Abstract
Vinasse is a by-product resulting from the ethanol production process. It is generated in huge volumes which are destined to fertirrigation, although has high polluting potential. Otherwise, microalgae-guided sequestration is seen as one of the more promising and sustainable solutions to mitigate CO2. Another advantage of microalgae production systems is their potential to utilize wastes, including sewage, leachates, and gas emissions. In this context, this research aimed to evaluate the efficiency of microalgae for vinasse preliminary treatment in terms of the remotion of nutrients (phosphorus and ammoniacal nitrogen) and organic matter. Moreover, the resultant microalgae biomass amount was characterized for bioproducts generation, such as carbohydrates, proteins, lipids content, and antioxidant, bioactive substances with high potential for both commercial and industrial use. The media cultivation supplemented with 20% or 30% of vinasse resulted in 3.16 g L−1 of biomass, 30% of carbohydrate and 20% of lipids and 3.05 g L−1 biomass, 24% of carbohydrate and 51% of lipids, respectively, in 96 h. Both treatments also resulted in a significant reduction of nutrients and chemical oxygen demand (COD). The obtained results indicate the increasing of vinasse in media cultivation can modulate the microalgae metabolism. Furthermore, is the first time that the microalgae Coelastrella sp., utilized in this work, is reported in Brazil and has its potential evaluated for the treatment of vinasse with concomitant generation of bioproducts.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, industrial development and social progress have led to an increase in water system pollution (Zhao et al. 2023). The production of ethanol from sugarcane has emerged as an energy alternative from renewable sources, but its production generates large amounts of highly polluting waste (Sydney et al. 2019). The vinasse is the main waste generated, obtaining 13 L for each liter of ethanol produced, and is characterized by being a by-product with high content of organic matter and nutrients (de Mattos and Bastos 2016). Moreover, due to its low pH, high corrosion capacity, and high rates of biochemical (BOD) and chemical (COD) oxygen demand, it is considered an effluent with high polluting potential (Soto et al. 2021).
Different alternatives for the reuse of this waste have already been tested. The primary use of vinasse is in soil fertirrigation due to the concentration of nutrients present in this effluent (Candido et al. 2022). However, this use has been questioned and restricted due to several factors (Quintero-Dallos et al. 2019). First, the use of vinasse for this purpose should be controlled due to its high potential for eutrophication (Candido and Lombardi 2018). In addition, there is the possibility of producing methane from the digestion of sugarcane vinasse (Oliveira et al. 2017). Besides, the low pH, electric conductivity, and chemical elements present in sugarcane vinasse may cause changes in the chemical and physical–chemical properties of soils, rivers, and lakes with frequent discharges over a long period of time, and also have adverse effects on agricultural soils and biota in general. And, in the long term, there is a tendency to accumulate certain minerals that make the soil more toxic for cultivation, impairing sugarcane growth (Trevisan et al. 2020). Given this scenario, it is necessary to point out sustainable alternatives for using vinasse, aiming to reduce environmental impacts and to associate it with generating products with added value. One of the possibilities is the use of vinasse in biotechnological processes, e.g., biodigestion for biogas generation or for the cultivation of microorganisms, such as microalgae (Candido and Lombardi 2018). Considering the advantages of microalgae, such as high photosynthetic efficiency and their ability to avoid competition with terrestrial crops, as well as being solar-powered microbial factories, they emerge as promising solution to address issues related to energy crisis, food security, and pollution (Ammar et al. 2020). Due to their high carbon demand, microalgae present significant potential as an agent capable effectively mitigating these problems (Leong et al. 2021).
Microalgae-based bioproducts have attracted the industry's attention (Ahmad et al. 2022); however, the costs associated with cultivating these microorganisms, especially those related to the necessary nutrients, are considered a significant barrier to their commercialization (Faé Neto et al. 2018; Silva et al. 2021). In this scenario, using vinasse as part of the culture medium for microalgae growth is considered a potential alternative. Besides reducing costs compared to synthetic culture media, microalgae can act in the degradation of toxic compounds present in vinasse and, concomitantly, in the generation of value-added products (Trevisan et al. 2020; Candido et al. 2022) such as lipids, pigments, and carotenoids (Santana et al. 2017).
It highlights the potential of microalgae as a promising resource for the production of value-aided products, as biofuels. It emphasizes its several possibilities, with specific focus on proteins for human nutrition and the production of biofuels such as gasoline, diesel, jet fuel and ethanol (Al Raie et al. 2020).
The use of sugarcane vinasse as a culture medium to study various green and blue microalgae is not new. dos Santos et al. (2016) studied a cyanobacterium Spirulina maximum supplemented with sugarcane vinasse to obtain different bioproducts: lipid, carbohydrate, protein, and ashes. In the same way, Micractinium sp. ME05 was studied with sugarcane vinasse in different types of metabolism (heterotrophic and mixotrophic), both to accumulate lipids and obtain biodiesel (Engin et al. 2018). Sydney et al. (2019) studied six species of microalgae (Botryococcus braunii SAG 801-7, Synechococcus nidulans LEB 25, Chlorella kessleri LEB15, Chlorella vulgaris LEB 104, Neochloris oleoabundans UTEX 1185 and Scenedesmus obliquus LEB 22) and three cyanobacteria (Arthrospira platensis SAG 257.80, Arthrospira laxissima SAG 256.80, and Arthrospira maxima SAG 84.79) and verified the potential of these microalgae to grow in pure and diluted sugarcane vinasse, and the possibility to have lipids accumulation. Other work has studied the potential of obtaining biofertilizers using two strains of C. vulgaris and Desmodesmus sp. growing in a medium supplemented with vinasse (Ferreira et al. 2021), which Desmodesmus sp. showed higher duplication cells (7 times), more proteins, and lipids production. It is worth mentioning that all the studies conducted with microalgae and vinasse have not been carried out with isolated strains from the mangrove.
The mangroves are unique ecosystems, rich in biodiversity, organic matter, salinity, and low oxygenation, requiring several physiological adaptations from the biota to overcome these problems. However, there are very limited studies on the mangrove-isolated microalgae, and it is well known that not all strains can grow under many adverse conditions (Thatoi and Behera 2013).
Therefore, this work aims to investigate the potential of microalgae biorefinery, which uses the sugarcane vinasse as a culture medium for microalgae strains isolated from mangroves to grow and characterize the main added-value bioproducts with biotechnological interest.
Materials and methods
Sugarcane vinasse and medium preparation
Crude sugarcane vinasse was kindly provided by an industrial ethanol plant. To make vinasse suitable for culture medium, it is necessary to use pre-treatment processes, given some of its characteristics, such as turbidity, suspension of solids, and acidity (Candido et al. 2022). Then, the process of clarification and decantation of solids was carried out according to the methodology of Santana et al. (2017). First, calcium hydroxide (Ca(OH)2) (Synth) was added to the crude vinasse at a rate of 3 g L−1. After 40 min, the material was centrifuged (High-Speed Refrigerated Centrifuge CR-22N Hitachi®) at 10,000 rpm, 20 °C for 5 min, and the precipitated material was discharged. Then, the pH is adjusted to 7.5 by adding sodium hydroxide (NaOH), and, finally, it was autoclaved at 121 °C for 20 min.
The culture medium BG-11 was used to dilute the previously prepared vinasse for the subsequent assays. The medium was formulated according to the methodology of (Gracioso et al., (2021), for maintenance of strains and biomass production.
Microalgae strains and inoculum procedure
Microalgae strains were obtained from mangrove samples from two different areas in the cities of Cubatão and Santos (SP, Brazil) (46 25′13.224″ W 23 53′46.428″ S and 46 23′7.567″ W 23 56′40.420″ S, respectively). Enrichment of the samples and isolating the strains were performed as described by Gracioso et al. (2021).
To promote the acclimatization of strains in vinasse, five different strains and one consortium (identified as A3, A5, A20, B7, B12, and MSC4P, respectively) were transferred to test tubes filled with increasing vinasse concentrations (10%, 20% and 30%, v/v) in BG-11 medium. The tubes were closed with a roller and exposed to a light intensity of 130 µmol m−2 s−1.
Microalgae selection and molecular identification
Microalgae strains were screened for growth in sugarcane vinasse. Initially, the cultures of each strain were inoculated in 250 mL Erlenmeyer flasks of diluted vinasse formulations at 10%, 20% and 30% in the BG-11 medium. The cultivation was performed with agitation (80 rpm) at 26 ± 1 °C and a light intensity of 130 µmol m−2 s−1. Microalgae growth was monitored visually along 15 days.
The A3 strain showed the best growth rate under the conditions tested in these selection experiments. Thus, it was chosen to proceed with subsequent experiments and identification by molecular biology.
For this, fresh culture pellets were submitted to DNA extraction using a protocol adapted from Lõoke et al. (2017). For cell disruption, a lysis buffer consisting of 0.2 M lithium acetate and 1% SDS (sodium dodecyl sulfate) in a solution associated with glass microspheres (150–212 μm, Sigma-Aldrich) was used. The mixture was kept under agitation for 20 min and then subjected to a temperature of 75 °C for 10 min. Next, a 5 M NaCl solution (100 μL) was added to the mixture to remove proteins and cellular debris. Subsequently, the DNA was precipitated using ice-cold 100% ethanol (Química Moderna, Brazil), keeping the mixture at −20 °C for approximately 30 min. Next, the precipitate was cleaned using 70% ethanol and kept at 37 °C for complete evaporation of the solvent. Finally, the extracted DNA was resuspended in 50 μL of deionized water.
The ITS gene was amplified by PCR using ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) primers. The reaction was performed using 1 μL of the previously extracted DNA, 1 μL of each primer (10 μM), 22 μL of PCR grade water, and 25 μL of ReadyMix™ Taq PCR Reaction Mix with MgCl2 (Sigma-Aldrich), totalizing 50 μL of the final volume. The mixture was submitted to amplification (GeneAmp PCR System 9700, Thermofisher) according to the parameters proposed by Ristaino et al. (1998). Amplification efficiency was observed on agarose gel electrophoresis (0.8% in TAE buffer) in AmershamTM Imager 600 (Cytiva, Sweden). The obtained sequences were compared with known sequences from the BLAST database (https://blast.ncbi.nlm.nih.gov/Blast.cgi).
Growth conditions of A3 strain
The cellular growth of A3 strain in vinasse-based media at concentrations of 0%, 20% and 30% (v/v) was carried out in sterile Drechsler flasks of 5 cm in diameter and a working volume of 250 mL, with the injection of air at 0.04% CO2 and an illumination rate of 130 µmol m−2 s−1. The biomass growth was determined by biomass dried weight (described below) during 96 h. By plotting the Ln (OD) in a graphic, the maximum specific growth rate was calculated during the exponential phase (µmax).
Biomass dry weight determination
Dry biomass was determined by gravimetric methods from 10 mL samples of the culture. In this procedure, polyethersulfone membranes with a pore size of 0.22 µm and 0.47 mm in diameter (Sartorius Stedim Biotech®) were dried in the microwave for 5 min at maximum power. After that, the membrane was weighed (Wi) and submitted to vacuum filtration of microalgae cultivation. After filtration, the membrane was dried in the microwave again, under the same conditions as described, and then the final weight (Wf) was annotated. To determine the biomass, Eq. (1) was used.
Evaluation of vinasse-based culture medium treatment
Determination of chemical oxygen demand (COD)
To quantify the initial and final COD of the cultures, it was utilized a colorimetric assay (Spectroquant® COD Merck kit) following the manufacturer’s instructions. To reduce the generated wastes, the methodology was adapted by using 100 µL of oxidant solution A and 950 µL of solution B in Test Tubes COD vials. The solutions were mixed and 1 mL of culture sample, previously centrifuged at 10,000 rpm for 5 min, was added to avoid the influence of algal biomass on the test. These samples were mixed and inserted into the digester block at 148 °C for 2 h. At the end of this period, the samples were left to stand for 10 min, shaken, and left to stand again for another 10 min. After that, they were analyzed in a UV/Vis spectrophotometer at λ = 446 nm. A calibration curve was performed with known concentrations of standard potassium biphthalate solution in water, following the same procedure reported above.
Determination of phosphorus
The determination of phosphate concentration in vinasse-based media was performed by the colorimetric assay (Phosphate-test Merck) following the manufacturer’s instructions. A calibration curve was performed with known concentrations of potassium phosphate standard solution in water, performing the same procedure. The phosphorus concentration represents one third of the obtained phosphate value.
Determination of ammoniacal nitrogen (N-NH3)
The determinations of N-NH3 in vinasse-based media were performed according to the colorimetric method 4500-NH3 F-Indophenol Method (Standard Methods for the Examination of Water and Wastewater—APHA). For this, the following solutions were used: (a) 1.26 mol L−1 of alcoholic phenol solution, (b) 0.017 mol L−1 of sodium nitroprusside solution, (c) 0.25 mol L−1 of alkaline citrate solution, (d) 10 mL of alkaline citrate solution and 2.5 mL of sodium hypochlorite (2.5%) to obtain oxidant solution. To each 500 µL of cultivation sample, 20 µL of solution (a), 20 µL of solution (b), and 50 µL of solution (d) were added, then shaken. After adding the oxidizing solution, the samples were kept at rest for 1 h, under low light. Subsequently, 200 µL of each sample were transferred to a 96-well plate and analyzed in a UV/Vis spectrophotometer at 640 nm.
Biomass characterization
Qualitative and quantitative characterization of lipids
To perform the qualitative analysis of lipids, 5–10 mg of cells in dry weight were used after 72 h of cultures in triplicate. For the extraction of total lipids, the National Renewable Energy Laboratory (NREL) method was used (Wychen et al. 2015). Briefly, 200 µL of chloroform/methanol (2:1) (Synth and Vetec, respectively), plus 300 µL of 0.6 M hydrochloric acid/methanol solution were added to the sample. The closed tubes were placed in a heating block and kept for 1 h at 85 °C. Then, were removed from the block after 15 min for cooling, and 1 mL of hexane was added and mixed. After stirring, the tubes were kept open in a hood at room temperature for solvent evaporation.
Fatty acids percentages were determined via GC-FID (Agilent Technologies 7890A) with: HP-Innowas column (19091N-133l—30 cm × 0.25 mm × 0.25 µm), helium as drag gas; FID detector at 300 °C; He (30 mL min−1), 40 mL min−1 H2 flow, 400 mL min−1 air flow; heating ramp (adapted of NREL) for analysis as 100 °C for 1 min; 25 °C min−1 till 200 °C, kept for 1 min; 5 °C min−1 till 250 °C, kept for 7 min. FAMEs were quantified using a calibration curve previously developed from the FAME Standard Mixture of 25 known FAMES (FAME STANDARD MIXTURE 100 mg USP), comparing peak areas to the known standards.
Determination of carbohydrates profile
The carbohydrate profile was determined by the NREL method. Briefly, 25 ± 2.5 mg of freeze-dried algal biomass were placed into 10 mL glass tubes. At this biomass, 250 μL of 72% (w/w) sulfuric acid was added and mixed vigorously. The tubes were transferred to an oven at 30 ± 3 °C and incubated for 1 h, vortexing each tube vigorously every 5–10 min. After 1 h, the tubes were removed from the oven, and 7 mL of deionized water was added to each tube, obtaining a concentration of 4% (v/v) sulfuric acid. The sealed samples were autoclaved for 1 h at 121 °C. After this cycle, the tubes were removed from the autoclave and cooled until room temperature.
The hydrolyzed samples were neutralized using calcium carbonate (CaCO3) to reach a pH between 6–7. So, the neutralized samples were centrifuged (10,000 rpm, 5 min) and filtered through a 0.22 μm nylon filter to remove all solids and precipitate before the HPLC analysis. The HPLC analysis was performed with a refractive index detector (RID), using a Shodex Sugar SP0810 column at 85 °C. The mobile phase used was deionized water at a flow rate of 0.6 mL min−1. The volume sample used was 50 μL.
For proper quantification of sugars, a calibration curve was performed with known concentrations of glucose, xylose, galactose, arabinose, and mannose.
Determination of total protein
To quantify the total of proteins, 5 mg of lyophilized cells were resuspended in 2 mL of buffer lysis (50 mM Tris, pH 8.0, 150 mM NaCl, and 1 mM dithiothreitol). To disrupt the cells, sonication was used (Digital Sonifer 450, Branson) at 90% of amplitude, for six cycles of 30 s on and 59 s off, on ice (Vidotti et al. 2020). The proteins extracted on the buffer were recovered by centrifugation, 10,000g at 4 °C for 30 min. After that, the proteins were quantified by a 2D-Quant kit (Cytiva), according to the manufacturer.
Extraction of carotenoids and determination of antioxidant activity
The protocol for carotenoid extraction was described by Zavřel et al. (2015) and adapted for microalgae. Briefly, cold methanol was added to 1 mg of lyophilized cells, incubating at 4 °C for 20 min. After this period, the sample was centrifuged under the same conditions.
The percentage of antioxidant activity (AA%) was assessed by DPPH free radical assay and calculated by Eq. (2). The measurement of the DPPH radical scavenging activity was performed according to the methodology described by Brand-Williams et al. (1995). The samples were reacted with the stable DPPH radical in a methanol solution (100 µL of sample and 400 µL of DPPH radical solution in methanol, 0.6 mM). The DPPH, when reacted with an antioxidant compound, donates hydrogen, which is reduced, changing the color from deep violet to light yellow. This reaction was read at 515 nm using a UV–Vis spectrophotometer until color stabilization. The mixture of methanol and sample served as blank, and the positive control solution was prepared by mixing ascorbic acid and DPPH radical solution.
The results are evaluated according to the percentage of reduction, where a higher percentage means the greater oxidizing capacity of the sample.
Results and discussion
Microalgae selection and identification
The initial selection of strains capable of growing in vinasse was carried out using this effluent at a concentration of 10% (v/v). Under this condition, only isolates A3 and A5 and the MSC4P consortium presented visual growth over 15 days of cultivation. Then, the concentration of vinasse was increased to 20% (v/v) where, under this condition, only strain A3 could grow (data not shown), being selected for future trials.
The isolate A3 (GenBank accession number: ON921055) was identified as Coelastrella sp. 99.40% similarity with Coelastrella sp. CORE-2, isolated from the Yucatan Peninsula, a tropical area in North America (Fig. 1). Coelastrella belongs to the phylum Chlorophyta and the family Scenedesmusceae (Karpagam et al. 2018), which has spherical, subspherical, ellipsoidal or fusiform cells with a diameter ranging from 6 to 15 μm. Reproduction is asexual, by autospores (Wang et al. 2019; Nayana et al. 2022). The genus is characterized by having parietal chloroplast and meridional ribbing on the cell wall surface (Vasistha et al. 2023).
Phylogenetic tree of the mangrove-isolated green-microalgae identified by rDNA ITS as Coelastrella sp. The tree was constructed by MEGA11: Molecular Evolutionary Genetics Analysis version 11 (Tamura et al. 2021) with sequences obtained from NCBI databases and then aligned using ClustelW in automatic mode. The bootstrap method was used with 500 replications in a Jukes–Cantor model. The black square marks Coelastrella sp. isolated from the mangrove
According to Nayana et al. (2022), this strain is distributed all over the planet. However, to the best of our knowledge, this is the first record of this strain isolated from mangrove. The strain presents a recognized capacity for effluent treatment (Luo et al. 2016) and production of bioproducts of interest, such as pigments relevant to the food and textile industries, in addition to having antioxidant potential, for example, and lipid-rich biomass for biofuel generation (Karpagam et al. 2018; Goecke et al 2020; Nayana et al. 2022).
Cellular growth in vinasse medium
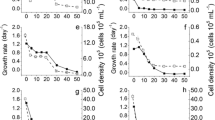
Assays containing two different concentrations of vinasse (20% and 30%, v/v) and control with BG-11 vinasse-free medium were tested to evaluate the growth of Coelastrella sp strain A3. As shown in Fig. 2, there was a significant difference in cell growth comparing the cultures with and without vinasse. Evaluating the cell growth phases of the strain, without vinasse, the onset of the exponential phase occurred within 48 h of testing. In the assays containing vinasse, this growth phase occurred in the first 24 h. The stationary phase occurred after 120 h in the control assay; with vinasse, it occurred after 48 h. This difference in growth can be attributed to the different carbon sources in the vinasse. Microalgae have a diversified metabolism, which allows them to perform not only photosynthesis in photoautotrophic processes but also mixotrophy, performing photosynthesis associated with the use of organic carbon to generate energy and biomass (Pang et al. 2019).
Different kinetic parameters were evaluated in all cultures performed (without and with—20% and 30%—vinasse). To evaluate the maximum growth rate in both concentrations of vinasse, the first 24 h of the assay were observed. In the concentration of 20% (v/v), the maximum growth rate (µmax) calculated during the exponential phase was 0.0063 h−1, with a generation time of 110.23 h (R2 = 0.98). In the concentration of 20% vinasse (v/v), the µmax was determined between 2 and 14 h of the assay, equal to 0.0432 h−1, with a generation time of 16.05 h (R2 = 0.98). In the concentration of 30% vinasse (v/v), the µmax was obtained between 2 and 22 h of the experiment, equal to 0.0338 h−1, with a generation time of 20.50 h (R2 = 0.97). With these results, it could be observed that under the concentration of 30%, the strain was more significantly stressed, since its kinetic data were directly affected.
Ramirez et al. (2014) studied Scenedesmus sp. growing in a supplemented medium with vinasse concentrations varying from 0–50% (v/v). In that research, it was observed that the exponential growth phase reached its peak between 180 and 240 h with 0.5 g L−1 of maximum dry biomass production. Ding et al. (2020) verified the growth capacity of Coelastrella sp. in grow in palm oil effluent. In this study, the biomass production for the strain was 0.936 g L−1 in 120 h of assay. In this work, the maximum biomass production by Coelastrella sp. in 20% (v/v) vinasse occurred after 14 h of the assay, reaching 1.43 g L−1. In 30% (v/v) vinasse, the maximum biomass production was obtained after 22 h of the assay, reaching 2.24 g L−1.
In our work, at the end of the experiment, the biomass production obtained was equal to 1.18 g L−1 (± 0.12) for the control culture (without vinasse), 3.16 g L (± 0.44) for the cultures containing 20% (v/v) vinasse and 3.05 g ± 0.30 for the cultures containing 30% (v/v) vinasse. About this, no significant differences (p > 0.05) were evidenced between the cultures containing different concentrations of sugarcane vinasse. However, there is a significant difference between the control and both cultures with effluent (p < 0.05) (Fig. 3).
Vinasse treatment
This work analyzed the efficiency of Coelastrella sp. in removing N-NH3, phosphorus and COD. The efficiency of N-NH3, phosphorus, and COD remotion can be better visualized in Fig. 4. N-NH3 values reached 100% removal in both treatments, with 20 and 30% vinasse. For 20% of the vinasse, the initial phosphorus content was 16.00 mg L−1 (± 0.41). On the last day of growth, it reached 6.0 mg L−1 (± 0.41). While the initial COD values were 76.30 mg L−1 (± 4.14) and the final values were 42.10 mg L−1 (± 1.46). On the other hand, the initial values for 30% vinasse were 26.00 mg L−1 (± 0.71) and 83.50 mg L−1 (± 10.37), phosphorous and COD respectively. And the final values were 1 mg L−1 (± 0.02) and 42.3 mg L−1 (± 8.78) for phosphorus and COD, respectively.
In both growth conditions, the N-NH3 was completely consumed by the strain. One of the essential nutrients for cells is nitrogen, which participates in various biological routes such as protein and peptides, chlorophylls, energy transfer molecules, and material genetic production (Luo et al. 2016).
De Mattos and Bastos (2016) observed nitrogen consumption in the cultivation of the Desmodesmus sp. strain in vinasse, resulting in a removal of approximately 50% nitrogen. Considering that stillage is used for fertirrigation, the removal of ammoniacal nitrogen minimizes the environmental impacts of its discharge into the soil, since it reduces its polluting potential. Similar to this work, Lee et al. (2021) demonstrated the nitrogen removal potential of Coelastrella sp. in piggery wastewater, where it was able to remove 99% of ammoniacal nitrogen present in the samples.
Regarding the phosphorus present in the stillage, it was observed that removal of 63.12% and 95.8% in 20% and 30% (v/v) of the stillage, respectively. Different studies point out the potential of the use of microalgae for the removal of phosphorus from wastewater of different processes. Scherer et al. (2017) observed the removal of approximately 52% of available phosphorus in a medium with 30% (v/v) bovine manure effluent by the consortia containing Scenedesmus species. Lee et al. (2021) verified the total removal of phosphorus present in piggery effluent by the Coelastrella sp. strain after 96 h of cultivation.
COD is a parameter that measures the amount of organic matter; however, it does not determine the concentration of specific and individual substances but the consumption of oxygen through chemical oxidation reactions (Dias et al. 2015). In the present study, the COD reduction was 53.9% and 50.7% in cultivation with 20% and 30% vinasse, respectively.
The potential for COD reduction by microalgae has also been described in several works. De Mattos and Bastos (2016) found a reduction of approximately 35% of COD by the strain Desmodesmus sp. in vinasse. Scherer et al. (2017) found a reduction of approximately 54% of COD by a consortium containing Scenedesmus species. Using Coelastrella sp. strain, Lee et al. (2021) found a 92% of COD reduction in 96 h of the experiment, using piggery effluent as a culture medium.
Biomass characterization (total and lipids profile, carbohydrates profile, antioxidant-carotenoids, total protein)
Lipid and carbohydrate production
Microalgae, when submitted to stressful conditions, mainly related to nitrogen limitation, store energy in the form of lipids and carbohydrates. However, under this condition their growth is decreased (Lee et al. 2021).
In this work, the production of lipids and total carbohydrates was evaluated by Coelastrella sp. after 96 h of cultivation in different concentrations of vinasse (Fig. 5). During the growth with 20% of vinasse there was a higher production of carbohydrate 30.89% (± 1.56), whereas in 30% this production decreased (24.36% ± 4.2), increasing the production of lipids 51% (± 0.32).
Previous investigations also endorse the fact that lipids content is one of the major indicators of bioproducts generation in biomass regarding to microalgae cultivation. As in the current work, Maltsev et al. (2021) also noticed significant lipids production in a medium with nitrogen and phosphorus stringency, in which 57% dry weight content was reached.
A work developed by Santana et al. (2017) studied the growth and biomass characterization of two microalgae strains, Chlamydomonas biconvexa and Micractinium sp. using vinasse at percentages of 50% and 100%. For the Chlamydomonas biconvexa strain, the increase in the concentration of vinasse also resulted in the decrease of carbohydrate concentration. For Micractinium sp. the increase of vinasse increased the production of carbohydrates.
Future studies on the characterization of the carbohydrates produced by the Coelastrella sp. strain would help in understanding the potential of this strain to produce ethanol from its biomass.
Previous studies developed with Coelastrella sp. indicate that, in fact, a higher proportion of BG-11 medium tends to inhibit the lipid accumulation, given the high concentration of nitrates from this medium (Lee et al. 2021).
When subjected to a higher concentration of vinasse (30%), there was a considerable increase in the content of stored lipids and a decrease in carbohydrate accumulation. Probably, the stress caused by increasing vinasse favored the lipid production pathway and inhibited the carbohydrate production pathway. Indeed, Coelastrella sp. is recognized for its potential production of lipids, including those of interest for biodiesel (Nayana et al. 2022).
Scherer et al. (2017) tested Scenedesmus sp. growing the strain in medium containing cattle raising wastewater at 10% v/v and obtained 15% total lipids concentration at 120 h cultivation time. Viêgas (2010) observed that an extraction method applying chloroform/methanol 2:1 v/v provided 20% of total lipids concentration for Chlorella pyrenoidosa. In comparison with the previous studies, it can be stated that Coelastrella sp. is not only highly adaptable in a medium containing wastewater, but also presents a high lipid production performance.
Through GC-FID analysis it was possible to identify the profile of fatty acids extracted from Coelastrella sp. in 20% and 30% of vinasse in BG-11 medium (Fig. 6).
Generally, the chain length of suitable fatty acids for biodiesel production ranges between 14 and 18 carbons. Most common FAMEs found in biodiesel are oleic, linoleic acid and palmitic acid which is a saturated fatty acid and is known as the most common fatty acid found in biodiesel (Ho et al. 2014).
The profile of fatty acids presents in the microalgae showed chains between 10 to 20 carbons. The lipid profiles showed predominant formation of arachidic acid (C20:0) followed by α-linolenic acid (C18:3). It is noted that most of the fatty acids are saturated, a preferable feedstock for biodiesel generation because of its properties such as: high oxidative stability, low ignition delay, and lower potential for NOX emission, an intensely aggressive pollutant for the ozone layer (Lee et al. 2021).
D’Oca et al. (2011) found a higher amount of linolenic acid (C18:3) ranging from 29.04% to 42.47% in the fatty acid profile of Chlorella pyrenoidosa under different culture conditions. However, lipids composed of higher concentrations of unsaturated fatty acids tend to lead to instabilities with oxygen during the combustion of biodiesel synthesized from this feedstock (Lee et al. 2021). Therefore, considering the results observed in the present work, it is suggested that Coelastrella sp. may be useful for the production of biodiesel with higher combustion stability.
The monosaccharides commonly present in hydrolyzed algal biomass are glucose, xylose, mannose, galactose, and arabinose (Hernández et al. 2015). Thus, the concentration of these was analyzed in the final biomass of strain A3. The HPLC characterized carbohydrate profile of the vinasse culture growth is shown in Fig. 7.
The most concentrated carbohydrate in both cultivations was glucose, which is the main monosaccharide fermented when producing bioethanol. It reached 8.87% (± 0.58) and 7.10% (± 2.11) for 20% and 30% vinasse, respectively. For the other sugars, the percentage of accumulation ranged from 1.2 to 6.1. As for the fatty acids, the profile of the carbohydrates produced was different in the two concentrations of vinasse used.
Antioxidants and protein
Some metabolites produced by microalgae are molecules with biotechnological potential, which can be added to human and animal nutrition, biofertilizers, pharmaceuticals, cosmetics, textile dye, food colorants, and others (Coulombier et al. 2021).
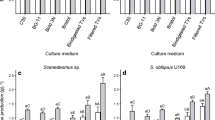
The antioxidant potential of Coelastrella sp. grown with different percentages of vinasse, and the total protein is shown in Table 1.
The results for antioxidant activity were promising, once 1 mg of biomass was enough to reduce 50% of DPPH. Between 20% or 30% of vinasse, no significant difference may be observed.
The same can be observed for proteins production, where with 20% of vinasse, 25.3 μg μL−1 of proteins were produced and with 30% of vinasse, 27.3 μg μL−1 were produced, with no significant difference between the growths.
In previous works, regarding the action of antioxidants naturally produced by microalgae, the experiments use different methodologies for extraction, quantification, concentration, and reaction time. This makes the comparison between studies complex, highlighting the need to standardize the protocols. In the results obtained in this study, we have compared the antioxidant activity with the positive control (ascorbic acid). The percentage of DPPH reduction for the control was about 75%, and for the extracted antioxidant from microalgae grown on sugarcane vinasse, the percentage reached 50% with 1 mg of biomass. These results show the potential for antioxidant production by Coelastrella sp. isolated from mangrove. Further studies can bring better results such as, for example, optimization of the cultivation to obtain the antioxidants, different extraction protocols, and data analysis can further show the potential of this microalgae.
Conclusions
The use of microalgae isolated from mangroves proved to be a potential tool for treating vinasse generated in bioethanol production from sugarcane. Besides the reduction of COD, phosphate, and ammoniacal nitrogen, it was observed the production of lipids, carbohydrates, proteins, and antioxidants. It is worth noting that the potential treatment with the generation of bioproducts occurred in only 4 days of cultivation. However, the main challenge of this technology is the scaling-up, a critical step in all biotechnological processes. In this sense, future studies with isolated Coelastrella sp. must be performed to check its potential to grow in larger concentrations of vinasse, with the concomitant bioproducts accumulation.
References
Ahmad A, Banat F, Alsafar H, Hasan SW (2022) Algae biotechnology for industrial wastewater treatment, bioenergy production, and high-value bioproducts. Sci Total Environ. https://doi.org/10.1016/J.SCITOTENV.2021.150585
Al Raie HH, Al Hassany JS, Rasheed KA (2020) Wastewater treatment by using locally isolated algae species Coelastrella terrestris (Reisigl). Plant Archiv 20(2):1691–1695
Ammar EM, Arora N, Philippidis GP (2020) The prospects of agricultural and food residue hydrolysates for sustainable production of algal products. Energies 13:6427. https://doi.org/10.3390/EN13236427
Brand-Williams W, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol. https://doi.org/10.1016/S0023-6438(95)80008-5
Candido C, Lombardi AT (2018) The physiology of Chlorella vulgaris grown in conventional and biodigested treated vinasses. Algal Res. https://doi.org/10.1016/j.algal.2018.01.005
Candido C, Cardoso LG, Lombardi AT (2022) Bioprospecting and selection of tolerant strains and productive analyses of microalgae grown in vinasse. Braz J Microbiol. https://doi.org/10.1007/s42770-022-00692-7
Coulombier N, Jauffrais T, Lebouvier N (2021) Antioxidant compounds from microalgae: a review. Mar Drugs. https://doi.org/10.3390/md19100549
D’oca MGM, Viêgas CV, Lemões JS, Miyasaki EK, Morón-Villarreyes JA, Primel EG, Abreu PC (2011) Production of FAMEs from several microalgal lipidic extracts and direct transesterification of the Chlorella pyrenoidosa. Biomass Bioenergy. https://doi.org/10.1016/j.biombioe.2010.12.047
De Mattos LFA, Bastos RG (2016) COD and nitrogen removal from sugarcane vinasse by heterotrophic green algae Desmodesmus sp. Desalin Water Treat. https://doi.org/10.1080/19443994.2015.1028454
Dias FP, Nascimento BLM, Nascimento RF, Stefanutti R, Braga EAS (2015) Efeitos de reagentes químicos no efluente da indústria de biodiesel: contribuição na carga orgânica e ecotoxicidade. Rev AIDIS Ingen Ciênc Ambient 8:1–13
Ding GT, Mohd Yasin NH, Takriff MS, Kamarudin KF, Salihon J, Yaakob Z, Mohd Hakimi NIN (2020) Phycoremediation of palm oil mill effluent (POME) and CO2 fixation by locally isolated microalgae: Chlorella sorokiniana UKM2, Coelastrella sp. UKM4 and Chlorella pyrenoidosa UKM7. J Water Process Eng. https://doi.org/10.1016/j.jwpe.2020.101202
dos Santos RR, Araújo OQF, de Medeiros JL, Chaloub RM (2016) Cultivation of Spirulina maxima in medium supplemented with sugarcane vinasse. Bioresour Technol 204:38–48. https://doi.org/10.1016/j.biortech.2015.12.077
Engin IK, Cekmecelioglu D, Yücel AM, Oktem HA (2018) Evaluation of heterotrophic and mixotrophic cultivation of novel Micractinium sp. ME05 on vinasse and its scale up for biodiesel production. Bioresour Technol. https://doi.org/10.1016/j.biortech.2017.12.023
Faé Neto WA, Borges Mendes CR, Abreu PC (2018) Carotenoid production by the marine microalgae Nannochloropsis oculata in different low-cost culture media. Aquac Res. https://doi.org/10.1111/are.13715
Ferreira GF, Pinto LFR, Filho RM, Fregolente LV (2021) Effects of cultivation conditions on Chlorella vulgaris and Desmodesmus sp. grown in sugarcane agro-industry residues. Bioresour Technol. https://doi.org/10.1016/j.biortech.2021.125949
Goecke F, Noda J, Paliocha M, Gislerød HR (2020) Revision of Coelastrella (Scenedesmaceae, Chlorophyta) and first register of this green coccoid microalga for continental Norway. World J Microbiol Biotechnol. https://doi.org/10.1007/s11274-020-02897-0
Gracioso LH, Bellan A, Karolski B, Cardoso LOB, Perpetuo EA, Nascimento CAO, Giudici R, Pizzocchero V, Basaglia M, Morosinotto T (2021) Light excess stimulates Poly-beta-hydroxybutyrate yield in a mangrove-isolated strain of Synechocystis sp. Bioresour Technol. https://doi.org/10.1016/j.biortech.2020.124379
Hernández D, Riaño B, Coca M, García-González MC (2015) Saccharification of carbohydrates in microalgal biomass by physical, chemical and enzymatic pre-treatments as a previous step for bioethanol production. J Chem Eng. https://doi.org/10.1016/j.cej.2014.10.049
Ho SH, Ye X, Hasunuma T, Chang JS, Kondo A (2014) Perspectives on engineering strategies for improving biofuel production from microalgae—a critical review. Biotechnol Adv. https://doi.org/10.1016/j.biotechadv.2014.09.002
Karpagam R, Jawaharraj K, Ashokkumar B, Sridhar J, Varalakshmi P (2018) Unraveling the lipid and pigment biosynthesis in Coelastrella sp. M-60: genomics-enabled transcript profiling. Algal Res. https://doi.org/10.1016/j.algal.2017.11.031
Lee SA, Ko SR, Lee N, Lee JW, Le VV, Oh HM, Ahn CY (2021) Two-step microalgal (Coelastrella sp.) treatment of raw piggery wastewater resulting in higher lipid and triacylglycerol levels for possible production of higher-quality biodiesel. Bioresour Technol. https://doi.org/10.1016/j.biortech.2021.125081
Leong YK, Chew KW, Chen WH et al (2021) Reuniting the biogeochemistry of algae for a low-carbon circular bioeconomy. Trends Plant Sci 26:729–740. https://doi.org/10.1016/j.tplants.2020.12.010
Lõoke M, Kristjuhan K, Kristjuhan A (2017) Extraction of genomic DNA from yeasts for PCR-based applications. Biotechniques. https://doi.org/10.2144/000114497
Luo L, He H, Yang C, Wen S, Zeng G, Wu M, Zhou Z, Lou W (2016) Nutrient removal and lipid production by Coelastrella sp. in anaerobically and aerobically treated swine wastewater. Bioresour Technol. https://doi.org/10.1016/j.biortech.2016.05.059
Maltsev Y, Krivova Z, Maltseva S et al (2021) Lipid accumulation by Coelastrella multistriata (Scenedesmaceae, Sphaeropleales) during nitrogen and phosphorus starvation. Sci Rep 11:19818. https://doi.org/10.1038/s41598-021-99376-9
Nayana K, Sudhakar MP, Arunkumar K (2022) Biorefinery potential of Coelastrella biomass for fuel and bioproducts—a review. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-022-02519-9
Oliveira NG, Carvalho JLN, Chagas MF, Cerri CEP, Cerri CC, Feigl BJ (2017) Methane emissions from sugarcane vinasse storage and transportation systems: comparison between open channels and tanks. Atmos Environ. https://doi.org/10.1016/j.atmosenv.2017.04.005
Pang N, Gu X, Chen S, Kirchhof H, Lei H, Roje S (2019) Exploiting mixotrophy or improving prodctivities of biomass. Renew Sustain Energy Rev. https://doi.org/10.1016/j.rser.2019.06.001
Quintero-Dallos V, García-Martínez JB, Contreras-Ropero JE, Barajas-Solano AF, Barajas-Ferrerira C, Lavecchia R, Zuorro A (2019) Vinasse as a sustainable medium for the production of Chlorella vulgaris UTEX 1803. Water. https://doi.org/10.3390/w11081526
Ramirez NNV, Farenzena M, Trierweiler JO (2014) Growth of microalgae Scenedesmus sp. in ethanol vinasse. Braz Arch Biol Technol. https://doi.org/10.1590/S1516-8913201401791
Ristaino JB, Madritch M, Trout CL, Parra G (1998) PCR amplification of ribosomal DNA for species identification in the plant pathogen genus Phytophthora. Appl Environ Microbiol. https://doi.org/10.1128/aem.64.3.948-954.1998
Santana H, Cereijo CR, Teles VC, Nascimento RC, Fernandes MS, Brunale P, Campanha RC, Soares IP, Silva FCP, Sabaini PS, Siqueira FG, Brasil BSAF (2017) Microalgae cultivation in sugarcane vinasse: selection, growth and biochemical characterization. Bioresour Technol. https://doi.org/10.1016/j.biortech.2016.12.075
Scherer MD, de Oliveira AC, Filho FJCM, Ugaya CML, Mariano AB, Vargas JVC (2017) Environmental study of producing microalgal biomass and bioremediation of cattle manure effluents by microalgae cultivation. Clean Technol Environ Policy. https://doi.org/10.1007/s10098-017-1361-x
Silva TL, Moniz P, Silva C, Reis A (2021) The role of heterotrophic microalgae in waste conversion to biofuels and bioproducts. Processes. https://doi.org/10.3390/pr9071090
Soto MF, Diaz CA, Zapata AM, Higuita JC (2021) BOD and COD removal in vinasses from sugarcane alcoholic distillation by Chlorella vulgaris: environmental evaluation. Biochem Eng J. https://doi.org/10.1016/j.bej.2021.108191
Sydney EB, Neto CJD, de Carvalho JC et al (2019) Microalgal biorefineries: Integrated use of liquid and gaseous effluents from bioethanol industry for efficient biomass production. Bioresour Technol 292:121955. https://doi.org/10.1016/j.biortech.2019.121955
Tamura K, Stecher G, Kumar S (2021) MEGA11: molecular evolutionary genetics analysis version 11. Mol Biol Evol 38(7):3022–3027. https://doi.org/10.1093/molbev/msab120
Thatoi H, Behera BC (2013) Biodiversity and biotechnological potential of microorganisms from mangrove ecosystems: a review. Ann Microbiol. https://doi.org/10.1007/s13213-012-0442-7
Trevisan E, Godoy RFB, Radomski FAD, Crisigiovanni EL, Branco KBZF, Arroyo PA (2020) Chlorella vulgaris growth in different biodigested vinasse concentrations: biomass, pigments and final composition. Water Sci Technol. https://doi.org/10.2166/wst.2020.192
Vasistha S, Balakrishnan D, Manivannan A, Rai MP (2023) Microalgae on distillery wastewater treatment for improved biodiesel production and cellulose nanofiber synthesis: a sustainable biorefinery approach. Chemosphere. https://doi.org/10.1016/j.chemosphere.2022.137666
Vidotti ADS, Riaño-Pachón DM, Mattiello L, Giraldi LA, Winck FV, Franco TT (2020) Analysis of autotrophic, mixotrophic and heterotrophic phenotypes in the microalgae Chlorella vulgaris using time-resolved proteomics and transcriptomics approaches. Algal Res 51:102060. https://doi.org/10.1016/j.algal.2020.102060
Viêgas CV (2010) Extracao e caracterizacao dos lipídeos da microalga Chlorella pyrenoidosa visando à producao de ésteres graxos. Dissertacao para obtencao do título de mestre; Programa de pós-graduacao em química tecnológica e ambiental, FURG, Rio Grande, RS
Wang Q, Song H, Liu X, Liu B, Hu Z, Liu G (2019) Morphology and molecular phylogeny of coccoid green algae Coelastrella sensu lato (Scenedesmaceae, Sphaeropeales), including the description of three new species and two new varieties. J Phycol. https://doi.org/10.1111/jpy.12915
Wychen SV, Ramirez K, Laurens LML (2015) Determination of total lipids as fatty acid methyl esters (FAME) by in situ transesterification. National Renewable Energy Laboratory (NREL), Golden, USA
Zavřel T, Červený J, Sinetova MA (2015) Measurement of chlorophyll a and caratonoids concentration in cyanobacteria. Bio-Protoc 5:1–5
Zhao D, Cheah WY, Lai SH et al (2023) Symbiosis of microalgae and bacteria consortium for heavy metal remediation in wastewater. J Environ Chem Eng 11:109943. https://doi.org/10.1016/J.JECE.2023.109943
Acknowledgements
The authors gratefully acknowledge support from Shell Brasil together with FAPESP through the Research Centre for Greenhouse Gas Innovation—RCGI (Fapesp Proc. 2020/15230-5), hosted by the University of São Paulo, and the strategic importance of the support given by ANP (Brazil’s National Oil, Natural Gas, and Biofuels Agency) through the R&D levy regulation. The author Borrego B.B. thank The Brazilian National Council for Scientific and Technological Development (CNPq), grant 140997/2021-0. The author Melo L.B.U. thank Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Silva, S., Melo, L.B.U., Borrego, B.B. et al. Sugarcane vinasse as feedstock for microalgae cultivation: from wastewater treatment to bioproducts generation. Braz. J. Chem. Eng. (2023). https://doi.org/10.1007/s43153-023-00399-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43153-023-00399-8