Abstract
Phycoremediation is the use of algae for removal or reduction of inorganic nutrients and xenobiotics from wastewaters. It is a reliable process for biotransformation and detoxification of a variety of pollutants. This study focused on the potential of a strain of the green microalga, Chlorella vulgaris, to reduce various pollutants in tannery wastewater (TW). The microalga was grown in TW for a culture period of 21 days and the resultant removal/reduction/biotransformation of biochemical oxygen demand (BOD), chemical oxygen demand (COD), nitrates (NO3–N), phosphates (PO4–P), sulphates (SO4–S), dissolved solids and chromium (Cr) was monitored. The isolate was efficient in the removal of excess nutrients in wastewater. Most notably, complete removal (100 % reduction) of NO3–N and Cr was observed by the 6th and 12th day of culture period, respectively. Removal of phosphates was as high as 91.73 % by day 6 and over 99 % by day 21. This strain also reduced sulphate concentrations to 67.4 % by day 21. Levels of chemical oxygen demand (COD) and biochemical oxygen demand (BOD) in TW were reduced by 94.74 and 95.93 %, respectively, after 21 days. Our results are useful to suggest that this isolate of C. vulgaris is promising for bioremediating and detoxifying tannery wastewater to improve its quality to meet up recommended effluent discharge limits.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Globally, an estimated total of more than 52 billion domesticated animals are slaughtered every year for human consumption (FAO 2004) and the hides of larger animals are processed for varied uses. Common commercial hides include leather from cattle and other livestock animals which are used for manufacturing shoes, clothes, upholstery, interior decoration among many others.
The tanning industry is a significant contributor to economy and provides large scale employment opportunities in particular to unskilled and/or semiskilled people. Unfortunately, it is one of the worst anthropogenic polluters (Khwaja et al. 2001). Tanneries process the foul-smelling, damp, hairy, and assorted-sized hides employing cheap labor. In spite of the impending deleterious ecological consequences, the developing world still practices traditional, archaic processing technologies (Kennedy 1999). To circumvent the obnoxious impacts of tannery wastewater, several treatment methods have emerged during the early 1990s (Durai and Rajasimman 2011; Saranraj and Sujitha 2013).
Notably, most of the world’s tanneries are located in the Indian subcontinent. India alone has about 3,000 tanneries with an annual processing capacity of 700,000 t of hides and skins. These tanneries produce and discharge an estimated 30 billion liters of effluent annually (Srivastav 2012). Conventional leather tanning technology is highly polluting and produces large amounts of organic and chemical pollutants (Kabdasli et al. 2002). These pollutants, in the discharged effluent, pose serious threats to the environment.
Tannery wastewaters are characterized mainly by high levels of biochemical oxygen demand (BOD), chemical oxygen demand (COD), suspended solids (SS), total dissolved solids (TDS), chromium, and sulphides (Leta et al. 2004). Conventional wastewater treatment schemes typically include primary and secondary treatment. Primary treatment consists of sulphide oxidation, solids separation, and chromium precipitation. Secondary treatment is usually accomplished by activated sludge systems (Dotro 2003). The large amounts of sludge generated by wastewater treatment processes must be sent off-site for disposal. Handling and disposal of this sludge is typically the largest single cost component in the operation of a wastewater treatment plant. Activated sludge systems require very high capital as well as operation and maintenance costs. The cost factor coupled with the recent but increasingly stringent environmental regulations and standards (Bosnic et al. 2000; Kabdasli et al. 2002), has led the industry to search for new effective treatment technologies.
With these concerns, there has been increased interest in using biological methods for remediation of different industrial wastewaters. Most studies have concentrated on the use of fungi and bacteria to treat wastewater (McMullan et al. 2001; Tastan et al. 2010). However, additional carbon sources are required for such systems. Hence, the use of microalgae in bioremediation of industrial wastewaters is of practical interest due to their central role in autotrophic carbon dioxide fixation. The process of wastewater treatment using algae has low energy requirement, reduced sludge formation as well as GHG emission along with production of variously useful algal biomass. It has been shown to be a more cost effective method for removal of BOD, certain microbial pathogens, phosphorus, and nitrogen than activated sludge process and other secondary treatment processes (Sheehan et al. 1998).
Various techniques are in place for exploiting faster growth rates and nutrient removal efficiencies of certain microalgae for the treatment/detoxification of a variety of wastewaters. During the last four decades, phycoremediation has been evaluated by numerous investigators (e.g., Oswald 1988; Mara et al. 1996; Tadesse et al. 2003; Shi et al. 2007; Chu et al. 2008; Craggs et al. 2012; Mustafa et al. 2012; Dixit and Singh 2014; Posadas et al. 2014). Dunn (1998) and Dunn et al. (2013) examined the detoxification of tannery wastewater and nutrient removal using high-rate algal pond systems. Different microalgae and cyanobacteria such as species of Oscillatoria, Phormidium, Ulothrix, Chlamydomonas, Scenedesmus have been evaluated for their ability to grow in tannery effluent and accumulate chromium (Rai et al. 2005; Balaji et al. 2015; Ajayan et al. 2015). Notable reports on the use of Chlorella for bioremediation of tannery wastewater are those by Chellam and Sampathkumar (2012) and by Jaysudha and Sampathkumar (2014). Both free as well as immobilized cells of Chlorella salina or Chlorella marina have been examined mainly for removal of nutrients in tannery wastewater. Chlorella vulgaris was used by Rao et al. (2011) for removal of nutrients from tannery wastewater and by Hernández-Zamora et al. (2015) for removal of Congo Red, an azo-dye used for dyeing cotton, jute, leather, paper, silk, and wool.
In view of such advantages offered by microalgae, this study aimed at evaluating the microalga, C. vulgaris for its potential in reducing BOD, COD, sulphates, inorganic nutrients, dissolved solids, and chromium in the effluent. Our results demonstrate a very highly efficient, minimal energy requiring improvement of tannery wastewater by this salt-tolerant microalgal strain.
Materials and methods
Salt tolerant microalga Chlorella vulgaris NIOCCV was used in this study. It was grown in algal culture medium (ACM, HiMedia, Mumbai, India) at a constant temperature of 28 ± 0.5 °C under fluorescent lights at 150–300 μmol photons m−2 s−1 (with 10:14-h light/dark photoperiod).
Characteristics of tannery wastewater
The tannery wastewater (TW) was collected from outside the discharge point of a tannery industry located in Kanpur, in Northern India on the banks of River Ganges. Various physical and chemical parameters of the wastewater collected were analyzed using standard methods in APHA (American Public Health Association 2005).
Experimental design
Initially, the growth of NIOCCV was tested in different strengths of TW viz., 100 % (no dilution), 70 % (7:3), 50 % (1:1), 30 % (3:7), and 10 % (1:9). The original TW was diluted to these strengths using tap water. Even after several trials of altering inoculum size, light periods, and aeration, very poor growth was observed in the 100 and 70 % TW unlike the vigorous growth (vis-a-vis that in standard algal culture medium) in 50, 30, or 10 % TW. Hence, we tested the bioremediation potential of this strain in 1:1 diluted TW.
Tannery wastewater was diluted to 50 % (1:1) with tap water and 100 mL aliquots of this dilution were dispensed into several 250-mL Erlenmeyer flasks. Ten milliliter of exponential algal culture, standardized to an optical density (OD 620 nm) of 0.2, were inoculated into eight triplicate sets of 250-mL flasks containing 100 mL of 1:1 TW. The culture was grown for 21 days at a constant temperature of 28 ± 0.5 °C under fluorescent lights at 150–300 μmol photons m−2 s−1 (10:14-h light/dark photoperiod). Cells from a set of three flasks each were harvested on day 0, 3, 6, 9, 12, 15, 18, and 21. Two controls (one of TW without any algae while another of standard algae culture broth (HiMedia) with algal cells) were included to confirm and check the effect of TW on the growth of algae during the experiment. On all sampling days, analysis of different parameters was carried out both from un-inoculated and algae-inoculated flasks with 1:1 TW.
For chlorophyll a measurement, 10 mL filtered sample (Whatman GF/C), extracted with acetone overnight and measured spectrophotometrically (Strickland and Parsons 1968). Cells were also counted microscopically at ×400 on all sampling days.
Analytical procedures
The physico-chemical parameters BOD, COD, nitrates (NO3–N), phosphates (PO4–P), sulphates (SO4–S), total dissolved solids (TDS), total chromium (Cr), and hexavalent chromium (Cr6+) were measured from the samples harvested during the experiment. Measurements of BOD and COD were made following standard methods described in APHA (2005). The BOD was determined using the 5-day BOD test and the COD, using open reflux oxidation method. TDS was determined by gravimetric method as per APHA (2005). TDS was calculated by measuring the residual weight after drying known sample volumes filtered through 0.7-μm glass microfiber filters at 180 °C. The algal cells were separated from wastewater through filtration using 0.45-μm glass microfiber filters, and the filtrate was used for measurement of dissolved nitrates, phosphates, and sulphates. Nitrate concentrations were colorimetrically measured following the Cd column reduction method (APHA 2005). Phosphates were measured using the ascorbic acid method (APHA 2005). Sulphates were measured following the barium chloride precipitation method (APHA 2005). For total chromium concentrations, the samples were digested with concentrated nitric acid followed by filtration through 0.45-μm filter paper, while for hexavalent chromium, 0.45-μm filtered samples without any pre-digestion were analyzed. The chromium contents were measured using the colorimetric diphenylcarbazide (DPC) method (APHA 2005).
Toxicity bioassay
Brine shrimp (Artemia salina) bioassay (Kiviranta et al. 1991) was used to assess the reduction in toxicity in the remediated tannery effluent. Briefly, 1-day-old Artemia hatchlings were placed in multi-well plates (10 well−1). Survival was monitored from 1 mL treated and untreated wastewater samples and seawater (positive control). The assay was conducted in five replicates. The number of dead nauplii after 24, 48, and 72 h were counted and percent survival calculated.
Statistical analysis
Statistical analyses were carried out using XLSTAT 7. One-way analysis of variance (ANOVA) was used to check the effect of C. vulgaris on its ability to reduce various toxic parameters from the wastewater. Subsequent pair-wise comparisons were performed using post hoc Tukey’s HSD (Honestly Significant Difference) tests.
Results
Characteristics of tannery wastewater
The physico-chemical characteristics of the raw tannery wastewater are presented in Table 1. The wastewater, collected from the outlets of a tannery, had dark brown color, salinity of 15 PSU, high BOD, COD, nitrates, phosphates, sulphates, TDS, and chromium concentrations. Both BOD and COD were quite high with average concentrations of 1,350 (±42.5) mg L−1 and 4,000 (±51.2) mg L−1, respectively. Untreated TW had very high concentrations of nitrate (0.93 ± 0.01 mg L−1), phosphate (6.01 ± 0.05 mg L−1), and sulphate (178.69 ± 0.98 mg L−1). Total dissolved solids (TDS) were higher compared to total suspended solids (TSS), indicating high contents of dissolved salts in the wastewater. Total chromium (3.22 mg L−1) concentrations in the wastewater were found to be higher than the permissible limits (<2.0 mg L−1) for discharge of effluents. Concentrations of chlorides, Ca, and Mg in the wastewater were also higher than those permitted by Bureau of Indian Standards (BIS 1994).
Growth of C. vulgaris in tannery wastewater
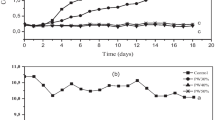
Cell counts of C. vulgaris, enumerated once every 3 days during the culture period of 21 days, increased by over seven times the initial counts of 5 × 103 cells mL−1. Also the chlorophyll a, 0.25 mg mL−1 was higher on day 21 (Fig. 1). In comparison to standard algal culture medium, the growth of cells was lower in the 1:1 diluted tannery effluent.
Reduction of different physico-chemical parameters
The concentrations of physico-chemical parameters analyzed from the 1:1 TW before treatment (day 0) and after treatment with C. vulgaris (day 21) are presented in Table 1. The concentrations of different parameters in tannery wastewater at 3-day intervals after treatment with C. vulgaris are shown in Table 2. After 21 days of treatment, the BOD level was reduced from 672 to 27 mg L−1, and the COD from 1,680 to 88 mg L−1 (Table 2). In case of BOD, the reduction was 95.92 %, whereas for reduction of COD, the efficiency rate was 94.74 % by day 21 (Fig. 2). Similarly, significant reduction of nitrates, phosphates and sulphates occurred within 21 days (Table 2). Indeed, 100 % reduction of nitrates was seen by day 6. Removal of phosphates by C. vulgaris was as high as 91.74 % by day 6 and over 99 % by day 21. The sulphate concentrations in TW were reduced by 67.4 % by day 21. The very high concentration of TDS of >3,000 mg mL−1 in untreated wastewater was reduced to 2,066 mg mL−1 by C. vulgaris after 12 days (Fig. 2) and to 1,766 mg mL−1 by day 21 (Table 2) with an overall TDS removal of 41 % (Fig. 2). Concentrations of total chromium in the tannery effluent, following treatment with C. vulgaris are presented against control TW (Fig. 3). Hexavalent chromium was completely transformed from the TW by day 12.
One-way ANOVA with post hoc Tukey’s HSD test showed that the concentrations of all the measured parameters decreased significantly after the treatment process of 21 days. For TDS, the reduction was significant (p < 0.001), and highly significant (p < 0.0001) for BOD, COD, NO3–N, PO4–P, SO4–S, total and hexavalent chromium (Table 3).
Toxicity assay
Percent survival of brine shrimp (A. salina) nauplii/hatchlings subjected to treated and untreated effluent for 24, 48 and 72 h differed rather significantly (Table 4). For instance, in the C. vulgaris treated effluent the survival of Artemia nauplii was 83.9 % on Day 1 and increased to 100 % by day 9. A positive trend was apparent in the survival of Artemia hatchlings in the treated effluent when exposed for 24, 48, and 72 h. Survival was significantly less in the untreated effluent. It was at least 28–32 % lower compared to that in treated effluent.
Discussion
Industrial effluent generation is on the rise in fast growing economies as India. While stringent regulatory norms are available statutorily, their implementation at fundamental levels is an obvious missing reality, in particular for tannery effluent discharges.
Concentrations of different physico-chemical parameters of the tannery wastewater were comparable to those reviewed by Durai and Rajasimman (2011). The observations of Munawar (1970) and Kannan (2006) suggest that algae grow luxuriantly with great variety and abundance in waters rich in calcium. The present data of the effluent characteristics also showed that calcium is possibly one of the favorable factors for growth of C. vulgaris in Kanpur tannery effluent. Besides calcium, high amounts of nitrates and phosphates and other oxidizable organic matter in the effluent also could be contributing to the growth of this microalgal strain as suggested by Murugesan and Sivasubramanian (2005) and Burch et al. (2001).
The biochemical oxygen demand (BOD) and chemical oxygen demand (COD) are important parameters to determine the water quality. Both of these parameters are greatly affected by the pollution load resulting from tannery industries (Islam and Suravi 2010). BOD in TW was reduced significantly (p < 0.001) by 95.93 % and that of COD by 94.74 % after treatment. Our strain, C. vulgaris, was efficient in reducing the levels to within the standards set by BIS (1994). These reductions were much higher within 21 days than those reported by Nanda et al. (2010) for Nostoc sp.-treated TW. Their strain of Nostoc sp. could bring down BOD by only 57.5 % and COD by only 37.8 % in 28 days using 1:5 TW diluted with BG11 medium. Therefore, our approach of diluting the TW by 50 % merely with tap water seems a more pragmatic and inexpensive approach. In order to achieve adequate improvement of the TW for safe discharge, the wastewater could be diluted and growing C. vulgaris in it for a fortnight or so would prove ecologically advantageous.
Results from this study demonstrate higher reduction of nutrients (nitrates, phosphates and sulphates) by C. vulgaris from TW when compared with those reported by Ajayan et al. (2015), for Scenedesmus sp. and Adam et al. (2015), for Tetraselmis sp. Remarkably, the nutrient levels were reduced to well below the maximum permissible limits of BIS.
Total dissolved solids (TDS) is an important chemical parameter of water, which mainly indicates the presence of various minerals including nitrate, nitrite, phosphate, sulphates, metallic ions, alkalis, and acids in both colloidal and dissolved forms (Rahman et al. 2012; Kabir et al. 2002). The C. vulgaris strain we tested has only a moderate potential for reducing the TDS contents in the tannery wastewater when compared to the BIS (1994) permissible limit.
Most pertinently, the strain of C. vulgaris we studied could efficiently biotransform Cr6+ from Kanpur tannery wastewater. This culture could achieve 100 % biotransformation of Cr6+ from the TW in about 12 days. As Cervantes et al. (2001) also noted in the case of Oscillatoria, Phormidium, Scenedesmus, and Pandorina spp., the present isolate seems to be useful for bio-sorption studies for the removal and/or biotransformation of Cr from contaminated sources. These kinds of algae biotransform/detoxify heavy metal ions usually through the process of biosorption, adsorption, and bioaccumulation (Rehman and Shakoori 2001; Gin et al. 2002; Rehman et al. 2007). Rehman (2011) reported 68 % reduction in Cr concentrations from TW in 20 days using Euglena proxima. In comparison to the previous results, the transformation potential of C. vulgaris is far superior.
Toxicity tests are indispensable tools to evaluate the quality and pollutant charge of the effluent. The brine shrimp, Artemia salina is a microcrustacean used frequently in toxicity studies, due to the ease of culture, short generation time, low commercial cost as dormant eggs (cysts), and cosmopolitan distribution (Vanhaecke et al. 1981). Hasegawa et al. (2014) determined the toxicity of tannery wastewater, before and after zinc oxide-assisted photocatalytic treatment, using dry shrimp eggs. Islam et al. (2014) studied the toxic effects of varying dilutions of tannery wastewater on brine shrimp nauplii.
Toxicity testing of tannery wastewater in this study, ascertained that the untreated wastewater is deleterious to the hatchlings of A. salina. The treatment of tannery wastewater with C. vulgaris unambiguously promoted the survival of the nauplii which can be directly ascribed to the reduction of toxic effects of treated effluent on A. salina. The results are useful to ascertain the decrease in toxicity in C. vulgaris-treated effluent to Artemia larvae. This can be attributed to the substantial reduction in concentrations of the toxicants that were in far higher concentrations than BIS (1994) permitted limits. Further, the biotransformation of toxic metals, in general, hexavalent chromium from the wastewater by C. vulgaris ought to be facilitating the prolonged survival. Therefore, the toxicity results of this work showed that survival of A. salina was an important tool to evaluate the efficacy of TW bioremediation by C. vulgaris.
Our salt-tolerant Chlorella strain grew the least in 100 % and quite poorly in 70 % TW, as explained in Methods section. This was the case even after increasing inoculum size, lengthening of light periods and elevated aeration. While the growth of this strain was far superior in the 3:7 or 1:9 TW, to ensure that the salt-tolerant strain would not experience undue hypo-osmotic stress with more diluted TW, we resorted to test its bioremediation efficiency in 1:1 TW. Since tannery effluent is a mixture of innumerable alkalis/chorides/salts, our approach of using 1:1 dilution is very likely to be effective in the reduction of COD, BOD, nutrients, and chromium. Further, as can be deciphered from the growth curve, the profuse growth of Chlorella NIOCCV in 1:1 TW generates substantial biomass that can be useful variously, as has been proposed by Huang et al (2010), for biofuel production in particular. Notably, the accumulation of troublesome sludge in conventional treatment methods is certainly avoided by phycoremediation. Additionally, unlike the use of costly chemical medium, as done for instance by Nanda et al (2010), our approach of using fresh water for dilution ought to be beneficial for the industry in some production processes. In that, by separating the algal biomass—that could be of use as a feeder material in a variety of other uses—the treated water can be recycled in the industry itself or for controlled irrigation of forests, lawns, and other greeneries. The latter intent of the use of microalga-treated TW for irrigating the non-food crops is an eco-friendly approach of carbon sequestration. Further, as Orandi and Lewis (2013) also proposed, the rigorous recovery of accumulated metals from the algal biomass can be achieved through washes with dilute acid or via adsorption/desorption cycles of alga itself.
In conclusion, this study is useful to ascertain that treatment by C. vulgaris has significance in reducing pollution load from tannery wastewater with high efficiency. Therefore, this algal species can be used as an alternate, potentially low cost method for treating tannery effluent before releasing into natural systems. Such methods seem to offer economic treatment and may be effective in minimizing the environmental impact. These very promising results call for elaborated studies on the use of algal species for promulgating functional phycoremediation strategies in the future.
References
Adam S, Suresh Kumar P, Santhanam P, Dinesh Kumar S, Prabhavathi P (2015) Bioremediation of tannery wastewater using immobilized marine microalga Tetraselmis sp.: experimental studies and pseudo-second order kinetics. J Mar Biol Oceanogr 4:1
Ajayan KV, Selvaraju M, Unnikannan P, Sruthi P (2015) Phycoremediation of tannery wastewater using microalgae Scenedesmus species. Int J Phytoremed 17:907–916
APHA (2005) Standard methods for the examination of water and wastewater, 20th edn. APHA-AWWA-WPCF, Washington DC
Balaji S, Kalaivani T, Sushma B, Varneetha Pillai C, Shalini M, Rajasekaran C (2015) Characterization of sorption sites and differential stress response of microalgae isolates against tannery effluents from Ranipet industrial area—an application towards phycoremediation. Int J Phytoremediation. doi:10.1080/15226514.2015.1115960
Bosnic M, Buljan J, Daniels RP (2000) Pollutants in tannery effluents. US/RAS/92/120, United Nations Industrial Development Organization, Regional Programme for Pollution Control in the Tanning Industry in South-East Asia
Burch MD, Chow CWK, Hobson P (2001) Algaecides for control of toxic cyanobacteria. Proceedings of the 2001 Water Quality Technology Conference. American Water Works Association, Nashville
Bureau of Indian Standards (BIS) (1994) Quality tolerance for water for Tanning industry. IS: 4221, New Delhi
Cervantes C, Campos-Garcia J, Devars S, Gutierrez-Corona F, Loza-Tavera H, Torres-Guzman JC, Moreno-Sanchez R (2001) Interactions of chromium with microorganisms and plants. FEMS Microbiol Rev 25:335–347
Chellam CT, Sampathkumar P (2012) Bio removal of nutrients in tannery effluent water using marine micro algae, Chlorella marina. Proceedings of International Forestry and Environment Symposium, Sri Lanka. Published by Department of Forestry and Environmental Science, University of Sri Jayewardenepura, Vol. 17
Chu W-L, See Y-C, Phang S-M (2008) Use of immobilised Chlorella vulgaris for the removal of colour from textile dyes. J Appl Phycol 21:641–648
Craggs R, Sutherland D, Campbell H (2012) Hectare-scale demonstration of high rate algal ponds for enhanced wastewater treatment and biofuel production. J Appl Phycol 24:329–337
Dixit S, Singh DP (2014) An evaluation of phycoremediation potential of cyanobacterium Nostoc muscorum: characterization of heavy metal removal efficiency. J Appl Phycol 26:1331–1342
Dotro GC (2003) Assessment and design framework for the use of constructed wetlands to treat tannery effluents. Master Thesis, University of Calgary
Dunn K (1998) the biotechnology of high rate algal ponding systems in the treatment of saline tannery wastewaters. PhD thesis. Rhodes University, South Africa.
Dunn K, Maart B, Rose P (2013) Arthrospira (Spirulina) in tannery wastewaters. Part 2: evaluation of tannery wastewater as production media for the mass culture of Arthrospira biomass. Water SA 39:279–284
Durai G, Rajasimman M (2011) Biological treatment of tannery wastewater—a review. J Envi Sci Technol 4:1–17
Food and Agriculture Organization (FAO) (2004) The State of Food and Agriculture. FAO Agriculture Series No. 35
Gin KY, Tangm YZ, Aziz MA (2002) Derivation and application of a new model for heavy metal biosorption by algae. Water Res 36:1313–1323
Hasegawa MC, de Souza Daniel JF, Takashima K, Batista GA, da Silva SMCP (2014) COD removal and toxicity decrease from tannery wastewater by zinc oxide-assisted photocatalysis: a case study. Environ Technol 35:1589–1595
Hernández-Zamora M, Cristiani-Urbina E, Martínez-Jerónimo F, Perales-Vela HV, Ponce-Noyola T, Montes-Horcasitas MC, Cañizares-Villanueva RO (2015) Bioremoval of the azo dye Congo Red by the microalga Chlorella vulgaris. Environ Sci Pollut Res 22:10811–10823
Huang G, Chen F, Wei D, Zhang X, Chen G (2010) Biodiesel production by microalgal biotechnology. Appl Energ 87:38–46
Islam MS, Suravi MNT (2010) Investigation on water quality in the Ashuliabeel, Dhaka. Bangladesh J Fish Res 14:55–64
Islam LN, Mahmud ARZ, Nurun Nabi AHM, Hossain M, Mohasin M (2014) Assessment of physicochemical and biochemical qualities of tannery effluents of Hazaribagh, Dhaka, and comparison with non-tannery water samples. Int J Environ 4:68–81
Jaysudha S, Sampathkumar P (2014) Nutrient removal from tannery effluent by free and immobilized cells of marine microalgae Chlorella salina. Int J Environ Biol 4:21–26
Kabdasli I, Tunay O, Cetin MS, Olmez T (2002) Assessment of magnesium ammonium phosphate precipitation for the treatment of leather tanning industry wastewaters. Water Sci Technol 46:231–239
Kabir ES, Kabir M, Islam SM, Mia C, Begum N, Chowdhury DA, Sultana SM, Rahman SM (2002) Assessment of effluent quality of Dhaka export processing zone with special emphasis to the textile and dying industries. Jahangirnagar Univ J Sci 25:137–138
Kannan S (2006) Biodiversity of cyanobacteria in freshwater ponds of Poondi, Thanjavur. M.Phil. dissertation, Bharathidasan University, Tiruchirapalli
Kennedy L (1999) Cooperating for survival: tannery pollution and joint action in the Palar Valley (India). World Dev 27:1673–1691
Khwaja AR, Singh R, Tandon SN (2001) Monitoring of Ganga water and sediments vis-a-vis tannery pollution at Kanpur (India): a case study. Environ Monit Assess 68:19–35
Kiviranta J, Sivonen K, Niemela SI, Huovinen K (1991) Detection of toxicity of cyanobacteria by Artemia salina bioassay. Environ Toxicol Water Qual 6:423–436
Leta S, Assefa F, Gumaelius L, Dalhammar G (2004) Biological nitrogen and organic matter removal from tannery wastewater in pilot plant operations in Ethiopia. Appl Microbiol Biotechnol 66:333–339
Mara DD, Pearson HW, Silva SA, Rose PD, Maart BA, Dunn KM, Rowswell RA, Britz P (1996) Waste Stabilization Ponds: Technology and Applications. High rate algal oxidation ponding for the treatment of tannery effluents. Water Sci Technol 33:219–227
McMullan G, Meehan C, Conneely A, Kirby N, Robinson T, Nigam P, Banat IM, Marchant R, Smyth WF (2001) Microbial decolourization and degradation of textile dyes. Appl Microbiol Biotechnol 56:81–87
Munawar M (1970) Limnological studies on freshwater ponds of Hyderabad, India. II The biocoenose-distribution of unicellular and colonial phytoplankton in polluted and unpolluted environments. Hydrobiologia 36:105–128
Murugesan S, Sivasubramanian V (2005) Cyanobacteria of Porur Lake, Chennai, Tamilnadu. Indian Hydrobiol 8:49–54
Mustafa E-M, Phang S-M, Chu W-L (2012) Use of an algal consortium of five algae in the treatment of landfill leachate using the high-rate algal pond system. J Appl Phycol 24:953–963
Nanda S, Sarangi PK, Abraham J (2010) Cyanobacterial remediation of industrial effluents, I. Tannery effluents. N Y Sci J 3:32–36
Orandi S, Lewis DM (2013) Biosorption of heavy metals in a photorotating biological contactor—a batch process study. Appl Microbiol Biotechnol 97:5113–5123
Oswald WJ (1988) Micro-algae and waste-water treatment. In: Borowitzka MA, Borowitzka LJ (eds) Micro-algal biotechnology. Cambridge University Press, Cambridge, pp 305–328
Posadas E, Bochon S, Coca M, García-González MC, García-Encina PA, Muñoz R (2014) Microalgae-based agro-industrial wastewater treatment: a preliminary screening of biodegradability. J Appl Phycol 26:2335–2345
Rahman MS et al (2012) Water quality characterization of the Sundarbans Reserve Forest (SRF), Khulna for biodiversity consideration, Bangladesh. In: Silver jubilee conference. Bangladesh Chemical Society 20:67–68
Rai UN, Dwivedi S, Tripathi RD, Shukla OP, Singh NK (2005) Algal biomass: an economical method for removal of chromium from tannery effluent. Bull Environ Contam Toxicol 75:297–303
Rao HP, Ranjith Kumar R, Raghavan B, Subramanian V, Sivasubramanian V (2011) Application of phycoremediation technology in the treatment of wastewater from a leather-processing chemical manufacturing facility. Water SA 37:7–14
Rehman A (2011) Heavy metals uptake by Euglena proxima isolated from tannery effluents and its potential use in wastewater treatment. Russ J Ecol 42:44–49
Rehman A, Shakoori AR (2001) Heavy metal resistant Chlorella spp. isolated from tannery effluents, and their role in remediation of hexavalent chromium in industrial wastewater. Bull Environ Contam Toxicol 66:542–547
Rehman A, Farah RS, Shakoori AR (2007) Heavy metal resistant Distigma proteus (Euglenophyta) isolated from industrial effluents and its possible role in bioremediation of contaminated wastewaters. World J Microbiol Biotech 23:753–758
Saranraj P, Sujitha D (2013) Microbial bioremediation of chromium in tannery effluent: a review. Int J Microbiol Res 4:305–320
Sheehan J, Dunahay T, Benemann J, Roessler P (1998) A look back at the U.S. Department of Energy’s Aquatic Species Program—biodiesel from algae, Golden, CO, National Renewable Energy Institute, Report No. NREL/TP-580-24190, p. 328
Shi J, Podola B, Melkonian M (2007) Removal of nitrogen and phosphorus from wastewater using microalgae immobilized on twin layers: an experimental study. J Appl Phycol 19:417–423
Srivastav AK (2012) Chromium removal from tannery industry waste water by fungus. Thesis, Thapar University, Punjab
Strickland JDH, Parsons TR (1968) A practical handbook of seawater analysis. Bull Fish Res Board Canada 167:49–62
Tadesse I, Isoaho S, Green F, Puhakka J (2003) Removal of organics and nutrients from tannery effluent by advanced integrated wastewater pond systems technology. Water Sci Technol 48:307–314
Tastan BE, Ertugrul S, Donmez G (2010) Effective bioremoval of reactive dye and heavy metals by Aspergillus versicolor. Bioresour Technol 101:870–876
Vanhaecke P, Persoone G, Claus C, Sorgeloos P (1981) Proposal for a short-term toxicity test with Artemia nauplii. Ecotox Environ Saf 5:382–387
Acknowledgments
We thank the Director, CSIR—National Institute of Oceanography for facilities and encouragement. The work was supported under the CSIR project BSC0111 “Integrated NextGen Approaches in Health, Disease and Environmental Toxicity (INDEPTH)”. This is CSIR-NIO contribution number 5913.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Das, C., Naseera, K., Ram, A. et al. Bioremediation of tannery wastewater by a salt-tolerant strain of Chlorella vulgaris . J Appl Phycol 29, 235–243 (2017). https://doi.org/10.1007/s10811-016-0910-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-016-0910-8