Abstract
Vinasse is a residue of the sugarcane industry. It can be biodigested or not, in this case referred as conventional. The conventional or biodigested vinasses have high content of organic matter and mineral elements, leading to their common use as soil fertilizer for the sugarcane crop. However, vinasses are toxic residues and they can salinize the soil if used too much. On the other hand, the production of photosynthetic microalgae is costly and using a residue to support its growth may contribute to cost reduction. However, because of the vinasse dark color and toxicity, high dilution is necessary to accomplish microalgal growth. Here we present results on the growth and biomass yield of Chlorella vulgaris in conventional and in biodigested vinasses that have been treated by filtration or centrifugation before their use as microalgae culture medium. A concentration range of 10 to 100 % was tested and microalgal growth occurred in vinasse concentration as high as 80 %, with no nutrient addition. We evaluated pH, electrical conductivity, absorbance at 570 nm, and cell density every 24 h in a 6-day incubation experiment. Specific growth rates were calculated and the results showed that in 60 % filtered conventional and 80 % biodigested vinasses, C. vulgaris performed as well as the controls in nutrient rich synthetic culture media, with growth rates of up to 1.2 day−1. Thus, we propose the use of treated vinasse as culture medium for lowering the costs of microalgae production, with the advantage of increasing the residue value.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ethanol is a biofuel produced from the fermentation of carbohydrate rich plants, such as sugar cane, sugar beet, soybean, and corn. After the oil crisis in 1975, the Brazilian production of ethanol from sugar cane has increased. As a consequence, the generation of its waste product, called vinasse, has also increased. In spite of the investments in new technologies to increase the ethanol productivity decreasing the residue generation, 12 to 18 L of vinasse are still produced per liter of ethanol. It has been registered an increasing demand for ethanol as biofuel, leading to the production of 286 × 1011 L vinasse in 2014 (Brasil 2015).
Vinasse is a dark brown liquid that has high turbidity, high content of organic matter and mineral nutrients, high biochemical oxygen demand and low pH (Silva et al. 2007). It is fetid and corrosive. This residue can be toxic and difficult to dispose of, with a contaminating potential about one hundred times greater than domestic sewage. Its disposal in aquatic environments is harmful to the microorganisms and wildlife in general (Silva et al. 2007). As a consequence, its release into water bodies is prohibited, forcing industries to seek ways for its disposal. An alternative has been its use as fertilizer in sugar cane crops from the alcohol production plant itself because of its high nutrient and organic matter contents. This use ends up stimulating the soil microbial activity and provides economy of fertilizers and water in the sugar cane crops. However, Silva et al. (2007) showed that such practice can result in soil salinization because of the high content of potassium in the vinasse. In addition, this can lead to groundwater contamination, turning such excessive application into a problem, not a solution for the residue disposal.
Vinasse can be biodigested in the alcohol production plants, a technology that, at the same time, modifies vinasse and generates energy for the sugar and ethanol production (Szymansky et al. 2010). Vinasse biodigestion is an anaerobic fermentation whose main product is a biogas composed of methane, carbon dioxide, nitrogen, water vapor and hydrogen sulfide. This biogas has the advantages of lower price and less polluting potential in comparison with fossil fuel, in addition to being a renewable resource (Szymansky et al. 2010). Literature has shown that burning this biogas can make the ethanol production plant sustainable in relation to its electricity consumption (Lamonica 2006), since this technique allows the industry to generate all the energy required. So, this practice is increasing. However, the vinasse inorganic nutrients persist after its biodigestion. Therefore, a proper destination for this residue, the biodigested vinasse, is still required.
Microalgae are a known source of carotenoids, carbohydrates, vitamins, unsaturated fatty acids, and other bioactive substances (Borowitzka 2013). In addition, they promote CO2 fixation through photosynthesis, a desirable ability related to the greenhouse effect. However, microalgae production is expensive and demands high volumes of water and requirements of energy (Pires et al. 2013). Thus, growing them using a residue as a complement in the culture medium can reduce costs and save water (Pires et al. 2013). Because vinasses not biodigested (conventional) or biodigested are liquid and rich in nutrients, their use as substrate for microalgae growth can be a means for reducing the residue’s eutrophic potential, at the same time that decreases costs in microalgae production.
Oliveira (1988) was a pioneer on the cultivation of microalgae in vinasse. She used 0.1 to 0.5 % conventional (not biodigested) vinasse mixed with artificial culture medium to grow Chlorella vulgaris. The author observed heterotrophic growth of C. vulgaris in the absence of light and mixotrophic growth under illumination. It indicates the importance of both mineral nutrients and organic materials provided by vinasse in the growth of this alga. Recently, Marques et al. (2013) demonstrated that diluting biodigested vinasse with wastewater at the concentration of 0.2 % enabled the growth of C. vulgaris. Confirming these results, Vieira (2013) showed that vinasse is better than glycerol for the mixotrophic growth of microalgae.
In common, studies about microalgae cultures in vinasse have been achieved at high dilution of the residue. In most cases, the conventional or biodigested vinasses are used at 0.1–20 % concentrations, making this use not a solution for the disposal of the residue. We consider that this is an indication that previous treatment of the conventional or biodigested vinasses can improve their quality and support better microalgae growth.
This research aimed at growing the chlorophyte microalga C. vulgaris in high concentrations of pretreated conventional or biodigested vinasses from the sugar cane industry. A filtration in smectite clay and, after, in activated charcoal was performed in the raw vinasses, making them less dark, allowing light to enter in the medium and increasing the photosynthetic activity of the microalgae. Conventional and biodigested filtered vinasses were compared as medium to support microalgal growth and cell yield.
Material and methods
The strain of Chlorella vulgaris LBA 01, used in this research, was isolated from the Sewage Treatment Station (STS) of São Carlos, an organic rich environment. It was maintained in the culture collection of the Algae Biotechnology Laboratory at the Federal University of São Carlos (UFSCar) in LC Oligo culture medium (AFNOR 1980).
Conventional (vinasse without biodigestion) and biodigested vinasses were used for the experiments. They were obtained from Usina São Martinho (São Paulo, Brazil), an alcohol production plant, approximately 30 days after the beginning of the harvest of vinasse generation. Polypropylene flaks (20 L) were used for vinasse collection (40 L of each vinasse). The flasks were washed with neutral detergent and 10 % HCl, and rinsed with distilled water. After collection vinasses were let cool to approximately 40 °C. Then they were distributed into previously washed 1 L polypropylene bottles and then frozen at −8 °C until use. Due to the high organic matter content of the vinasses (Silva et al. 2007), freezing is necessary to avoid degradation of the material.
Before use, both conventional and biodigested vinasses were subjected to one of the two treatments described below in an attempt to clarify them. One of the treatments consisted of filtering the conventional or biodigested vinasses through a mixture of smectite clay and coarse sand and then through activated charcoal (Synth, Brazil) as described in a patent resulting from our work (Candido and Lombardi 2016). The other vinasses clearance treatment was centrifugation at 3920×g for 10 min, 10 °C. According to preliminary tests, the speed and time of centrifugation were the minimum necessary to separate the yeast from the vinasse, resulting in reduced competition of this microorganism with microalgae of interest in cultures. Before microalgae inoculation, the vinasses were defrosted and submitted to one of the two described clearance treatments. Both the filtration and centrifugation procedures removed most of the yeast, resulting in cleaner residues. Yeast cell counts revealed that about 105 cells mL−1 were originally present in the conventional vinasse while 108 cells mL−1 were detected in the biodigested one. The filtration or centrifugation treatments removed more than 95 % of these yeasts. In this way, such procedures have made the vinasses more appropriate media for microalgae growth. Figure 1 shows the treatments of the conventional and biodigested vinasses.
Experimental cultures were grown in 250-mL flasks containing 150 mL of non-sterilized medium, which consisted of treated conventional or treated biodigested vinasses at 10, 20, 30, 40, 50, 60, 70, 80, 90, and 100 % concentrations, which were achieved with distilled water. No extra nutrients were added to the media. Controls were performed in LC Oligo synthetic media (AFNOR 1980), which contained no vinasse. As a reference of high microalgae cell yield, cultures of C. vulgaris were also grown in BG11 culture medium (Rippka et al. 1979) used as a second control. This is a highly nutritive medium commonly used in large-scale microalgal cultures because of the high biomass yield it supports. Four experimental treatments were performed considering the concentrations mentioned above: filtered conventional vinasse, centrifuged conventional vinasse, filtered biodigested vinasse, and centrifuged biodigested vinasse. Vinasses were not sterilized, but the distilled water used for the vinasses dilutions and the controls was sterile (autoclave, 121 °C, 20 min).
Controls and treatments had the pH adjusted to 7.0 before inoculating to achieve a better microalgal growth. The cultivations were carried out in a residue that allows coexistence of contaminants (bacteria and fungi, including yeast) that results in acidification of the medium, so the pH needed to be adjusted to ∼7.0; otherwise, an extended lag phase would be observed. Although C. vulgaris grows well in mildly acidic—near neutrality pH (Mayo 1997), adjusting the pH was important to avoid a major impact on the microalgae. However, it should be mentioned here that vinasses can have an inherent buffering effect (Lopes et al. 2013), so it takes more alkaline or acidic solutions in comparison to controls to adjust its pH. Three experimental replicates were performed for all conditions described.
Cultures lasted 6 days and the experiments had 5 × 104 cells mL−1 initial cell density, whose inoculants were obtained from exponentially growing cultures. Inoculum was obtained from physiologically activated algal cells that were obtained through three transfers of microalgae culture in LC Oligo medium in their exponential phase. Experiments were kept under controlled conditions of temperature (24 ± 1 °C) and light intensity (150 μmol photons m−2 s−1) inside the flasks, obtained by varying the distance between the flasks and the light sources depending on the concentrations of vinasse.
Cultures were monitored for pH (Logen Scientific pH meter, Brazil), conductivity (Hach HQ, Multimeter, USA), cell density and optical density at 570 nm (FEMTO Scan 800 XI spectrophotometer, Brazil). Although unusual, the 570 nm wavelength was used because it is related with total particulate material in the medium, being algal cells or other non-chlorophyll containing contaminants, such as bacteria and fungi, including yeast (Costa et al. 2003; Lourenço 2006). Cell density was measured by cell counts using a Fuchs-Rosenthal chamber under an optical microscope (Nikon Eclipse E200, Japan). Formaldehyde at 4 % was added to fresh culture aliquots, preserving cells to be counted later. Growth rates were obtained by plotting the natural logarithm of cell density (cell mL−1) as dependent variable vs time (days) as independent variable. This resulted in straight lines during the exponentially growing phases that were determined by linear regression. The slopes of the linear regression equations represent the specific growth rates.
Physicochemical characterization of conventional and biodigested vinasses in their raw, centrifuged and filtered forms before being used in the cultures were carried out at the ASL Environmental Analysis, Laboratory St. Luke, Rio Claro/SP, Brazil. The analyses of vinasses were also performed after cultivation in the conditions that allowed the best microalgal growth, e.g., the 60 % conventional and 80 % biodigested filtered vinasses.
Data were plotted using the program Origin 8.5. Statistical analysis was performed using the program R. Result comparison was performed using the ANOVA and Tukey tests (p < 0.05).
Results
The mean values of pH and optical density at 570 nm for conventional and biodigested vinasses in their raw and treated forms before the beginning of the experiments, as well as the physicochemical characterization of the vinasses are reported in Table 1. In general, the filtering process promoted a greater reduction of minerals than the centrifugation, and the algal cultivation caused further reductions in these values. The conventional vinasse pH was acid, but near neutral for the biodigested vinasse in their raw forms. However, after the filtering process, the pH increased. High absorbances at 570 nm, which indicates the presence of particulate material (Costa et al. 2003; Lourenço 2006), can be a problem for photosynthetic microalgae growth due to reduction in light penetration in cultures. After filtering vinasses, a 90 % decrease in 570 nm absorbance was obtained.
Figure 2 shows the daily pH variation, represented by the average values from all the concentrations of each treatment, as function of time. It shows an initial pH decrease for the conventional vinasses (filtered or centrifuged), but an increase in the biodigested ones (filtered or centrifuged).
pH. Daily average of pH values as function of time (days) for the (×) control in LC Oligo and the vinasse treatments: (○) filtered conventional vinasse, (●) centrifuged conventional vinasse, (□) filtered biodigested vinasse and (■) centrifuged biodigested vinasse. Error bars represent the standard deviation of n = 3
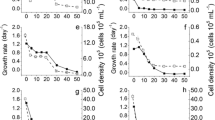
Figure 3 shows the mean values of conductivity and absorbance in 570 nm for the cultures. The conductivity values varied in accordance with the vinasses concentrations. The higher the vinasse concentration, the higher the conductivity value. However, as observed in the experiments, they remained constant in all treatments throughout the experimental period. In the controls it ranged from 370 to 420 μS cm−2, while in the highest vinasse concentration, it reached 14,000 μS cm−2 in filtered vinasses at 100 %.
Conductivity and particulate material. Mean values of a conductivity (μS cm−2) and b particulate material detected as absorbance at 570 nm (arbitrary unit) at the 6th culture day for the (×) LC Oligo control and the vinasses treatments (○) filtered conventional vinasse, (●) centrifuged conventional vinasse, (□) filtered biodigested vinasse and (■) centrifuged biodigested vinasse during the experiments. Error bars represent the standard deviation of n = 3
The absorbance at 570 nm suggested an increase of the particulate material in the cultures as function of concentration of vinasses and experimental time. As observed under an optical microscope, these absorbance values are mainly due to contaminants, such as fungi and bacteria, which develop further in increasing concentration of vinasse.
Figure 4 shows that 80 and 100 % filtered biodigested vinasse supported the best exponential growth of C. vulgaris in filtered vinasses. This treatment had a 1-day lag phase only, whereas most filtered conventional vinasse cultures presented longer lag phase.
Growth curves. Natural logarithm of the average values of cell densities (× 105 cells mL−1) for the controls in (×) LC Oligo and (+) BG11 and for the treated vinasses concentrations of: (  ) 10 %, (
) 10 %, (  ) 20 %, (
) 20 %, (  ) 30 %, (
) 30 %, (  ) 40 %, (
) 40 %, (  ) 50 %, (
) 50 %, (  ) 60 %, (
) 60 %, (  ) 70 %, (
) 70 %, (  ) 80 %, (
) 80 %, (  ) 90 %, and (
) 90 %, and (  ) 100 %. a Filtered conventional vinasse, b centrifuged conventional vinasse, c filtered biodigested vinasse, d centrifuged biodigested vinasse. Error bars represent the standard deviation of n = 3
) 100 %. a Filtered conventional vinasse, b centrifuged conventional vinasse, c filtered biodigested vinasse, d centrifuged biodigested vinasse. Error bars represent the standard deviation of n = 3
Figure 5 shows that the highest final biomass yield was obtained for 10–20 % centrifuged conventional vinasse. However, considering higher vinasse concentrations, filtered biodigested vinasse showed best results, with high algal growth in concentrations of up to 100 %.
Final cell yield. Cell yield (× 105 cells mL−1) for the controls in (×) LC Oligo and (+) BG11 and for vinasses treatments: (○) filtered conventional vinasse, (●) centrifuged conventional vinasse, (□) filtered biodigested vinasse, and (■) centrifuged biodigested vinasse on the 6th culture day. Error bars represent the standard deviation of n = 3
Figure 6 shows that, generally, higher growth rates were obtained in C. vulgaris cultures kept in filtered vinasses in comparison with cultures at the centrifuged ones, what applies to both conventional and biodigested vinasses. With a growth rate of 0.9 day−1, 5.0 × 105 cells mL−1 were obtained in the last day of the LC Oligo control. Already in the BG11 control, the growth rate of 1.1 day−1 provided 1.5 ×6 cells mL−1 by the end of the experiment. These results suggest that the best growth conditions for C. vulgaris were provided by the 80–100 % filtered biodigested vinasse. The centrifugation procedure supported comparable growth rates, but at lower vinasse concentrations, from 10 to 30 %.
Growth rates (day−1) for the controls in (×) LC Oligo and (+) BG11 and for the vinasses treatments with a conventional vinasse and b biodigested vinasse. Black bars refer to the cultures in filtered vinasses and white bars in the centrifuged vinasses. Error bars represent the standard deviation of n = 3. Similar letters above bars indicate values do not differ significantly (ANOVA, p > 0.05)
In general, the filtering promoted a greater reduction of minerals than centrifugation. The algal cultivation caused further reductions in these values.
Discussion
The treatment of filtration from conventional and biodigested vinasses resulted in cleaner residues that supported C. vulgaris growth as well as the controls in LC Oligo and BG11. It clarified and increased the vinasse pH. The present results agree with those of Cogo (2011), which showed that the adsorptive capacity of smectite clay is effective for pigment removal from aqueous solutions. Similarly, the activated charcoal which can have basic or acid adsorptive properties (Legrouri et al. 2005) may have contributed for the residue transformations from dark brown acid liquids to cleared near neutral pH residues, suitable for microalgal growth. The properties of activated charcoal have been investigated by Pereira et al. (2008). They reported that activated charcoal can purify and clarify liquids and gases, being commonly used in waste treatment and industrial processes. In addition, a direct relation between absorbance at 570 nm and particulate material has been demonstrated in Costa et al. (2003). As the filtering reduced the values of this parameter, we can assign to this process of filtration the ability to retain particulate materials from vinasses.
Chlorella vulgaris growth in the control lead to the usual pH increase observed in synthetic culture medium in the first 3 days of culture, while in the vinasses, this was observed when the microalgae reached the exponential growth phase. According to Raven (2007), this indicates a positive balance of photosynthesis in relation to respiration. However, the tendency observed in the vinasse pH to return to its original values during the first two days, from near pH 4 for the conventional and pH 8 for the biodigested vinasse, signals the buffering effect of the residue that was noticed while adjusting the pH in the beginning of the experiment. This buffering effect has been confirmed by Lopes et al. (2013) who used vinasse in soil and detected increased soil pH buffering in the presence of the residue. On subsequent days, the increase in culture pH, especially those in filtered vinasses, corresponded to the algal growth and photosynthetic activity, which were higher than the respiration of the contaminants.
The conductivity increase related with the vinasses concentrations are in agreement with literature information. Kadioglu and Algur (1992) reported that the organic matter and mineral contents of vinasse are responsible for their high conductivity. According to the authors, this may cause osmotic problems for freshwater organisms, which can further lead to the degradation of the cells. In the present work, we observed that in conventional and biodigested centrifuged vinasses, the more concentrated the media, the lower the growth rates. This may indicate that, in these treatments, increases in concentrations of vinasse and, consequently, in the conductivity of the medium, inhibited algal development. In filtered biodigested vinasse at 80 %, our best growth condition, the conductivity was 11 mS cm−1. In the 90 and 100 % concentrations, in which there was a small reduction in cell growth, the conductivity was 12.5–13 mS cm−1. The difference in the conductivity values together with the different results related to C. vulgaris growth in the 80, 90, and 100 % vinasses, suggest the growth inhibition at the two highest vinasse concentrations can be due to the conductivity of the medium.
The absorbance in 570 nm was higher in higher vinasse concentration, but it does not correspond to increased algal growth, particularly in the centrifuged vinasse cultures. This parameter is associated with the total number of cells and particulate material (Costa et al. 2003; Lourenço 2006), so the more vinasse in the medium, the higher the value obtained at 570 nm and the higher the contamination. Despite C. vulgaris being a good competitor (Safi et al. 2014), excessive amounts of contaminants can make it expend resources that could otherwise be invested in reproduction. It is difficult to control contamination in microalgae cultures kept in residues (Costa and de Morais 2013). Thus, algal growth in residues depends on a balance between the amount of contaminants and the availability of growth factors, such as nutrients and light. Furthermore, due to its own competitiveness, C. vulgaris may control contamination. The absorbance analysis (570 nm) showed that contamination is higher in media where vinasses were centrifuged (and not filtered), which at high concentrations may have reduced light penetration, so affecting algal growth.
In general, the filtered vinasses allowed better microalgal growth, resulting in higher cell yield in comparison with the centrifuged vinasses. Within the filtered vinasses, the 60 % conventional and 80 % biodigested vinasses yielded the highest cell density. Filtering vinasses enabled increased algal growth in higher concentrations of vinasse than the centrifugation process, especially with biodigested vinasse. This is probably due to higher light penetration because of lower particulate material. Furthermore, the best algal growth in biodigested vinasse confirms the results of Marques et al. (2013) who showed that the anaerobic digestion pretreatment of vinasse allowed an increase in the production of C. vulgaris.
The present results are in agreement with others in the literature that showed that treated vinasse supports better growth of photosynthetic microalgae than the untreated residue. Mitra et al. (2012) observed C. vulgaris growth in settled and siphoned corn vinasse, using the supernatant at 100 % concentration as culture medium. Similar to our treatment, theirs sharply decreased the turbidity of corn vinasse. Using diluted raw vinasses, Marques et al. (2013) obtained growth rates of 0.76 day−1 for C. vulgaris in 0.2 % of raw biodigested vinasse diluted with wastewater. Just as in this present study, they obtained better algae response in biodigested than in conventional vinasse. However, while the authors used the concentration of only 0.2 % of raw biodigested vinasse, we used media with 80 % filtered biodigested vinasse, resulting in an increase of approximately 64 % in the growth rates.
Most investigations on microalgae growth in untreated vinasse use highly diluted solutions, as was the case of the pioneer study of Oliveira (1988). The author used 0.1–0.5 % sugar cane vinasse in synthetic culture medium to grow C. vulgaris and identified heterotrophic and mixotrophic growth. Similarly, Budiyono et al. (2014) grew Spirulina platensis in 0.8 % biodigested vinasse, with higher concentrations being toxic to this microalgae. The authors observed cell membrane and photosynthetic pigment degradations that were responsible for reducing the cyanobacterial growth rate to 0.15 day−1. Barrocal et al. (2010) optimized Spirulina maxima growth in 0.5 % beet vinasse in Schlösser culture medium, increasing the cyanobacterial biomass from 3.5 g L−1 in the control to 4.8 g L−1. More recently, Coca et al. (2015) obtained high concentrations of proteins in S. platensis with the equivalent of 0.01 % of vinasse in synthetic culture medium. Olguín et al. (2015), using media with 6 % digested vinasse complemented with sodium bicarbonate, obtained an increase in total lipid in Neochloris oleoabundans biomass of up to 38 %, signaling a possible destiny as biofuel.
The results in Table 1, in which the filtering process caused greater nutrient removal than centrifugation in addition to the higher light penetration, are an indication that the filtering process favored algal development. According to Kadioglu and Algur (1992), the high amount of organic matter and mineral nutrients in vinasse causes osmotic effects in the algal cells, which can restrict their development. Additionally, algal growth in vinasse further contributed to the consumption of the inorganic and possibly, the organic substances, decreasing the eutrophication and polluting potential of the residue (Kadioglu and Algur 1992; Olguín et al. 2015; Santos et al. 2016). Similar to what we have shown for C. vulgaris, Mattos and Bastos (2016) demonstrated that Desmodesmus sp. cultured in vinasse reduced nitrogen and chemical oxygen demand in the residue. Given that the main limitation to the vinasse application on sugar cane crops soil is its high content of potassium, which can salinize the soil, reduction of this nutrient up to 50 % promoted by the filtering process and algal growth can expand the subsequent use of the treated waste in the soil.
The present results showed that the growth pattern of C. vulgaris in the vinasses showed a characteristic adaptation or lag phase before the exponential growth larger than in the controls. However, in spite of the lag phase, the best conditions of treated vinasses rendered higher final biomass than the LC Oligo control. We obtained a C. vulgaris increase of 32 times, considering the inoculated cell concentration and final cell yield, in these best conditions. Vieira (2013) grew Chlorella sp. in 10 % vinasse and obtained a 3.5 times increase of cell density, while Barrocal et al. (2010) obtained an increase of 1.4 times only. So, the results represented in this work include a higher cell increase than those reported in literature so far.
In conclusion, specific growth rates for C. vulgaris in filtered biodigested vinasse at 80 % and in filtered conventional vinasse at 60 % were as high as that obtained in the control in BG11 (1.1 day−1) and in the same vinasses centrifuged, but at much lower concentrations. It is the highest growth rate obtained so far for C. vulgaris grown in vinasse. Filtering the vinasses through smectite clay and activated charcoal allowed better microalgal growth at higher vinasse concentrations than in centrifuged vinasses. The highest C. vulgaris cell yield was obtained in 80 % filtered biodigested vinasse being followed by the 60 % filtered conventional vinasse.
Based in the present results, which showed that the vinasse filtering technique is effective as conventional and biodigested vinasses treatment for their use as microalgae culture media, we suggest it as a promising strategy to reduce the costs of microalgae production industry. In addition, it has the advantage of valorizing these residues. However, an optimization of the filtering process is still necessary for its application in industrial scale.
Specific studies on C. vulgaris biomass produced in vinasse can better direct the application of both products, the algae that is a known source of proteins and lipids along with other molecules, and the vinasse that will have lower nutrient content so resulting in less problems of soil salinization when applied to sugarcane crops as fertilizer.
References
AFNOR–Association Française of Normalization (1980) Essais des eaux. Determination of inhibition of Scenedesmus subspicatus par une substance. Norme Experimentale T90–304
Barrocal VM, García-Cubero MT, González-Benito G, Coca M (2010) Production of biomass by Spirulina maxima using sugar beet vinasse in growth media. New Biotech 27:851–856
Borowitzka MA (2013) High-value products from microalgae—their development and commercialisation. J Appl Phycol 25:743–756
Brasil. Ministério da Agricultura, Pecuária e Abastecimento (2015). Anuário estatístico da agroenergia/Ministério da Agricultura, Pecuária e Abastecimento. – Brasília: Mapa/ACS, 2009; 160p. http://www.agricultura.gov.br/arq_editor/file/Desenvolvimento_Sustentavel/Agroenergia/anuario_agroenergia_web_2012.pdf. Acessed 20 June 2015.
Budiyono IS, Sumardiono S, Sasongko SB (2014) Production of Spirulina platensis biomass using digested vinasse as cultivation medium. Trends in applied sciences. Research 9(2):93–102
Candido C, Lombardi AT (2016) The growth of Chlorella vulgaris in filtered vinasse. Rev Bras de Ciên Ambientais 35:55–62
Coca M, Barrocal VM, Lucas S, González-Benito G, García-Cubero MT (2015) Protein production in Spirulina platensis biomass using beet vinasse-supplemented culture media. Food Bioprod Process 94:306–312
Cogo JM (2011) Caracterização e funcionalização de argila esmectita de alteração basáltica e utilização na remoção de corante com processo de adsorção. Dissertation, Federal University of Mato Grosso, Instituto de Ciências Exatas e da Terra
Costa JAV, de Morais MG (2013). An open pond system for microalgal cultivation. In: Pandey A, Lee DJ, Chisti Y, Soccol CR. (eds) Biofuels from algae, Elsevier Amsterdam pp 1–22
Costa PHA, Silva JV, Bezerra MA, Enéas Filho J, Prisco JT, Gomes Filho E (2003) Growth and organic and inorganic solute contents in NaCl-stressed cultivars of Vigna unguiculata. Rev Bras Bot 26:289–297
Kadioglu A, Algur OF (1992) Tests of media with vinasse for Chlamydomonas reinhardii for possible reduction in vinasse pollution. Bioresour Technol 42:1–5
Lamonica, HM (2006) Potencial de geração de excedentes de energia elétrica com o biogás produzido a partir da biodigestão da vinhaça na indústria sucro-alcooleira brasileira. Encontro de energia no meio rural. Campinas: 6ª ed. http://www.proceedings.scielo.br/scielo.php?pid=MSC0000000022006000200027& script =sci_arttext. Acessed 22 June 2015
Legrougi K, Khouya E, Ezzine M, Hannache H, Denoyel R, Paller R, Naslain R (2005) Production of activated carbon from a new precursor molasses by activation with sulfuric acid. J Hazard Mat 118:259–263
Lopes O, Costa L, Lopes-Assad ML (2013) Solubilização de pó de basalto por meio de vinhaça: variação de pH e nutrientes disponíveis. Engenharia Ambiental: Pesquisa e Tecnologia 10:175–188
Lourenço SO (2006) Cultivo de microalgas marinhas: princípios e aplicações. RiMa, São Carlos Brazil
Marques SSI, Nascimento IA, de Almeida PF, Chinalia FA (2013) Growth of Chlorella vulgaris on sugarcane vinasse: the effect of anaerobic digestion pretreatment. Appl Biochem Biotechnol 171:1933–1943
Mattos LFA, Bastos RG (2016) COD and nitrogen removal from sugarcane vinasse by heterotrophic green algae Desmodesmus sp. Desalin Water Treat 57:9465–9473
Mayo AW (1997) Effects of temperature and pH on the kinetic growth of unialga Chlorella vulgaris cultures containing bacteria. Water Environ Res Env Res 69:64–72
Mitra D, Van Leeuwen JH, Lamsal B (2012) Heterotrophic/mixotrophic cultivation of oleaginous Chlorella vulgaris on industrial co-products. Algal Res 1:40–48
Olguín EJ, Dorantes E, Castillo OS, Hernández-Landa VJ (2015) Anaerobic digestates from vinasse promote growth and lipid enrichment in Neochloris oleoabundans cultures. J Appl Phycol 27:1813–1822
Oliveira HT (1988) Utilização de vinhaça como meio de cultura para Chlorella vulgaris (CCAP – 211/11b). Dissertation. Federal University of São Carlos
Pereira E, Oliveira LCA, Vallone A, Sapag K, Pereira M (2008) Preparação de carvão ativado em baixas temperaturas de carbonização a partir de rejeitos de café: utilização de FeCl3 como agente ativante. Química Nov 31:1296–1300
Pires JCM, Alvim-Ferraz MCM, Martins FG, Simões M (2013) Wastewater treatment to enhance the economic viability of microalgae culture. Environ Sci Pollut Res 20:5096–5105
Raven, PH (2007). Vegetal physiology. Guanabara Koogan: 7th ed.
Rippka R, Deruelles J, Waterbury J, Herdman M, Stanier R (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 111:1–61
Safi C, Zebib B, Merah O, Pontalier PY, Vaca-Garcia C (2014) Morphology, composition, production, processing and applications of Chlorella vulgaris: a review. Renew Sust Energ Rev 35:265–278
Santos RR, Araújo OQF, Medeiros JL, Chaloub RM (2016) Cultivation of Spirulina maxima in medium supplemented with sugarcane vinasse. Bioresour Technol 204:38–48
Silva MAS, Griebeler NP, Borges LC (2007) Uso de vinhaça e impactos nas propriedades do solo e lençol freático. Rev Bras de Eng Agrícola Ambiental 11:108–114
Szymansky MSE, Balbinoti R, Schimer WN (2010) Biodigestão anaeróbia da vinhaça: aproveitamento energético do biogás e obtenção de créditos de carbono–estudo de caso. Semina: Ciências Agrárias, Londrina 31(4):901–902
Vieira TQ (2013) Uso de resíduo líquido no cultivo da microalga Chlorella sp. com potencial para a produção de biocombustíveis. Completion of course work in Sanitary and Environmental Engineering. Estadual University of Paraíba, Campina Grande, p 61
Acknowledgments
The authors acknowledge the Brazilian National Council for Scientific and Technological Development (CNPq—Proc. No. 302837/2012-4; 302175/2015-6) and the São Paulo State Research Foundation (FAPESP—Proc. No. 2014/15894-0) for financial support. FAI-UFSCar is also acknowledged for assistance in the patent process.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Candido, C., Lombardi, A.T. Growth of Chlorella vulgaris in treated conventional and biodigested vinasses. J Appl Phycol 29, 45–53 (2017). https://doi.org/10.1007/s10811-016-0940-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-016-0940-2