Presentation MB04-111, Winnipeg
Kou Z, Bei S, Sun J, Pan J (2013) Fluorescent measurement of lipid content in the model organism Chlamydomonas reinhardtii. J Appl Phycol 25:1633–1641
Leonardi RJ, Niizawa I, Irazoqui HA, Heinrich JM (2018) Modeling and simulation of the influence of fractions of blue and red light on the growth of the microalga Scenedesmus quadricauda. Biochem Eng J 129:16–25
Lima GM, Teixeira PCN, Teixeira CMLL, Filócomo D, Lage CLS (2018) Influence of spectral light quality on the pigment concentrations and biomass productivity of Arthrospira platensis. Algal Res 31:157–166
Lin K-H, Huang M-Y, Huang W-D, Hsu M-H, Yang Z-W, Yang C-M (2013) The effects of red, blue, and white light-emitting diodes on the growth, development, and edible quality of hydroponically grown lettuce (Lactuca sativa L. var. capitata). Sci Hortic 150:86–91
Massa GD, Emmerich JC, Morrow RC, Bourget CM, Mitchell CA (2007) Plant-growth lighting for space life support: a review. Gravitat Space Res 19:19–29
Matula EE, Nabity JA (2019) Failure modes, causes, and effects of algal photobioreactors used to control a spacecraft environment. Life Sci Space Res 20:35–52
Meijer G, Engelsma G (1965) The synergistic influence of a pre-irradiation on the photoinhibition of gherkin seedlings. Photochem Photobiol 4:251–258
Mitchell CA, Both A-J, Bourget C, Burr J, Kubota C, Lopez R, Morrow R, Runkle E (2012) LEDs: The future of greenhouse lighting! Chronica Hortic 52:6–12
Nhut DT, Nam NB (2010) Light-Emitting Diodes (LEDs): An artificial lighting source for biological studies. In: Van Toi V, Khoa TQD (eds) The Third International Conference on the Development of Biomedical Engineering in Vietnam. Springer, Berlin, pp 134–139
Nwoba EG, Parlevliet DA, Laird DW, Alameh K, Moheimani NR (2019) Light management technologies for increasing algal photobioreactor efficiency. Algal Res 39:101433
Oldenhof H, Zachleder V, Van Den Ende H (2006) Blue- and red-light regulation of the cell cycle in Chlamydomonas reinhardtii (Chlorophyta). Eur J Phycol 41:313–320
Olle M, Viršile A (2013) The effects of light-emitting diode lighting on greenhouse plant growth and quality. Agric Food Sci 22:223–234
Orefice I, Chandrasekaran R, Smerilli A, Corato F, Caruso T, Casillo A, Corsaro MM, Piaz FD, Ruban AV, Brunet C (2016) Light-induced changes in the photosynthetic physiology and biochemistry in the diatom Skeletonema marinoi. Algal Res 17:1–13
Pereira S, Otero A (2019) Effect of light quality on carotenogenic and non-carotenogenic species of the genus Dunaliella under nitrogen deficiency. Algal Res 44:101725
Pereira S, Otero A (2020) Haematococcus pluvialis bioprocess optimization: effect of light quality, temperature and irradiance on growth, pigment content and photosynthetic response. Algal Res 51:102027
Ra CH, Sirisuk P, Jung J-H, Jeong G-T, Kim S-K (2018) Effects of light-emitting diode (LED) with a mixture of wavelengths on the growth and lipid content of microalgae. Bioprocess Biosyst Eng 41:457–465
Ramanna L, Rawat I, Bux F (2017) Light enhancement strategies improve microalgal biomass productivity. Renew Sust Energ Rev 80:765–773
Rumin J, Bonnefond H, Saint-Jean B, Rouxel C, Sciandra A, Bernard O, Cadoret J-P, Bougaran G (2015) The use of fluorescent Nile red and BODIPY for lipid measurement in microalgae. Biotechnol Biofuels 8:42
Schulze PS, Pereira HG, Santos TF, Schueler L, Guerra R, Barreira LA, Perales JA, Varela JC (2016) Effect of light quality supplied by light emitting diodes (LEDs) on growth and biochemical profiles of Nannochloropsis oculata and Tetraselmis chuii. Algal Res 16:387–398
Schulze PSC, Barreira LA, Pereira HGC, Perales JA, Varela JCS (2014) Light emitting diodes (LEDs) applied to microalgal production. Trends Biotechnol 32:422–430
Severes A, Hegde S, D'Souza L, Hegde S (2017) Use of light emitting diodes (LEDs) for enhanced lipid production in micro-algae based biofuels. J Photochem Photobiol B 170:235–240
Sirisuk P, Ra C-H, Jeong G-T, Kim S-K (2018) Effects of wavelength mixing ratio and photoperiod on microalgal biomass and lipid production in a two-phase culture system using LED illumination. Bioresour Technol 253:175–181
Tan W (2018) ROI-Integrated commercialization: an adaptive pathway for microalgae technology. Ind Biotechnol 14:17–24
Vadiveloo A, Moheimani NR, Cosgrove JJ, Bahri PA, Parlevliet D (2015) Effect of different light spectra on the growth and productivity of acclimated Nannochloropsis sp. (Eustigmatophyceae). Algal Res 8:121–127
Velmurugan N, Sung M, Yim SS, Park MS, Yang JW, Jeong KJ (2013) Evaluation of intracellular lipid bodies in Chlamydomonas reinhardtii strains by flow cytometry. Bioresour Technol 138:30–37
Wang X, Fosse HK, Li K, Chauton MS, Vadstein O, Reitan KI (2019) Influence of nitrogen limitation on lipid accumulation and EPA and DHA content in four marine microalgae for possible use in aquafeed. Front Mar Sci 6:95
Wells ML, Potin P, Craigie JS, Raven JA, Merchant SS, Helliwell KE, Smith AG, Camire ME, Brawley SH (2017) Algae as nutritional and functional food sources: revisiting our understanding. J Appl Phycol 29:949–982
Wynne D, Rhee G (1986) Effects of light intensity and quality on the relative N and P requirement (the optimum N:P ratio) of marine planktonic algae. J Plankton Res 8:91–103
Li X, Huff J, Crunkleton DW, Johannes TW (2020) Light saturation and inhibition of Chlamydomonas reinhardtii under three light qualities, growth kinetics, and nutrient composition Algal Res, Manuscript submitted for publication
Li X, Slavens S, Crunkleton DW, Johanns TW (2021) Interactive effect of light quality and temperature on Chlamydomonas reinhardtii growth kinetics and lipid synthesis. Algal Res 53:1–2127
Yan C, Zheng Z (2014) Performance of mixed LED light wavelengths on biogas upgrade and biogas fluid removal by microalga Chlorella sp. Appl Energy 113:1008–1014
Yan C, Zhu L, Wang Y (2016) Photosynthetic CO2 uptake by microalgae for biogas upgrading and simultaneously biogas slurry decontamination by using of microalgae photobioreactor under various light wavelengths, light intensities, and photoperiods. Appl Energy 178:9–18
Zaiontz C (2020) Real Statistics Using Excel. www.real-statistics.com. Accessed Aug 2020
Zhang Y, Hu W, Peng X, Sun B, Wang X, Tang H (2018) Characterization of anthocyanin and proanthocyanidin biosynthesis in two strawberry genotypes during fruit development in response to different light qualities. J Photochem Photobiol B 186:225–231
Zhao B, Su Y (2014) Process effect of microalgal-carbon dioxide fixation and biomass production: a review. Renew Sust Energ Rev 31:121–132
Zhao Y, Sun S, Hu C, Zhang H, Xu J, Ping L (2015) Performance of three microalgal strains in biogas slurry purification and biogas upgrade in response to various mixed light-emitting diode light wavelengths. Bioresour Technol 187:338–345
Funding
This work was supported by NASA (grant number 15-EPSCoR2015-0026).
Author information
Authors and Affiliations
Contributions
XL conceived the research and designed the experiments. XL performed the experiments, analyzed, and interpreted the data. XL wrote the original draft. TWJ, and DC, funding acquisition, supervised and edited the manuscript. JM assisted the experiment and edited the manuscript. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, X., Manuel, J., Crunkleton, D.W. et al. Effect of blue and red-orange LEDs on the growth and biochemical profile of Chlamydomonas reinhardtii. J Appl Phycol 33, 1367–1377 (2021). https://doi.org/10.1007/s10811-021-02411-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-021-02411-5