Abstract
The sugarcane industry generates vinasse waste that could have serious environmental effects if not properly disposed of. This study investigated the use of vinasses generated in the sugarcane industry in Arequipa, Peru, as a growth medium for the microalga Chlorella sp. and lipid production. The results demonstrate that the best culture conditions for this microalga in a 4-L photobioreactor included a pretreatment of crude vinasse and an inoculum size of 1.0 × 106 cells mL−1. In these conditions, Chlorella sp. attained a growth rate of 1.19 day−1, showing high efficiency in nutrient removal from this residue and attaining a lipid content of 11.5 mg L−1 when cultured with a vinasse concentration of 10%. However, the highest lipid productivity (2.6 mg L−1 day−1) was recorded in cultures of 20% vinasse. Overall, our results suggest that it is possible to integrate microalgae culture as a biological strategy to properly dispose of the vinasse generation of Arequipa’s sugarcane industry; thus, modern technologies can be used for the purpose of valorizing this agro-industrial residue.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The distillation of ethanol from sugarcane molasses is a significant industrial activity in several countries. Particularly in Peru, the nationwide production of ethanol reached 58 million liters by 2020 (PRODUCE 2021). Vinasses are the main liquid by-product of the sugarcane industry and are generated in a proportion of 9–14 L for each liter of distilled alcohol (España-Gamboa et al. 2011). Vinasse is a dark brown liquid with a high content of organic/inorganic compounds, nutrients, and minerals (España-Gamboa et al. 2011) and a high polluting capacity due to its acid pH (3.5 − 5.0), as well as its biochemical oxygen demand (BOD) and chemical oxygen demand (COD). The index of its polluting character ranges between 35–50 and 100–150 g L−1, respectively (Pant and Adholeya 2007). To date, the rich nutrient content of vinasses has been used as fertilizer for sugarcane crop production. However, the direct discharge of vinasses can negatively affect the physicochemical properties of soil and groundwater, generating a severe environmental impact (Altenhofen et al. 2017). Therefore, the U.S. Environmental Protection Agency forbids the inappropriate disposal of vinasses into rivers, lakes, oceans, and soil without prior conditioning treatment (Colin et al. 2016). Nevertheless, in Peru, small and medium-sized distillery companies do not have the appropriate plants for effectively treating their vinasses before discharging them into waterbodies. Hence, it is evident that there is a need to seek creative solutions for the valorization and sustainable disposal of this agro-industrial residue in this country.
Recently, the circular economy based on reduction, reuse, and recycling is an approach that describes the valorization of agro-industrial residues as inputs for the production of value-added active components (Nagarajan et al. 2020). In this regard, the valorization of vinasses through biomass and high-valuable metabolite production by microalgae is an important strategy to dispose of this residue and comply with government regulations (Nagarajan et al. 2020; Fernández et al. 2021). Such approaches also simultaneously reduce the costs of microalgae production, since nutrients such as carbon, nitrogen, phosphorous, and minerals represent 50% of the total cost of microalgal production (Zhang et al. 2016). Today, vinasses generated from different feedstocks, such as sugarcane, beet, grape, and corn, have been used as nutrient sources for culturing microalgae in different countries (Carrilho et al. 2016). Different microalgae genera, such as Chlorella, Scenedesmus, Chlamydomonas, Neochloris, and Micractinium, have been cultured in vinasses. However, each microalga shows different growth patterns and biochemical composition due to the different capacities of each strain, as well as the different culture strategies used (Choix et al. 2021). For instance, the concentration of vinasse and the inoculum size of microalga are critical factors in successfully valorizing this residue (Bohutskyi et al. 2016; Li et al. 2017).
Several years ago, Olguín et al. (2015) demonstrated that the effluents of diluted vinasses can be used as nutrient sources with the potential for lipid production by Chlorella. A few years later, Tan et al. (2018) demonstrated that vinasses with a high sugar content can also stimulate lipid production in microalgae growing under a mixotrophic regime. In particular, under a mixotrophic growth regimen, microalgae can assimilate organic and inorganic carbon, attaining higher biomass and valuable-compound production than when cultured under a heterotrophic and autotrophic regime (Pang et al. 2019; Patel et al. 2020). Thus, the use of industrial residues to support microalgal biomass production with high lipid content could constitute a sustainable strategy for valorizing industrial by-products in an environmentally friendly way (Engin et al. 2018). Furthermore, microalgae have attributes, such as resistance, that make them candidates for further valorization systems of agro-industrial residues, combining the environmental benefits of using waste with the production of biomolecules and/or biomass of commercial interest (Candido et al. 2022).
Considering that microalgae culture supported by agro-industrial residues is considered a strategy to valorize nutrient content and produce highly valuable metabolites, this research aimed to evaluate the use of sugarcane vinasses generated in the region of Arequipa, Peru, as a culture media. Specifically, vinasses can be used to support microalgal biomass and lipid production by the native microalga Chlorella sp., which is a sustainable strategy for valorizing this agro-industrial residue. Furthermore, the effects of the concentration of vinasses and inoculum size on the growth and lipid production of Chlorella sp. were studied. The cultivation of this microalga in vinasses was also scaled up in a 4-L photobioreactor.
Materials and methods
Microalga
The Chlorophyceae strain was Chlorella sp. isolated from Chucarapi, Cocachacra, Arequipa-Peru (latitude 17°4′24'' S, longitude 71°43′18'' W) and identified according to Bellinger and Sigee (2015). This microalga is maintained and protected at the Laboratory of Genetics of the Professional School of Biology at the Universidad Nacional de San Agustín de Arequipa (UNSA) and cultured in BG11 medium (Rippka et al. 1979) at 24 ± 2 °C, 12 h/12 h light/dark regimen, with a light intensity of 48 μmol photons m−2 s−1. The substance was stirred by aeration supplied by an air pump operating at a flow rate of 2.4 L min−1, using cotton and activated carbon for air filtration.
Sugarcane vinasses
Crude vinasses were supplied by a local industry located in the Cocachacra district of Arequipa, Peru. A total of 100 L of crude vinasses were collected in the outlet of the separation column and preserved at –15 °C until their use. The physicochemical composition of the crude vinasses is shown in Table 1. Before experimentation, samples of the crude vinasses were centrifuged at 3000 xg for 10 min. Subsequently, the centrifuged vinasses were diluted by the addition of distilled water at the following proportions: 5, 7.5, 10, 15, 20, 25, 30, 35, and 40%. The composition of the centrifuged vinasses is shown in Table 4.
Experimental setup
First, the suitable inoculum size of Chlorella sp. cultured in sugarcane vinasses was determined. Briefly, from a culture of Chlorella sp. with a cell density of 2.0 × 107 cells mL−1, 250 mL of centrifuged vinasse diluted at 5, 7.5, and 10% with distilled water (treatments), as well as 250 mL of BG11 medium (control), were inoculated until they reached an initial cell density of 5.0 × 104, 1.2 × 105, 1.0 × 106, and 1.8 × 106 cells mL−1, respectively. The pH of each treatment was adjusted to 7.0 using a 5 M NaOH solution. Each culture was carried out using flat plate glass bioreactors (Supplementary Fig. 1) and maintained at 25 ± 2° C, with a light intensity of 60 μmol photons m−2 s−1 under a regimen of 12/12 h light/dark over the course of 7 days. Bioreactors were stirred by a constant air flow supplied through air pumps.
Subsequently, the appropriate concentrations of centrifuged vinasses of a specific pH (7 or 8) to support the highest growth of Chlorella sp. were evaluated. Therefore, 250 mL of centrifuged vinasses diluted at 10, 15, 20, 25, 30, 35, and 40% with distilled water (treatments) and 250 mL of BG11 medium (control) were inoculated with the inoculum size of Chlorella sp. previously selected. Each culture was carried out and maintained under the aforementioned conditions. In both experimental setups, the cell density of Chlorella sp. was determined each 24 h using a Neubauer hemocytometer.
In the second set of experiments, 4 L of centrifuged vinasse diluted at 5, 10, 15, 20, and 25% were inoculated with the inoculum size of Chlorella sp. and the vinasse concentration formerly selected (Fig. 1). The pH was adjusted to 7.0 with the addition of a 10 M NaOH solution. Each culture was conducted at 25 ± 2 °C, with a light intensity of 60 μmol photons m−2 s−1 under a regimen of 12/12 h light/dark for 6 days. The physicochemical characterization of the vinasses was determined every third day.
Schematic diagram of the lab-scale airlift flat plate photobioreactors. [a] air pumps; [b] hydrophobic membrane filter; [c] pressure regulators; [d] sample outlet; [e] regulator thermostat; [f] Water distiller. [1] sample inlet; [2] Gas inlet; [3] Gas diffuser; [4] Liquid upflow; [5] Liquid downflow; [6] Riser; [7] Gas outlet
Analytical methods
Vinasses characterization was determined according to the standard method SMEWW-APHA-AWWA-WEF (2015/2017): Total Suspended Solids (Dried at 103 − 105 °C); BOD (5-Day BOD Test); COD (Closed Reflux, Colorimetric); Nitrate (Cadmium reduction method); Nitrite (Colorimetric method); Ammoniacal nitrogen (Ammonia-selective electrode method); Potassium (Direct air-acetylene flame method); Total Nitrogen (Nitrogen macro-Kjeldahl method); Sulfate (Turbidimetric method); and Phosphate (Ascorbic acid method). The efficiency of nutrients removal was calculated using Eq. 1.
where Ci is the initial concentration of nutrients, Cn is the final concentration of nutrients.
The yield and productivity of biomass, as well as the specific growth rate of Chlorella sp. in each treatment were determined through the Eqs. 2, 3, and 4 according to Gao et al. (2018).
where Xmax and X1 are the maximum concentration and initial concentration during the period of cultivation, respectively. Δt is interval of time (in days) between Xmax and X1. The specific growth rate was determined during the period of exponential growth phase.
At the end of experimental time (6 days), from the 4-L glass bioreactors, 1 L of sample was taken of each treatment and was acidified to pH ≤ 2 with 6 M H2SO4 solution. Subsequently, the concentration of lipids was determined using the EPA Method 1664 (Cheng et al. 2017). The yield and productivity of lipids in each treatment were calculated according to Eqs. 5 and 6.
where Lmax and L1 are the maximum concentration and initial concentration of lipids in the culture media, respectively. Δt is the interval of time (in days) between Lmax y L1.
Statistical analysis
Each experiment setup was performed in triplicate and repeated thrice (n = 9). The data from each treatment from the three replicates were combined for analyses of variance using Fisher’s least significant difference (LSD) post hoc analysis with significance p < 0.05, using Statistica 6.0 software (StatSoft).
Results
Inoculum size of Chlorella sp. cultured in centrifuged sugarcane vinasses
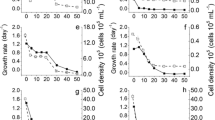
Chlorella species inoculated at 5.0 × 104 and 1.2 × 105 cells mL−1 showed low cell density cultured in each vinasse concentration used (5, 7.5, and 10%) with respect to the BG11 medium (control) during all experimental times (Fig. 2a, b). However, this microalga inoculated at 1.0 × 106 cells mL−1 attained a cell density statistically similar to BG11, recording 16.0 (BG11), 16.2 (5% vinasse), 15.8 (7.5% vinasse), and 16.4 (10% vinasse) natural logarithms (ln) of cell density−1 at 7 days. (Fig. 2c, lowercase analysis). Meanwhile, when cultured at 1.8 × 106 cells mL−1, C. vulgaris only grew up to the fourth day of incubation; subsequently, its cell density decreased in each vinasse concentration evaluated (Fig. 2d).
Cell density (natural logarithm) of Chlorella sp. inoculated in centrifuged sugarcane vinasses with different inoculum size. Points denoted by different lowercase letters differ significantly when Chlorella sp. was growing in different concentration of centrifuged sugarcane vinasses (n = 9). Statistical analyses were performed using Analysis of Variance (ANOVA) and Least Significant Difference (LSD) post hoc analysis at p < 0.05. Bars represent standard error
Similarly, the maximum biomass productivity attained by Chlorella sp. was also directly proportional to the growth curves recorded for each concentration of vinasse used (Supplementary Table 1). Thus, the inoculum size of Chlorella sp. with a concentration of 1.0 × 106 cells mL−1 was selected for further evaluation of the growth and lipid production from the centrifuged sugarcane vinasses.
Growth of Chlorella sp. using centrifuged sugarcane vinasses with different pH
Chlorella sp. cultured in vinasses recorded a growth indirectly proportional to vinasse concentration used either with pH 7 (Fig. 3a) or pH 8 (Fig. 3b). In both pHs this microalga had the capacity to grow with a concentration of 10 to 25% of centrifuged vinasses. In contrast, concentrations of 30; 35; and 40% of vinasses decreased the growth of Chlorella sp. during all incubation time. At the end of experimental time, cultured in pH 7 Chlorella sp. recorded a cell density of 16.51 ± 0.06; 16.29 ± 0.10; 15.95 ± 0.07; 15.43 ± 0.22; 15.04 ± 0.12 ln of cell density−1 growing in BG11 medium; 10; 15; 20; and 25% of vinasses, respectively (Fig. 3a). Similarly, growing under pH 8 the cell density attained by this microalga was 16.51 ± 0.06; 16.06 ± 0.03; 15.58 ± 0.09; 15.62 ± 0.29; 15.66 ± 0.12 ln of cell density−1, respectively (Fig. 3b). In both pHs, the cell density of Chlorella sp. attained in these vinasses concentration was statistically similar at 7 days (Fig. 3a, b lowercase analysis). Correspondingly, the highest biomass yield, and productivity as well as specific growth rates were obtained when Chlorella sp. was growing 10; 15; 20; 25% of centrifuged vinasses (Table 2).
Cell density (natural logarithm) of Chlorella sp. inoculated in centrifuged sugarcane vinasses with different pH. Points denoted by different lowercase letters differ significantly when Chlorella sp. was growing in different concentration of centrifuged sugarcane vinasses (n = 9). Statistical analyses were performed using Analysis of Variance (ANOVA) and Least Significant Difference (LSD) post hoc analysis at p < 0.05. Bars represent standard error
Lipid production by Chlorella sp. from centrifuged sugarcane vinasses
Figure 4 shows the lipids produced by Chlorella sp. as a function of the vinasse concentration. When cultured under each vinasse concentration used, Chlorella sp. recorded the highest lipid production at the end of the incubation period (7 days). Growing with a load of 10% of vinasses, this microalga attained the highest lipid production (15.4 ± 2.3 mg L−1), followed by a culture of 20% (12.3 ± 1.98 mg L−1) and 15% vinasse (10.9 ± 2.0 mg L−1). However, there were no significant differences between these treatments (Fig. 4, lowercase analysis). Conversely, the concentrations of 25 and 5% of vinasses induced the lowest lipid production by this microalga. Likewise, the highest productivity and yield lipid by Chlorella sp. were also recorded in the cultures with loads of 10 and 20% of vinasses, respectively (Table 3).
Lipid production by Chlorella sp. from different concentrations of centrifuged sugarcane vinasse. Points denoted by different lowercase letters differ significantly (n = 9). Statistical analyses were performed using Analysis of Variance (ANOVA) and Least Significant Difference (LSD) post hoc analysis at p < 0.05. Bars represent standard error
The physicochemical characteristics of vinasses after supporting the growth of this microalga, as well as the removal efficiency of nutrients such as nitrogen, phosphorous and carbon oxygen demand (COD) from the vinasse concentration used, are shown in Tables 4.
Discussion
This study evaluated the use of sugarcane vinasses generated in the region of Arequipa, Peru, as a culture medium to support microalgal biomass and lipid production by the native microalga Chlorella sp. as a sustainable strategy to valorize this by-product.
Our results demonstrate that centrifuged sugarcane vinasses can support biomass and lipid production by Chlorella sp. This can be attributed to suitable nutrient composition, such as carbon, nitrogen and phosphorous, as well as the capacity of this microalga to endure the specific physicochemical characteristics of this residue. Nonetheless, the inoculum size of Chlorella sp. is a vital factor in producing biomass from agro-industrial residues. The low cell density recorded by this microalga inoculated in small inoculum sizes (5.0 × 104 and 1.2 × 105 cells mL−1) in each vinasse concentration evaluated could be because their growth was surpassed by other microorganisms, such as bacteria and yeast, since non-sterile vinasses were used in this study. In a previous study, Bohutskyi et al. (2016) demonstrated that increasing the algal inoculum has a significant effect on relations between microalgae and wastewater-borne bacteria, especially in competition for organic carbon and nutrients. Meanwhile, when inoculated at 1.0 × 106 cells mL−1, Chlorella sp. recorded the highest cell densities and biomass productivities in each vinasse concentration assessed, indicating that in this inoculum size, the growth of Chlorella sp. was not surpassed by other microorganisms (Supplementary Fig. 1).
Li et al. (2017) demonstrated that C. vulgaris 1067 attained a higher cell density from post hydrothermal liquefaction wastewater when cultured with a high rather than low inoculum size. Surprisingly, the cell density of Chlorella sp. inoculated at 1.8 × 106 cells mL−1 decreased after 4 days of incubation in each concentration of vinasses. This could have happened due to fact that the ideal nutrient ratio for supporting microalgae growth from agro-industrial residues (127 carbon/16 nitrogen/8 phosphorous; Fernández et al. 2021) was modified in each dilution. Thus, the nutrient ratio used in this study could not support the growth of this inoculum size after 4 days. These results demonstrate the importance of selecting the appropriate inoculum size for each microalga strain to grow and produce biomass from sugarcane vinasses.
Each microalga strain has its own pH, ideal to maximize its growth. In this study, Chlorella sp. recorded a performance similar to the production of biomass when cultured in centrifuged vinasses of either pH 7 or 8. This result could be explained by Chlorella sp. being a robust strain that can grow in a wide pH range (Mayo 1997). Previously, Santana et al. (2017) reported the growth of non-axenic Micractinium sp. and Chlamydomonas biconvexa at pH 8 for the purpose of reducing contamination by heterotrophic microorganisms. In particular, Chlorella has the potential to endure the stressful conditions of agro-industrial residues because of its capacity to mediate anti-oxidative defense through the activities of various reactive oxygen species (ROS) scavenging enzymes, such as ascorbate peroxidase (APX; EC 1.11.1.11; Osundeko et al. 2014). To date, different methods of vinasse purification are well known. For instance, the filtration process with activated carbon can remove or diminish the load of phenolic compounds, allowing the growth of microalgae from the nutrimental characteristics of these effluents (Candido and Lombardi 2017; Choix et al. 2021). In our work, the pretreatment strategy included centrifugation and dilution to create an appropriate vinasse to allow for light penetration and to increase the photosynthetic activity of microalgae (Candido and Lombardi 2020). Nonetheless, the toxic effects of vinasses were observed in this study, since the increment of vinasse concentration (30, 35, and 40%) induced low biomass yield and productivities of Chlorella sp. The above could be attributed to the presence of phenolic compounds and melanoidins, since these compounds have toxic effects on algae growth (España-Gamboa et al. 2011).
In addition, the high level of organic material in vinasses could induce an osmotic effect in some microalgae, causing cell degradation (Kadioǧlu and Algur 1992). Furthermore, each microalga strain has a different capacity to endure the harsh conditions of each agro-industrial residue (Choix et al. 2021). For instance, Marques et al. (2013) claimed that sugarcane vinasses are highly toxic in concentrations higher than 4% to Chlorella vulgaris. Likewise, Barrocal et al. (2010) reported a decrease in biomass production of Spirulina maxima proportional to an increase in beet vinasse concentration. In another study, Budiyono et al. (2014) indicated that concentrations of vinasses higher than 0.8% inhibited the growth of S. platensis because the dark color and turbidity inhibited the penetration of light in the culture medium. The above findings suggest that Chlorella sp., as used in this study, has the ability to grow when supplied with a load of centrifuged sugarcane vinasses of up to 25% (Reference Table 5). This latter factor highlights the vital activity of determining the ideal concentration of each residue to maximize microalgal biomass production.
It should also be noted that Chlorella sp. showed the ability to produce lipids as a bioproduct of commercial interest for the industry due to the nutrient content of sugarcane vinasse. According to Heidari et al. (2016), the most important parameter for the success of any bioprocess based on microalgae is the production of lipids. Regardless, the different dilutions of vinasses evaluated in this study could have modified the nutrient ratio, thus inducing distinct growth patterns and biochemical compositions (Choix et al. 2018). This could explain the high growth and lipid content of this microalga when cultured in 10% of centrifuged sugarcane vinasses, suggesting that this concentration is suitable for lipid production by this microalga. As mentioned in the methods section, we worked with centrifuged non-sterile vinasses. Thus, the microbiota of this residue might also have contributed to the lipid content, thus hindering the accurate determination of lipid production by Chlorella sp. from this residue. This topic needs further investigation, but the result is similar to one reported by Barcia et al. (2020), who found that 10% of tequila vinasses induced the highest biomass productivity of microalgae-yeast flocs.
Previously, Yang et al. (2015) stated that the addition of vinasses as organic and inorganic carbon sources can simultaneously trigger biomass and lipid production. In another study, Tan et al. (2018) reported that wastewater as a carbon source enhances biomass and lipid production by C. vulgaris. Similarly, under our experimental conditions, Chlorella sp. assimilated the highest content of nitrogen when cultured in 10% sugarcane vinasses, recording the highest efficiency nutrient removal and confirming that in this concentration, the physiological performance of this microalga was appropriate. Recently, Rahimi and Jazini (2021) found that Chromochloris zofingiensis cultured in 1.2 g L−1 of vinasses almost completes the consumption of COD, TOC, nitrogen, potassium, magnesium, phosphorous, and sulfur of a particular medium. However, in this study, the growth of Chlorella sp. cultured in 10% of non-sterile vinasses was not surpassed by yeast and bacteria (Supplementary Fig. 1). The incidence of these microorganisms in nutrient assimilation should also be evaluated further.
In this study, after supporting the culture of Chlorella sp., the content BOD5, COD, and potassium (K+) in vinasses still remained high, which allows for the supernatant to be reused as a fertilizer in the sugar cane agroindustry following the concept of a circular bio-economy. Finally, our results demonstrate that the nutrient composition of centrifuged sugarcane vinasses can support the growth of Chlorella sp., which is native to Arequipa, Peru, thus providing a suitable strategy for the industrial sector of this country.
Conclusions
Overall, the use of vinasses from Arequipa’s sugarcane industry as a growth medium for Chlorella sp. resulted in the simultaneous reduction of organic and inorganic compounds and high biomass productivity. Moreover, the growth of Chlorella sp. along with the other microorganisms of this residue, could enable lipid production in this native strain. Ultimately, the results show the feasibility of integrating microalgae culture with the sugarcane industry for the purpose of valorizing this agro-industrial residue.
Data availability
The datasets analyzed during the current study are available from the Institutional Repository of Universidad Nacional de San Agustín de Arequipa (UNSA; http://repositorio.unsa.edu.pe/handle/UNSA/9726), but restrictions apply for the availability of these data which were used under license of UNSA. However, data are available upon reasonable request and permission of Universidad Nacional de San Agustín de Arequipa.
References
Altenhofen SM, Barbosa GH, Brito Codato C, Arjonilla de Mattos LF, Gaspar BR, Kieckbusch TG (2017) Heterotrophic growth of green microalgae Desmodesmus subspicatus in ethanol distillation wastewater (vinasse) and lipid extraction with supercritical CO2. J Chem Technol Biotechnol 92:573–579
Barcia GEC, Cervantes RAI, Zuniga IT, Van Den Hende S (2020) Converting tequila vinasse diluted with tequila process water into microalgae-yeast flocs and dischargeable effluent. Bioresour Technol 300:122644
Barrocal VM, García-Cubero MT, González-Benito G, Coca M (2010) Production of biomass by Spirulina maxima using sugar beet vinasse in growth media. New Biotechnol 27:851–856
Bellinger EG, Sigee DC (2015) Freshwater algae: identification and use as bioindicators. Wiley, London
Bohutskyi P, Kligerman DC, Byers N, Nasr LK, Cua C, Chow S, Su C, Tang Y, Betenbaugh MJ, Bouwer EJ (2016) Effects of inoculum size, light intensity, and dose of anaerobic digestion centrate on growth and productivity of Chlorella and Scenedesmus microalgae and their poly-culture in primary and secondary wastewater. Algal Res 19:278–290
Budiyono IS, Sumardiono S, Sasongko SB (2014) Production of Spirulina platensis biomass using digested vinasse as cultivation medium. Trends Appl Sci Res 9:93–102
Candido C, Lombardi AT (2017) Growth of Chlorella vulgaris in treated conventional and biodigested vinasses. J Appl Phycol 29:45–53
Candido C, Lombardi AT (2020) Mixotrophy in green microalgae grown on an organic and nutrient rich waste. World J Microbiol Biotechnol 36:20
Candido C, Lombardi AT, Lima MIS (2016) Cultivo de Chlorella vulgaris em vinhaça filtrada. Rev Bras Cienc Amb 35:55–62
Candido C, Cardoso LG, Lombardi AT (2022) Bioprospecting and selection of tolerant strains and productive analyses of microalgae grown in vinasse. Braz J Microbiol 53:845–855
Carrilho ENVM, Labuto G, Kamogawa MY (2016) Destination of vinasse, a residue from alcohol industry: Resource recovery and prevention of pollution. In: Prasad MNV, Shih K (eds) Environmental materials and waste. Academic Press, NY, pp 21–43
Cheng T, Wei CH, Leiknes T (2017) Polishing of anaerobic secondary effluent by Chlorella vulgaris under low light intensity. Bioresour Technol 241:360–368
Choix FJ, Ochoa-Becerra MA, Hsieh-Lo M, Mondragón-Cortez P, Méndez-Acosta HO (2018) High biomass production and CO2 fixation from biogas by Chlorella and Scenedesmus microalgae using tequila vinasses as culture medium. J Appl Phycol 30:2247–2258
Choix FJ, Ramos-Ibarra JR, Mondragón-Cortez P, Lara-González MA, Juárez-Carrillo E, Becerril-Espinoza A, Ocampo-Álvarez H, Torres JR (2021) Mixotrophic growth regime as a strategy to develop microalgal bioprocess from nutrimental composition of tequila vinasses. Bioprocess Biosyst Eng 44:1155–1166
Colin VL, Cortes ÁAJ, Aparicio JD, Amoroso MJ (2016) Potential application of a bioemulsifier-producing actinobacterium for treatment of vinasse. Chemosphere 44:842–847
de Melo RG, de Andrade AF, Bezerra RP, Correia DS, de Souza VC, Brasileiro-Vidal AC, Porto ALF (2018) Chlorella vulgaris mixotrophic growth enhanced biomass productivity and reduced toxicity from agro-industrial by-products. Chemosphere 204:344–350
Engin IK, Cekmecelioglu D, Yücel AM, Oktem HA (2018) Evaluation of heterotrophic and mixotrophic cultivation of novel Micractinium sp. ME05 on vinasse and its scale up for biodiesel production. Bioresour Technol 251:128–134
España-Gamboa E, Mijangos-Cortes J, Barahona-Perez L, Dominguez-Maldonado J, Hernández-Zarate G, Alzate-Gaviria L (2011) Vinasses: characterization and treatments. Waste Manag Res 29:1235–1250
Fernández FGA, Reis A, Wijffels RH, Barbosa M, Verdelho V, Llamas B (2021) The role of microalgae in the bioeconomy. N Biotechnol 61:99–107
Gao F, Peng YY, Li C, Yang GJ, Deng YB, Xue B, Guo YM (2018) Simultaneous nutrient removal and biomass/lipid production by Chlorella sp. in seafood processing wastewater. Sci Total Environ 640:943–953
Heidari M, Kariminia HR, Shayegan J (2016) Effect of culture age and initial inoculum size on lipid accumulation and productivity in a hybrid cultivation system of Chlorella vulgaris. Process Saf Environ 104:111–122
Kadioǧlu A, Algur ÖF (1992) Tests of media with vinasse for Chlamydomonas reinhardii for possible reduction in vinasse pollution. Bioresour Technol 42:1–5
Li Z, Haifeng L, Zhang Y, Shanshan M, Baoming L, Zhidan L, Na D, Minsheng L, Buchun S, Jianwen L (2017) Effects of strain, nutrients concentration and inoculum size on microalgae culture for bioenergy from post hydrothermal liquefaction wastewater. Int J Agric Biol Eng 10:194–204
Marques SSI, Nascimento IA, de Almeida PF, Chinalia FA (2013) Growth of Chlorella vulgaris on sugarcane vinasse: the effect of anaerobic digestion pretreatment. Appl Biochem Biotechnol 171:1933–1943
Mayo AW (1997) Effects of temperature and pH on the kinetic growth of unialga Chlorella vulgaris cultures containing bacteria. Water Environ Res 69:64–72
Nagarajan D, Lee D, Chen C, Chang J (2020) Resource recovery from wastewaters using microalgae-based approaches: A circular bioeconomy perspective. Bioresour Technol 302:122817
Olguín EJ, Dorantes E, Castillo OS, Hernández-Landa VJ (2015) Anaerobic digestates from vinasse promote growth and lipid enrichment in Neochloris oleoabundans cultures. J Appl Phycol 27:1813–1822
Osundeko O, Dean AP, Davies H, Pittman JK (2014) Acclimation of microalgae to wastewater environments involves increased oxidative stress tolerance activity. Plant Cell Physiol 55:1848–1857
Pang N, Gu X, Chen S, Kirchhoff H, Lei H, Roje S (2019) Exploiting mixotrophy for improving productivities of biomass and co-products of microalgae. Renew Sustain Energy Rev 112:450–460
Pant D, Adholeya A (2007) Biological approaches for treatment of distillery wastewater: a review. Bioresour Technol 98:2321–2334
Patel AK, Choi YY, Sim SJ (2020) Emerging prospects of mixotrophic microalgae: Way forward to sustainable bioprocess for environmental remediation and cost-effective biofuels. Bioresour Technol 300:122741
PRODUCE (2021) Anuario Estadístico Industrial, MIPYME y Comercio Interno 2020. Ministerio de la Producción del Perú. https://ogeiee.produce.gob.pe/index.php/en/shortcode/oee-documentos-publicaciones/publicaciones-anuales/item/download/801_a8c6a5d223b51aa720694c500fb6ea0d (retrieved on 25 Feb 2022)
Rahimi M, Jazini M (2021) Mixotrophic cultivation of Chromochloris zofingiensis on glycerol, acetate, and vinasse. J Appl Phycol 33:3579–3590
Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 111:1–61
Santana H, Cereijo CR, Teles VC, Nascimento RC, Fernandes MS, Brunale P, Campanha RC, Soares IP, Silva FCP, Sabaini PS, Siqueira FG, Brasil BSAF (2017) Microalgae cultivation in sugarcane vinasse: Selection, growth and biochemical characterization. Bioresour Technol 228:133–140
Tan XB, Zhao XC, Zhang YL, Zhou YY, Yang LB, Zhang WW (2018) Enhanced lipid and biomass production using alcohol wastewater as carbon source for Chlorella pyrenoidosa cultivation in anaerobically digested starch wastewater in outdoors. Bioresour Technol 247:784–793
Tedesque MG, Scardoeli-Truzzi B, Sipaúba-Tavares LH (2021) Messastrum gracile (Chlorophyceae) growth using sugarcane molasses-based macrophyte extract culture media. J Appl Phycol 33:2745–2754
Yang L, Tan X, Li D, Chu H, Zhou X, Zhang Y, Yu H (2015) Nutrients removal and lipids production by Chlorella pyrenoidosa cultivation using anaerobic digested starch wastewater and alcohol wastewater. Bioresour Technol 181:54–61
Zhang TY, Hu HY, Wu YH, Zhuang LL, Xu XQ, Wang XX, Dao GH (2016) Promising solutions to solve the bottlenecks in the large-scale cultivation of microalgae for biomass / bioenergy production. Renew Sustain Energy Rev 60:1602–1614
Acknowledgements
The authors are grateful to the Universidad Nacional de San Agustín de Arequipa (UNSA), UNSA INVESTIGA for supporting this work through the contract N° TP-002-2018-UNSA.
Author information
Authors and Affiliations
Contributions
MAMC performed and analyzed all experiments and drafted the manuscript. MVV assisted in monitoring and analysis of the experimental data. FAF and FJC served as critical reviewer and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Condori, M.A.M., Valencia, M.R.V., Fernández, F.G.A. et al. Evaluation of sugarcane vinasse as a medium for enhanced Chlorella sp. growth, lipids production, and process integration. J Appl Phycol 35, 581–591 (2023). https://doi.org/10.1007/s10811-022-02902-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-022-02902-z