Abstract
Produced water (PW) is an effluent from the petrochemical industry that has a significant environmental impact due to its high salinity and presence of chemical compounds and heavy metal. Microalgae have been considered promising to phytoremediation of this effluent and producing biomass with high added value. Therefore, this study aimed to stimulate the production of biomass and biomolecules from Chlorella vulgaris by supplementation with PW and evaluate the synergistic effect of sources of physical and chemical stress. C. vulgaris was cultivated in different PW concentations of PW (not autoclaved) and BG11 medium under a 24 h photoperiod, with inoculum pre-adapted the same photoperiod. The culture containing 70% of BG11 and 30% PW (PW 30%) was the most viable, with a biomass production of 1.35 g/L and a higher concentration of carbohydrates (37.46%) and ash (18.21%) than the control culture (100% BG11). Furthermore, PW 30% also resulted in considerable amounts of lipids (9.92%), proteins (21.94%), chlorophyll-a (6.64 μg/mL), chlorophyll-b (10.57 μg/ mL), and carotenoids (21.38 μg/mL). The major fatty acids were C18:3n6 (21.50%), C20:0 (19.96%), C16:0 (17.12%), and C18:0 (12.15%). The PW 30% treatment showed removal efficiencies for iron (61.80%), chlorides (79.64%), phosphates (97.18%), and petroleum hydrocarbons including total petroleum hydrocarbons (TPH; 45.39%) and resolved petroleum hydrocarbons (RPH; 57.57%). The analyzed fuel properties presented an ideal profile for the production of biodiesel and bioethanol, which can be obtained from carbohydrates. Simultaneously, treatment PW 30% resulted in the production of biomolecule-rich in biomass in addition to the bioremediation effect.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The effluent resulting from oil and gas production, also known as produced water (PW), is wastewater typically composed of hydrocarbons, heavy metals, and inorganic salts. The PW composition and volume depend on the location of the well, frequency of water injection, and constituents extracted from the oil [1]. In 1990, the global production of PW was 190 million barrels per day. Thirty years later, the production increased to approximately 320 million barrels per day, and it is estimated that this volume will grow to 600 million barrels per day over the next ten years [2, 3].
The large volume of PW, scarcity of pure water, and greater energy demand have increased interest in the use of microalgae for biomass production as energy sources for the treatment and reuse of effluents. The cultivation of microalgae stands out because of the non-necessity of using fresh water; microalgae can utilize wastewater due to their bioremediation capacity and assimilating of organic and inorganic pollutants and heavy metals [4].
Some of these pollutants, mainly heavy metals, are considered toxic and are increasingly found in water and soil owing to economic growth and accelerated industrialization. Therefore, various removal treatments are used, including chemical, electrochemical, redox, membrane separation, adsorption, ion exchange, and biological treatment [4, 5]. Biological treatment with microalgae uses organic matter from these wastewaters as a source of nutrients and carbon, reduces toxicity, removes pollutants, and provides biomass rich in fatty acids, proteins, carbohydrates, vitamins, minerals and pigments, making them a source of bioproducts used in the aquaculture, pharmaceutical, food, and biofuel industries [4, 6].
Although PW contains several toxic components that inhibit microalgae growth, it also contains nutrients necessary for cultivation, such as iron (Fe), potassium (K), and manganese (Mn) [6]. Research has been conducted to make the production of microalgae in PW feasible. Ammar et al. [1] cultivated Nannochloropsis oculata and Isocrysis galbana in PW for 15 days at three percentages (10, 25, and 50%). Silva et al. [7] produced 1.69 g/L of Chlorella vulgaris biomass for 26 days in 1.5 L of BG11 medium, when cultivated in medium supplemented with 50 mL of non-autoclaved PW per day.
The Chlorella genus is widely used for wastewater phycoremediation. Chlorella stands out for its tolerance of wastewater from industrial, agricultural, and municipal environments, making it useful in the treatment of effluents and ideal for biomass production [7]. Its potential is based on its plasticity, which can be modified according to cultivation conditions, allowing for modifications to its growth and biochemical composition. Some strategies are used to increase biomass production and the yields of some compounds, such as modulating chemical or physical stressors. Chemical stress is induced by modifying the composition of the culture medium, and physical stress is induced by changing the luminosity, temperature, salinity, and pH [8].
Light conditions are the main factors that affect phytoplankton physiology and directly affect microalgae photosynthesis. The quantity and quality of light are primordial and limiting factors for the energy available for photosynthesis and all metabolic activities [9]. According to Khoeyi et al. [9], increased light duration at different intensities (37.5; 62.5 and 100 µmol photons/m2s) was associated with increased specific growth rates of Chlorella vulgaris (0.81, 1.00, and 1.12 / d) for the highest light cycle (16:8 h light/dark). Moreover, other authors, such as Yusof et al. [10], have stated that with increased light exposure, there is an increase in the microalgal reproduction rate up to the saturation point. Moreover, each organism has different light requirements and life cycles.
Strategies to maximize microalgae growth grown in wastewater depend on changes in cultures and knowledge of microalgal physiology [11]. Therefore, modifications of physical and chemical factors are viable options to influence cell metabolism, increase growth, and stimulate the synthesis of biomolecules of interest based on their biochemical composition [12]. This study aimed to stimulate the production of biomass and biomolecules from Chlorella vulgaris by supplementation with PW and evaluate the synergistic effect of maximum luminosity and PW chemical composition on microalgal growth.
Material and Methods
Collection, the Chemical Composition of the Culture Medium with Produced Water, and Nutrient Removal Efficiency
The PW was collected from the Santo Amaro das Brotas district (latitude 10º46′44’’ south; 37º3′30’’ west), located 41 km from Aracaju, Sergipe (SE), Brazil. The samples were kindly supplied by a local oil exploration company and transported to the Federal University of Bahia (UFBA) in sterile polypropylene vessels under refrigeration. All PW samples were stored at -20 °C until use.
The physicochemical parameters were evaluated by previously reported consolidated methods using the standard method for the examination of water and wastewater [13] for the following analyses: chloride (Cl−), phosphate (PO43−), lead (Pb), arsenic (As), cadmium (Cd), nickel (Ni), manganese (Mn), vanadium (V), iron (Fe), zinc (Zn), and chromium (Cr). Determination of total petroleum hydrocarbons (TPH) and resolved petroleum hydrocarbons (RPH) by EPA 8015D (SW-846) followed the US standard method [14]. The nutrient removal efficiency (RE) from the treatment was evaluated using Eq. 1, previously reported by Cardoso et al. [15].
where, Ci and Cf are the initial and final concentrations, respectively.
Growing Conditions and Treatments
The C. vulgaris strain was obtained from the Iracema Nascimento Microalgae Bank of the Bioprospecting and Biotechnology Laboratory of the UFBA. To obtain the inoculum for used in the experiments, the strain was autoclaved and cultivated in a synthetic medium, BG11 (NaHCO3; K2PO4.3H2O; NaNO3; Na2CO3; MgSO4.7H2O; CaCl2.2H2O; (NH+)5Fe(C6H2O7)2; C6H8O7; and EDTA) and trace elements [16] under constant temperature (28 °C), aeration, and continuous photoperiod (24 h) with lighting provided by five fluorescent lamps at 41.60 μmol photons/m2s each. After 18 d of cultivation, the inoculum was centrifuged (Eppendorf 5702 R) at 3450 × g for 4 min.
Following this, the experiments were carried out in Erlenmeyer-type photobioreactors (1 L) with a total volume of 800 mL (BG11 medium + PW according to each treatment) and an initial concentration of approximately 0.2 g /L for 18 d. The treatments consisted of a control culture (100% BG11) and treatments containing 30% PW + 70% BG11 (PW 30%), 40% PW + 60% BG11 (PW 40%), and 50% PW + 50% BG11 (PW 50%), all performed in triplicate under conditions of constant temperature (28 °C), aeration, and continuous photoperiod (24 h) with lighting provided by five fluorescent lamps at 41.60 μmol photons/m2s each. PW proportions were determined based on the study by Ammar et al. [1] and in preliminary tests that pointed to a maximum concentration of 50% of PW.
After 18 d of cultivation, the biomass was recovered by centrifugation (Eppendorf 5702 R) at 3450 × g for 4 min, washed with distilled water, and centrifuged again (3450 × g for 4 min) to remove salts and interferents. After washing, the biomass was frozen at -80 °C and freeze-dried at -43 °C (L101, Liobras) for further analysis.
Growth and pH
The biomass concentration (X, g/L) was determined by optical density and evaluated daily in all cultures. A standard curve was constructed according to the method described by Costa et al. [17], using a spectrometer (Perkin–Elmer Lambda 35 UV–Vis). All readings were recorded at a wavelength of 680 nm. The productivity (P, (g/L)/d) was determined using Eq. 2, and the growth rate (µ, 1/d) was determined by linear regression of the biomass production curve in the log phase [18]. pH was measured daily using a digital pH meter (Gehaka PG2000).
where, Xt e X0 are the biomass concentrations (g/L) at times t (d) and t0 (d) initial.
Biomass Biochemical Composition
Protein concentration was calculated from the total nitrogen concentration determined by Kjeldahl method [19] with a microalgae conversion factor of 5.22 [20]. Total lipids were extracted and quantified using the method by Folch et al. [21] with chloroform–methanol solution (2:1). Thermogravimetric analysis (TGA) was used to determine ash and moisture contents at temperatures between 25 and 900 °C, as proposed by Jesus et al. [18]. Carbohydrates were determined by the difference in proteins, lipids, ash, and moisture contents [20].
Fatty Acid (FA) Composition
Fatty acid composition was determined by transmethylation of fat with boron trifluoride in hexane, followed by gas chromatography. The FA methyl esters were separated with a column (DB-FFAP; 30 m × 0.25 mm × 0.25 μm) in a gas chromatograph equipped with a flame ionization detector (CG-FID Clarus 680; Perkin–Elmer). Fatty acid methyl esters were identified by comparing the retention times obtained from the standard chromatogram (C4–C24, 189–19-AMP, Sigma-Aldrich) according to the method proposed by Souza et al. [22].
Evaluation of Biodiesel Properties
The most important biofuel properties were evaluated. The iodine value (IV), saponification value (SV), cetane number (CN), long-chain saturation factor (LCSF), degree of unsaturation (DU), and cold filter plugging point (CFPP) were determined from empirical equations based on the FA profile [23]. SV and IV calculations were based on the SV and IV values of soybean, palm, and peanut vegetable oils.
where, D = number of double bonds, M = molecular weight of the fatty acid, and N = percentage of each fatty acid present in the oil.
Measurement of Chlorophyll a, Chlorophyll b, and Total Carotenoids
The analysis of chlorophyll a and b and carotenoid contents was performed as described by Andrade et al. [20]. Briefly, 0.2 g of lyophilized sample was added to 25 mL of 80:20 acetone:water, vortexed (Phoenix AP 56), and centrifuged (Eppendorf 5702R) at 3450 g for 5 min. The supernatant was centrifuged again, and the absorbance was measured using a spectrophotometer (Perkin – Elmer Lambda 35 UV–Vis) at wavelengths 663, 647, and 470 nm. Yields were calculated following the equations proposed by Lichtenthaler and Buschmann [24] and expressed in μg/ mL.
where, Ca = Chlorophyll a; Cb = Chlorophyll b; CCT = Total Carotenoids.
Theoretical Conversion of Carbohydrates from Microalgae Biomass to Ethanol
The theoretical conversion of carbohydrate, obtained from C. vulgaris biomass, for the production of ethanol and carbon dioxide, originating from the alcoholic mixture, can be represented in stoichiometric doses. The Gay-Lussac prescription represents the transformation of carbohydrate into ethanol and carbon dioxide (Eq. 12) [25].
Theoretical calculations were performed to evaluate the conversion of carbohydrates produced in the biomass of C. vulgaris into bioethanol. The calculation considered 100 g of biomass, theoretical stoichiometric conversion of glucose into ethanol (kg) of 0.511 with 70% efficiency (EE), and an equivalence of ethanol gallons to GGE of 1.5:1.0 [26].
Thermogravimetric Analysis (TGA)
Thermogravimetric analyses (TGA) (PerkinElmer Model Pyris 1 TGA) was performed to determine the biomass pyrolysis behavior. A total of 5 mg of biomass was subjected to different temperatures ranging from 25 to 900 °C at a heating rate of 10 °C/min and a nitrogen flow rate of 20 mL/min [18]. The initial decomposition temperature and the final and maximum decomposition temperatures were denoted as (Tonset) and (Tdecomp), respectively.
Attenuated Total reflectance Fourier Transform Infrared Spectroscopy (FTIR-ATR)
The samples were analyzed by Fourier transform infrared spectroscopy using an attenuated total reflectance device (Spectrum two spectrometer, Perkin-Elmer) with a wavenumber ranging from 4000 – 600/cm, accumulating 64 scans [27].
Statistical Analysis
The results were analyzed using two statistical tests to compare and demonstrate the efficiency of the PW-supplemented treatments. An analysis of variance (ANOVA) and Tukey's test at a 95% confidence level were performed using the Statistica software (version 10.0) for comparison of the means. The Shapiro–Wilk test was used to test the normality of the data. The experimental results are presented as the mean ± standard deviation of triplicates experiments. Statistical treatment was applied to all quantitative analyzes performed in this study, with the exception of FTIR, TGA, and evaluation of the theoretical properties of biodiesel, as they are qualitative analyses.
Results and Discussion
Growth and pH
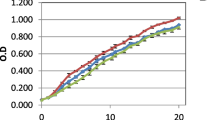
The growth curve of C. vulgaris at different PW concentrations (30%, 40%, and 50%) are shown in Fig. 1a. The PW 40% and PW 50% treatments did not show microalgal growth throughout cultivation. This behavior may be related to the disruption of the cell membrane caused by cell-PW contact, as PW is rich in contaminants such as metals, organic compounds, and nutrients, causing interactions between the toxic components and intracellular organelles of the microalgae and hindering their normal biological functions, including growth [1, 28]. Ammar et al. [1] also observed growth inhibition of Isocrysis galbana with increasing PW concentration (10%-50%).
In addition to the control, the only treatment that showed cell growth was PW 30%, which may be related to the ability of microalgae to degrade toxic constituents into less toxic products [28]. An adaptation period lasting two days (0 to 2 nd day) was observed in PW 30% and the control groups, followed by exponential growth until the 18th day of cultivation (Fig. 1a). According Cardoso et al. [15], the growth after two days of cultivation indicates the ability of microalgae to adapt to the imposed environmental conditions. In the present study, C. vulgaris showed good adaptation to PW 30% supplementation, similar to the results found by Mehta et al. [29] in cultivating Chlorella pyrenoidosa at different salt concentrations, with a comparable adaptation phase of two days. The similar exponential growth of the PW 30% treatment and the control demonstrates the existence of a positive synergism between the luminosity and the chemical composition of the culture, which allowed the cell growth and exponential development. The PW 40% and PW 50% treatments remained in the lag phase (latency) throughout the 18 days of culture, maintaining the same cell concentration as the initial inoculum concentration (~ 0.2 g/L).
The initial pH values (day 0) decreased with increasing PW concentration in the treatments. Figure 1b shows a variation in the pH from 10.31 (control) to 7.75 (PW50%). In the PW 40% and PW 50% treatments, the pH reduction may be associated with the chemical composition of PW, limiting growth. pH is affected by the availability of inorganic nutrients, carbonaceous species in the medium, and the presence of metals. According to Gómez-Luna et al. [30], pH reduction is usually lethal for microalgae because its tolerance limits are determined by the chemical composition of the culture medium and cell metabolic effects.
The PW 30% and the control treatments showed an increase and a decrease in pH over the cultivation period (Fig. 1b). The pH increase is due to the consumption of CO2 during photosynthesis and the release of hydroxide (OH−) by reducing the oxygen molecules produced [31]. Otondo et al. [4] observed higher growth rates during the cultivation of Nannochloropsis salina in domestic wastewater at pH 8.0 and 9.0. Here, the control and PW 30% treatments (pH > 8.0) showed better growth and biomass production. pH 8.0 is indicated for most freshwater species, with a pH of 10.0 being ideal for C. vulgaris [30]. Therefore, a pH range between 8.0 and 10.0 is ideal for culturing Chlorella supplemented with PW 30%.
The PW 30% culture showed a maximum production of 1.35 g/L on the 18th day (Table 1), compared to the control culture with a maximum production of 3.05 g/L on the 16th day. The lower values found in the PW 30% treatment occurred because of the stress caused to the microalgae by PW components, such as petroleum hydrocarbons, excess nutrients, and metals [1]. Even with these characteristics, the dilution of PW allowed the growth of C. vulgaris because of the ability of microalgae to degrade petroleum hydrocarbons into their less toxic forms, converting acetyl-CoA into H2O and CO2, enabling the production of biomass [32]. Church et al. [31] also observed a reduction in biomass production in the cultivation of Chlorella vulgaris in saline wastewater, from 1.30 g/L in the standard culture to 0.74 g/L in saline wastewater.
The specific growth rate (μ) of the control culture was higher than that of the PW 30% treatment (Table 1). This result may be related to the phosphorus (P) limitation of PW because P acts as a chemical energy carrier. The presence of P increases the growth of some microalgae species, including C. vulgaris [33]. In addition, low μ rates were found with increasing salt concentration in the medium [31]. According to Mehta et al. [29] and Matos et al. [34] moderate concentrations of NaCl (up to 1%) can help with nutrient absorption and intracellular regulation of photosynthesis. However, a 2–6% NaCl medium can cause cell osmotic stress, interfering with electron transport and photophosphorylation. Therefore, highly saline media tend to reduce algal growth, possibly by accumulating reactive oxygen species. Ammar et al. [1] reported similar behavior in cultivating Nanochloropsis oculata with 25% PW (0.1796/d). Thus, a higher growth rate occurs in treatments with higher biomass concentrations [31].
Chemical Composition and Treatment Removal Efficiency
The chemical composition and treatment efficiency of cultivating C. vulgaris in BG11 culture medium supplemented with PW 30% are shown in Table 2. The culture medium contained metals important for the metabolism and growth of microalgae. Iron (Fe), for example, participates in electron transport and cellular respiration, acting as an enzymatic cofactor that increases the degradation power of the medium [35].
The Fe removal rate of the PW 30% treatment reached 61.80%. Yaghmaeian and Jaafari [35] found an Fe removal of 86.7% in 2.6 g/L of C. Coloniales biomass grown in BG11 medium supplemented with some metals (Cr, Cd, Co, Fe, and As). The lower removal efficiency found in the present study may be related to the lower number of cells used in the inoculum in the PW 30% treatment (~ 0.2 g/L). A lower inoculum concentration represents fewer active biosorption sites. The cell wall contains polysaccharides, proteins, and lipids with functional groups composed of electronegative atoms. The high electron densities of these groups are responsible for the binding of cations to the cell wall surface [35]. Znad et al. [33] reported results similar to those found in the present study cultivating Chlorella vulgaris in petroleum effluents, with 57% Fe removal.
Even with low removal efficiency (16.45%), manganese (Mn) is another essential element in microalgae metabolism. Like iron (Fe), Mn also acts as a cofactor in enzyme systems, mainly to maintain a regular photosynthetic rate [33]. The components with the highest removal efficiency were phosphates (97.18%) and chlorides (79.64%). The Cl− removal efficiency may be related to the ability of microalgae to remove chlorine ions and produce lipids and polyols as an osmoregulatory response to extracellular solute concentration, minimizing osmotic stress and dehydration. Chloride ion are essential cofactor for photosynthesis and are vital for plant growth [36]. Brar et al. [36] reported similar findings to the present study regarding chloride removal (61.1%) from wastewater from the textile industry using C. pyrenoidosa.
The high efficiency of PO4− removal in the treatment is directly associated with the absorption of phosphorus (P) for microalgae growth, as microalgae accumulate phosphorus in their cells as polyphosphates. Microalgae use phosphorus to synthesize cellular constituents, such as phospholipids and nucleic acid, and for reactions related to cell division [36]. Brar et al. [36] reported a 70.8% phosphate removal from wastewater from the textile industry and 99% phosphorus removal in the cultivation of Chlorella vulgaris in synthetic wastewater.
Total petroleum hydrocarbons represent the sum of the fractions of RPH and the unresolved complex mixture (UCM). The TPH and RPH removals were 45.39 and 57.57%, respectively. These results indicate the synergistic action of microalgae and bacteria in the treatment, considering that the medium was not autoclaved. The removal of petroleum hydrocarbons depends on the microalgae species used, cultivation conditions, cell wall size, and extracellular and intracellular physiological processes. These processes are characterized by the adsorption of hydrocarbons on the surface, absorption of hydrocarbons into the cells, and transformation of hydrocarbons by enzymes into less toxic molecules [37]. Petroleum hydrocarbons are resistant to degradation by microorganisms mainly because of their high molecular mass. However, the synergistic action of microalgae and bacteria resulted in good removal efficiency. This process transforms hydrocarbons into cis-dihydrodiols by dioxygenases, leading to pyruvic, acetic, fumaric, succinic, aldehydes, and acids production [37].
Abid et al. [38] reported a TPH removal efficiency higher than that of the present study (99.18%) in Spongiochloris sp. cultivation in PW for 120 days. The high removal efficiency found by the authors is related to the time of cultivation and the adaptation of the symbiotic culture with aerobic bacteria in the bioreactor because, in the 50-day culture, the removal of TPH was only 22%. Silva et al. [7] presented similar results to those found in this study in cultivating Chlorella vulgaris with 10% of PW for day, removing 48.59% of TPH.
Biochemical Composition of Biomass
The biochemical compositions of the biomass obtained from the PW 30% and control treatments are shown in Fig. 2. The lipids concentration in the PW 30% treatment (9.92%) was lower than that in control (17.25%). This result may be related to the high salinity of PW and the stress caused by the metallic cations, forcing the degradation of macromolecules to maintain metabolism [31]. In addition, the high concentration of organic matter present in PW causes additional stress on microalgae, which can inhibit lipid synthesis [23].
In this study, the salinity of PW and the salts present in the synthetic medium BG11 may have been higher than that tolerated by C. vulgaris, causing a reduction in lipids. Jawaharraj et al. [39] also found a low concentration of lipids in Oscillatoria sp. in wastewater from the agro-industry (9.6%). Gao et al. [40] reported a high lipid content (29%) during Chlorella sp. Cultivation in untreated wastewater. The variation in lipid levels found in these studies is due to the diversity of chemicals present in wastewater [8].
The protein content in the PW 30% treatment (21.94%) was significantly reduced (p < 0.05) compared with that in the control treatment (32.03%). The poor protein content of PW is related to saline stress. Cells subjected to high salinities redirect the available energy to osmoregulation rather than protein synthesis [34]. In addition, stress caused by N limitation can decrease protein synthesis and increase the metabolism of biomolecules, resulting in higher carbohydrates and ash leves [15]. Matos et al. [34] reported results similar to those of the present study in Chlorella vulgaris grown in saline medium, with 20% protein content in the treatment group and 37.2% in the control.
The moisture content in the PW 30% and control groups was 12.48 and 13.18%, respectively. Matos et al. [34] reported 18.8% moisture from cultivating Chlorella vulgaris in a saline medium, and these values were considered typical. However, moisture affects the quality of bio-oil production because of the reduction in calorific value during pyrolysis [41]. The ash content was higher in the PW 30% treatment (18.21%) than that in the control (4.12%). It is possible that the excess salts found in PW, such as calcium carbonate, sodium, and chlorides, and the accumulation of alkali metals in microalgal cells hinder the degradation of biomass by pyrolysis [15]. High ash values decrease the bio-oil yield owing to the catalysis of the secondary cracking of the pyrolysis steam [41]. In the cultivation of Chlorella in sewage wastewater, Xu et al. [42] found an ash content (16.06%) similar to that observed in the present study.
The carbohydrate content in the PW 30% and control was 37.55% and 33.42%, respectively. The high levels may be related to the continuous photoperiod (24 h) used, which provides the formation of starch and sugars by the reduction of pentose phosphate [43]. Another factor for the higher carbohydrates concentration in PW cultivation is the stress caused by the salinity of the medium, which induces the microalgae to store energy for cellular protection and as a structural component [26]. Mata et al. [6] also found high carbohydrates levels (42.29%) in the cultivation of Spirulina sp. LEB18 in saline wastewater. The abundance of carbohydrates in this study is an important biotechnological source of bioethanol because microalgae produce lignin-free fermented carbohydrates [20].
Fatty Acids (FA) Composition
The fatty acids concentrations in the PW and control treatments groups are listed in Table 3. The PW 30% treatment resulted in 54.14% saturated fatty acids (SFA), 10.34% monounsaturated fatty acids (MUFA), and 21.50% of polyunsaturated fatty acids (PUFA). The control showed 66.41%, 7.26%, and 13.09% SFA, MUFA, and PUFA, respectively. The high SFA content was due to the continuous photoperiod. Continuous illumination stimulates ATP and NADPH production, which helps distribute excess light energy and prevents cell damage [9]. In addition, large amounts of salts stimulate desaturase synthesis, which helps in SFA production [15, 23]. Church et al. [31] also observed a difference in SFA levels, from 16.99% to 21.53%, when C. vulgaris was cultivated in a medium with high salinity. Zhang et al. [44] reported similar SFA values found to those found in this study (56.76%) by cultivating Chlorella sorokiniana SDEC-18 in wastewater from kitchen effluents at various dilutions. The degree of saturation is favorable for obtaining high-quality biodiesel. Higher amounts of SFA increase ignition quality, and a lower percentage of PUFA prevents auto-oxidation [15].
The major FAs from the PW 30% treatment were C18:3n6, C20:0, C16:0, and C18:0. Large concentrations of SFA from the C16 and C18 families occur due to the action of acetyl-CoA carboxylase. This enzyme converts the carbon present in the medium by elongation and desaturation into malonyl-CoA, mainly forming C16 and C18 carbon chains [15, 23]. Gao et al. [40] reported the predominance of C16:0 (26,4–31,5%), C18:0 (18,2–27,2%), and C18:1 (19,1–25,4%) in the cultivation of C. vulgaris in different seafood processing wastewater.
In contrast to the PW 30% treatment, the major FA in the control was C20:0. This difference was attributed to the composition of the culture, which consisted of only BG11 only, which has a higher nitrate concentration than PW 30%. Costa et al. [45] similary reported 22.62% C20:0 when cultivating Chlorella minutíssima in BG11 medium and attributed the high SFA content to a high nitrate concentration in the medium. The diversity and profile of FAs found in the PW 30% treatment reinforce the possibility of obtaining high-quality biodiesel from the produced biomass.
Evaluation of Biodiesel Properties
Table 4 presents the quality indices of the theoretical biodiesel produced using biomass from the PW 30% treatment as the substrate. The iodine value (IV) indicates the degree of fatty acid unsaturation. High unsaturation levels result in the deposition and deterioration of lubricating oil. In the present study, the IV was adequate for both Brazilian and European standards (< 120 g I2/100 g). The PW 30% treatment resulted in 68.48 g I2/ 100 g due to the higher SFA content (54.14%) than unsaturated fatty acids. Cardoso et al. [15] also found results within the recommended IV limit from the cultivation of Spirulina sp. LEB18 in different proportions of aquaculture wastewater (87.78–103 g I2/100 g), as did Jawaharraj et al. [39] in the cultivation of Oscillatoria sp. (65.6 g I2/100 g) and Synechocystis sp. (50.5 g I2/ 100 g) in industrial wastewater.
The saponification value index (SV) indicates the amount of oil needed to saponify 1.0 g of oil. The saponification reaction interferes with the transesterification reaction during biodiesel production. Therefore, low saponification level imply better biodiesel quality. Although there are no standardized SV parameters, these values were compared with biodiesel from vegetable oils, such as palm oil (192–202); soybean oil (189–195), and sunflower oil (188–194) [23]. In this study, the PW 30% treatment presented values close to those of the vegetable oils (182.07). The SV result found in this study were similar to those reported by Jawaharraj et al. [39] for the cultivation of Synechocystis sp. (193.4), Oscillatoria sp. (202.8), and Jatropha sp. (199.7) in industrial wastewater.
The cetane number (CN) defines the ignition quality of the fuel. The CN range indicated by American standards require a minimum of 47, while European standards require a minimum value of 51 [23]. The CN found in this study (60.85) was attributed to the high SFA concentrations and was above the level recommended by international standards. High cetane values promote good engine performance and reduce smoke formation, which are parameters used to evaluate combustion quality and ignition delay time [44]. Zhang et al. [44] found similar results (CN = 59.24) from the cultivation of Chlorella sorokiniana in kitchen wastewater.
The long-chain saturation factor (LCSF) determines the behavior of biodiesel at low temperatures. It represents long-chain saturated fatty acids (C16; C18; C20; C22, and C24) [44]. The PW 30% treatment showed a higher value (27.75%) than that indicated for biodiesel quality, which is different from what was reported by Cardoso et al. [23] from the cultivation of Spirulina sp. LEB18 in aquaculture wastewater (4.81 to 5.52%). High LCSF values decrease the quality of biofuels. Saturated FAs tend to crystallize at engine operating temperatures; therefore, the crystals rapidly clump together when the temperature is reduced, causing clogged filters and fuel lines [46].
The degree of unsaturation (DU) is related to the number of double bonds and the IV and describes the oxidation stability of biodiesel [39]. The DU value found in this study (53.34%) was proportional to the IV. However, both DU and IV were lower than those reported by Cardoso et al. [23] for Spirulina sp. LEB18 cultivation in different proportions of wastewater (74.54–86.83%). The DU was measured based on the mass of monounsaturated and polyunsaturated fatty acids. Therefore, the greater the number of unsaturations, the higher the DU and IV [46]. Low DU values are desirable, particularly for polyunsaturated and long-chain fatty acids. Linoleic and linolenic acids are more susceptible to oxidation than monounsaturated fatty acids because of the bis-allylic position at C11 in linoleic acid and at C11 and C14 in linolenic acid [46].
The cold filter plugging point (CFPP) is an index used to observe biodiesel performance at low temperatures. CFPP is related to the SFA content, as SFAs tend to crystallize at low temperatures and consequently clog filters, pumps, injectors, and pipes due to changes in viscosity and inadequate cold flow properties [44]. The Brazilian Petroleum Agency considers a maximum of 19 °C for this parameter. The CFPP found in the present study (70.70 ºC) exceeded the recommended value by nearly fourfold. This high index indicates low induction at low temperatures [44]. However, additives can be used to inhibit crystal agglomeration [23]. Jawaharraj et al. [39] measured CFPP values ranging from -8.1 to 3.1 °C in the cultivation of Oscillatoria sp., Synechocystis sp. and Jatropha sp. in industrial wastewater. The quality of biodiesel is related to its oxidative stability, which depends on peroxides, light, air, heat, and trace metals in the compounds [46]. Therefore, Chlorella vulgaris biomass has appealing properties and a high potential for biodiesel production.
Chlorophyll a, Chlorophyll b and Total Carotenoids
Chlorophyll a (chl a), and chlorophyll b (chl b) values in the PW 30% treatment and control groups were significantly different (p < 0.05) (Fig. 3). Both treatments resulted in appropriate levels of pigments, which could be related to high biomass production and luminosity. High biomass concentrations result in high pigment content associated with the growing conditions [47]. Luminosity affects the quantity and quality of energy used for the metabolic activities of photosynthetic organisms and stimulates the growth and accumulation of biocompounds such as pigments [11].
The chl a and chl b of the PW 30% treatment group were measured at 6.64 and 10.57 μg/mL, respectively. The control treatment had 15.81 μg/mL (chl a) and 24.21 μg/mL (chl b). The lower chlorophyll content found with the PW 30% treatment is attributed to the stress conditions under which the microalgae were subjected, resulting in microalgae degradation and reducing in pigments such as chlorophyll [10]. Gao et al. [40] reported a total chlorophyll content of approximately 18 mg/L after 20 d of cultivation of Chlorella sp. in seafood wastewater. The chlorophyll content found in this study was similar to that of the present study (17.21 μg/mL).
The carotenoid contents in PW 30% and control groups was 21.38 μg/mL and 44.37% μg/mL, respectively. The high levels found in both treatments were attributed to continuous exposure to light. Microalgae produce carotenoids as an antioxidant that inhibit the formation of peroxides and reactive oxygen species. These orange pigments are present in the chloroplast membrane and protect photosynthetic systems against the stress of lighting [10]. Ge et al. [47] obtained 12.9 μg/mL of carotenoids from cultivating Chlorella vulgaris in municipal wastewater, which is lower than that obtained in this study (21.38 μg/mL). High concentrations of carotenoids are related to the synergistic effect of high luminosity and pollutants in PW. Carotenoids are sensitive biomarkers for contaminants in aqueous media. The higher the carotenoids content, the greater the pollution in the culture medium [15, 23].
Theoretical Conversion of Carbohydrates from Microalgae Biomass to Ethanol
The theoretical production of ethanol from the carbohydrates in the C. vulgaris biomass was 17.02 mL/100 g in the PW 30% treatment and 15.15 mL/100 g in the control. These considerable theoretical ethanol values represent an alternative for the production of biofuels because microalgae biomasses has a high carbohydrate content. The high levels of starch and cellulose in biomass make it an excellent raw materials for bioethanol production, mainly due to the absence of lignin. In addition, microalgal biomass does not contain recalcitrant cells that efficiently convert sugars into ethanol, reducing the cost of processing and pre-treatment [26].
Bioethanol is promising sustainable and renewable fuel. In addition, it has a high octane number and lower CO2 emissions, despite its lower energy content compared to oil [26]. Biomass has great advantages as a substitute for fossil fuels because of its high organic content, low cost, and high yield, and it can be transformed into chemical products through biological and thermochemical processes [41].
Duarte et al. [26] found a theoretical ethanol value of 25.84 mL/100 g in the cultivation of Spirulina sp. LEB18 in brackish groundwater. The theoretical ethanol value in this study was similar to that reported by Duarte et al. [26]. Thus, the results showed that biomass has potential for use in bioethanol production.
Thermogravimetric Analysis (TGA)
The PW 30% and control treatments presented two and three thermal events, respectively. Figure 4a shows the TG profiles, and Fig. 4b shows the derivative (DTG) profiles of both samples. The first mass loss occurred in the temperature range of 30 to 190 °C and was attributed to the loss of free and crystallized water [18, 20] for the PW 30% treatment (12.48%) and control (13.18%). The second mass loss occurred between 190 and 520 °C, representing the devolatilization of the main components of the microalgae such as proteins, polysaccharides, and lipids, starting with unsatured fatty acids and followed by saturated fatty acids as they have a higher melting point [18, 20].
Among the polysaccharides, pyrolysis occurs first in cellulose, a crystalline, linear material composed of glucose, with pyrolysis at 250–350 °C, and then in hemicellulose, an amorphous polysaccharide composed of xylose, galactose and arabinose, with pyrolysis between 325 and 400 °C. The main by-product of pyrolysis, l-glucan, can be used in the production of fiber ethanol in the biogas industry [41]. The third mass loss occurred from 520 to 800 °C and was observed only in the control. This was attributed to the decomposition of carbonaceous residues from macromolecules and inorganic compounds, which is characterized by the decomposition of C–C and C-H bonds. These differences in treatments may be related to the presence of chemical compounds, such as Na, K, Mg, and silicates, which affect the degradation via pyrolysis of the biomass material as well as the char yield [44, 48].
Andrade et al. [20] reported three thermal decomposition events during cultivation of Spirulina sp. LEB18 in reused Zarrouk’s medium, similar to what was observed in this study. The first represented mass loss up to approximately 190 °C, the second at approximately 290 °C, and the third thermal event after 500 °C. Gai et al. [48] also reported three thermal events in cultivating Chlorella pyrenoidosa and Spirulina platensis. The first, second, and third events occurred from 25 to 127 °C, 127 to 327 °C, and 427 to 727 °C, respectively. The onset and endset temperatures were close to those reported in the present study.
Fourier Transform Infrared Spectroscopy (FTIR) Coupled to Attenuated Total Reflectance (ATR) Device
The spectroscopic profiles of the PW 30% and control treatments showed differences in the intensity and shape of some spectral bands (Fig. 5). The association of wavenumber with some functional groups is shown in Table 5. The PW 30% and control treatments presented a band between 3400 and 3275/cm referring to the –OH group, which mainly represents residual water [49]. A band between 2934 and 2915/cm was also observed, representing the elongation of symmetrical and asymmetrical vibrations of saturated C–H chains [49, 50]. Spectral bands from 2860 to 2847/cm can also be attributed to –CH2 and –CH3 groups, which form the lipid backbone.
PW 30% displayed shifted bands from 1745–1410/cm. According to Kumar et al. [9], this spectral region represents the C = O methyl esters groups from algal oil. Li et al. [49] and Bataller and Capareda [50] reported that the range between 1774 and 1583/cm is related to the type-I amide band in the C = O stretching vibrations, while bands from 1583 to 1484/cm are associated with type-II amide, resulting in a curvature N–H and C–N, therefore confirming the presence of a peptide bond between amino acids [48]. Protein side-chain and amide III COO− were observed in the spectrum between 1426–1377/cm and 1329–290/cm [49, 50]. The treatments also produced an intense band between 1031 and 1014/cm. These regions represent the elongation of C–O and C–C, typical of carbohydrates [50].
Li et al. [49] reported a spectral profile similar to that of the present study when cultivating Tetraselmis suecica at different CO2 concentrations. Spectral bands at 3402/cm (referring to the –OH bond), 3325/ cm (N–H bonds), 2998–2854/cm (C–H of lipids), 1734/cm (C = O of the esters), 1583/cm (amide I), 1521/cm—amide II (N–H and C–H), and 1423/cm (–CH3 and –CH2) were associated with proteins, and 1059/cm (C–O–C) was associated with polysaccharides. Bataller and Capareda [50] also reported a similar spectral profile in the cultivation of Spirulina platensis, 3960–3340/cm (–OH), 2981–2767/ cm (C–H of lipids), 1762–1719/cm (C = O of esters), 1583–1484/cm (amide II), 1482–1456/cm (–CH2 absorption band of proteins), 1426–1377/cm and 1329–1290/cm (protein side chain and amide III), in addition to 1186–955/cm (referring to carbohydrates). These similarities show that the spectral cell model is applicable for predicting biomass composition.
Conclusion
The cultivation of microalgae in produced water (PW 30%) showed viability and high biomass production among the treatments tested. The pre-adaptation of the inoculum to the continuous photoperiod, additional stress from the 24 h photoperiod, and chemical stress acted synergistically to favor biomass production and biomolecules synthesis by Chlorella vulgaris. The obtained biomass has great potential for use as a raw material in the biodiesel and bioethanol industries because of its fatty acid profile and high concentration of carbohydrates. Furthermore, microalgae also showed the environmental benefit of high removal efficiency of the analyzed chemical compounds, including petroleum hydrocarbons and heavy metals found in the medium, as a wastewater treatment method. Therefore, using 30% PW and a constant photoperiod stimulated the production of biomass-derived biomolecules with high added value that can be converted into bioproducts of interest for the petrochemical industry.
References
Ammar SH, Khadim HJ, Mohamed AI (2018) Cultivation of Nannochloropsis oculata and Isochrysis galbana microalgae in produced water for bioremediation and biomass production. Environ Technol Innov 10:132–142. https://doi.org/10.1016/j.eti.2018.02.002
Dudek M, Vik EA, Aanesen SV, Øye G (2020) Colloid chemistry and experimental techniques for understanding fundamental behaviour of produced water in oil and gas production. Adv Colloid Interface Sci 276:102105. https://doi.org/10.1016/j.cis.2020.102105
Amakiri KT, Canon AR, Molinari M, Angelis-Dimakis A (2022) Review of oilfield produced water treatment technologies. Chemosphere 298:134064. https://doi.org/10.1016/j.chemosphere.2022.134064
Otondo A, Kokabian B, Stuart-Dahl S, Gude VG (2018) Energetic evaluation of wastewater treatment using microalgae, Chlorella vulgaris. Environ Chem Eng 6(2):3213–3222. https://doi.org/10.1016/j.jece.2018.04.064
Qiu B, Tao X, Wang H, Li W, Ding X, Chu H (2021) Biochar as a low-cost adsorbent for aqueous heavy metal removal: A review. J Anal Appl Pyrolysis 155:105081. https://doi.org/10.1016/j.jaap.2021.105081
Mata SN, Santos TS, Cardoso LG, Andrade BB, Duarte JH, Costa JAV, Souza CO, Druzian JI (2020) Spirulina sp. LEB18 cultivation in a raceway type bioreactor using wastewater from desalination process: production of carbohydrate-rich biomass. Bioresour Technol 311:123495. https://doi.org/10.1016/j.biortech.2020.123495
Silva DA, Cardoso LG, Silva JSJ, Souza CO, Lemos PVF, Almeida PF, Ferreira ES, Lombardi AT, Druzian JI (2022) Strategy for the cultivation of Chlorella vulgaris with high biomass production and biofuel potential in wastewater from the oil industry. Environ Technol Innov 25:102204. https://doi.org/10.1016/j.eti.2021.102204
Kumar KS, Prasanthkumar S, Ray JG (2017) Biomass yield, oil productivity and fatty acid profile of Chlorella lobophora cultivated in diverse eutrophic wastewaters. Biocatal Agric Biotechnol 11:338–344. https://doi.org/10.1016/j.bcab.2017.08.006
Khoeyi ZA, Seyfabadi J, Ramezanpour Z (2012) Effect of light intensity and photoperiod on biomass and fatty acid composition of the microalgae, Chlorella vulgaris. Aquac Int 20:41–49. https://doi.org/10.1007/s10499-011-9440-1
Yusof NS, Yeong YS, Zakeri HA, Wahid MEA, Ghafar SN, Yusuf N (2021) Photoperiod influenced the growth and antioxidative responses of Chlorella vulgaris, Isochrysis galbana, and Tetraselmis chuii. J App Pharm Sci 11(04):125–134. https://doi.org/10.7324/JAPS.2021.110415
Benavente-Valdés JR, Aguilar C, Contreras-Esquivel JC, Méndez-Zavala A, Montanez J (2016) Strategies to enhance the production of photosynthetic pigments and lipids in chlorophycae species. BTRE 151, PII S2215-017X(16)30016-9. Biotechnol Rep. https://doi.org/10.1016/j.btre.2016.04.001
Chia MA, Lombardi AT, Melão MGG (2013) Growth and biochemical composition of Chlorella vulgaris in different growth media. J An Acad Bras Cienc 85–4:1427–1438. https://doi.org/10.1590/0001-3765201393312
APHA (2005) - Standard methods for the examination of water and wastewater, 21st edn. American Public Health Association/ American Water Works Association/ Water Environment Federation, Washington, DC
EPA, Environmental monitoring systems laboratory office research and development U.S. Environmental Protection Agency Cincinnati, Ohio 45268 (1993). Determination of inorganic anions by ion chromatography. Inorganic Chemistry branch chemistry research division. Method 300.0, Revision 2.1
Cardoso LG, Duarte JH, Andrade BB, Lemos PVF, Costa JAV, Druzian JI, Chinalia FA (2020) Spirulina sp. LEB18 cultivation in outdoor pilot scale using aquaculture wastewater: high biomass, carotenoid, lipid and carbohydrate production. Aquaculture 525:735272. https://doi.org/10.1016/j.aquaculture.2020.735272
Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 111:1–61. https://doi.org/10.1099/00221287-111-1-1
Costa JAV, Colla LM, Duarte Filho P, Kabke K, Weber A (2002) Modelling of Spirulina platensis growth in fresh water using response surface methodology. World J Microbiol Biotechnol 18:603–607. https://doi.org/10.1023/A:1016822717583
Jesus CS, Uebel LS, Costa SS, Miranda AL, Morais EG, Morais MG, Costa JAV, Nunes IL, Ferreira ES, Druzian JI (2018) Outdoor pilot-scale cultivation of Spirulina sp. LEB-18 in different geographic locations for evaluating its growth and chemical composition. Bioresour Technol 256:86–94. https://doi.org/10.1016/j.biortech.2018.01.149
AOAC (2005) Official methods of analysis, 18th edn. Association of Analytical Chemists, Washington DC, Method 935.14 and 992.24
Andrade BB, Cardoso LG, Assis DJ, Costa JAV, Druzian JI, Lima STC (2019) Production and characterization of Spirulina sp. LEB18 cultured in reused Zarrouk’s medium in a raceway-type bioreactor. BITE 21305, PII S0960-8524(19)30518-8. Bioresour Technol. https://doi.org/10.1016/j.biortech.2019.03.144
Folch J, Lees M, Sloane GHS (1957) A simple method for the isolation and purification of total lipids from animal tissues. Biol Chem 226:497–509. https://doi.org/10.1016/S0021-9258(18)64849-5
Souza CO, Leite MEQ, Lasekan J, Baggs G, Pinho LS, Druzian JI, Ribeiro TCM, Mattos AP, Menezes-Filho JA, Costa-Ribeiro H (2017) Milk proteins-based formulas containing diferente oils affect fatty acids balance in term infants: a randomized blinded crossover clinical trial. Lipids Health Dis 16:78. https://doi.org/10.1186/s12944-017-0457-y
Cardoso LG, Duarte JH, Costa JAV, Assis DJ, Lemos PVF, Druzian JI, Souza CO, Nunes IL, Chinalia FA (2020) Spirulina sp. as a bioremediation agent for aquaculture wastewater: production of high added value compounds and estimation of theoretical biodiesel. Springer Science+Business Media, LLC, part of Springer Nature 2020. Bioenergy Res. https://doi.org/10.1007/s12155-020-10153-4
Lichtenthaler H, Buschmann C (2001) Chlorophylls and carotenoids: measurement and characterization by UV-VIS spectroscopy. Res Gate. https://www.researchgate.net/publication/200037314. Accessed 15 Feb 2021
Jacques, KA, Lyons, TP, Kelsall, DR (2003) The Alcohol Textbook, A reference for the beverage, fuel and industrial alcohol industries, 4th edn. Nottingham University Press, Manor Farm, Main Street, Thrumpton
Duarte JH, Cardoso LG, Souza CO, Nunes IL, Druzian JI, Morais MG, Costa JAV (2019) Brackish groundwater from brazilian backlands in Spirulina cultures: potential of carbohydrate and polyunsaturated fatty acid production. Biotechnol Appl Biochem. https://doi.org/10.1007/s12010-019-03126-7
Campos MI, Figueiredo TVB, Sousa LS, Druzian JI (2014) The influence of crude glycerin and nitrogen concentrations on the production of PHA by Cupriavidus necator using a response surface methodology and its characterizations. Ind Crops Prod 52:338–346. https://doi.org/10.1016/j.indcrop.2013.11.008
Kurade MB, Kim JR, Govindwar SP, Jeon BH (2016) Insights into microalgae mediated biodegradation of diazinon by Chlorella vulgaris: microalgal tolerance to xenobiotic pollutants and metabolism. Algal Res 20:126–134. https://doi.org/10.1016/j.algal.2016.10.003
Mehta P, Rani R, Gupta R, Mathur AS, Puri SK (2018) Biomass and lipid production of a novel freshwater thermo-tolerant mutant strain of Chlorella pyrenoidosa ncim 2738 in seawater salinity recycled medium. Algal Res 36:88–95. https://doi.org/10.1016/j.algal.2018.10.015
Gómez-Luna L, Ortega-Díaz Y, Tormos-Cedeño L (2021) Effect of pH over growth and cellular viability of Chlorella vulgaris Beijerinck local strain. Tecnol Quím 41:252–276
Church J, Hwang JH, Kim KT, Mclean R, Oh YK, Nam B, Joo JC, Lee WH (2017) Effect of salt type and concentration on the growth and lipid content of Chlorella vulgaris in synthetic saline wastewater for biofuel production. Bioresour Technol 243:147–153. https://doi.org/10.1016/j.biortech.2017.06.081
Huo S, Chen J, Zhu F, Zou B, Chen X, Basheer S, Cui F, Qian J (2019) Filamentous microalgae Tribonema sp. cultivation in the anaerobic/oxic effluents of petrochemical wastewater for evaluating the efficiency of recycling and treatment. Biochem Eng J 145:27–32. https://doi.org/10.1016/j.bej.2019.02.011
Znad H, Al- Ketife AMD, Judd S, Almomanid P, Vuthaluru HB (2018) Bioremediation and nutrient removal from wastewater by Chlorella vulgaris. Ecol Eng 110:1–7. https://doi.org/10.1016/j.ecoleng.2017.10.008
Matos AP, Feller R, Moecke EHS, Sant’anna ES (2015) Biomass, lipid productivities and fatty acids composition of marine Nannochloropsis gaditana cultured in desalination concentrate. J Bioresour Technol 197:48–55. https://doi.org/10.1016/j.biortech.2015.08.041
Yaghmaeian K, Jaafari J (2018) Optimization of heavy metal biosorption onto freshwater algae (Chlorella coloniales) algae cells using response surface methodology (RSM). CHEM22467, PII S0045-6535(18)32080-0. Chemosphere. https://doi.org/10.1016/j.chemosphere.2018.10.205
Brar A, Kumar M, Vivekanand V, Pareek N (2018) Phycoremediation of textile effluent contaminated water bodies employing microalgae: nutrient sequestration and biomass production studies. Int J Environ Sci Technol. https://doi.org/10.1007/s13762-018-2133-9
Duarte IF, Ribeiro VS, Santos MIGR, Costa TAD, Santana MB, Oliveira ACV, Marques IM, Ñañez KB, Moreira ITA (2021) Remediation mechanisms of polycyclic aromatic petroleum hydrocarbons using microalgae and cyanobacteria with emphasis on circular bioeconomy. Res Soc Dev 10:512101119954.https://doi.org/10.33448/rsd-v10i11.19954
Abid A, Saidane F, Hamdi M (2017) Feasibility of Carbon dioxide sequestration by Spongiochloris sp microalgae during petroleum wastewater treatment in airlift bioreactor. Bioresour Technol 234:297–302. https://doi.org/10.1016/j.biortech.2017.03.041
Jawaharraj K, Karpagam R, Ashokkumar B, Pratheeba CN, Yaralakshmi P (2016) Enhancement of biodiesel potential in cyanobacteria: using agroindustrial wastes for fuel production, properties and acetyl CoA carboxylase d (ACCD) gene expression of Synechocystis sp.nn. Renew Energy 98:72–77. https://doi.org/10.1016/j.renene.2016.02.038
Gao F, Peng YY, Li C, Yang GJ, Deng YB, Xue B, Guo YM (2018) Simultaneous nutrient removal and biomass/lipid production by Chlorella sp. in seafood processing wastewater. Sci Total Environ 640–641:943–953. https://doi.org/10.1016/j.scitotenv.2018.05.380
Qiu B, Tao X, Wang J, Liu Y, Li S, Chu H (2022) Research progress in the preparation of high-quality liquid fuels and chemicals by catalytic pyrolysis of biomass: A review. Energy Conv Manag 261:115647. https://doi.org/10.1016/j.enconman.2022.115647
Xu D, Wang Y, Lin G, Guo S, Wang S, Wu Z (2019) Co-hydrothermal liquefaction of microalgae and sewage sludge in subcritical water: ash effects on bio-oil production. Renew Energy 138:1143–1151. https://doi.org/10.1016/j.renene.2019.02.020
Jacob-Lopes E, Scoparo CHG, Lacerda LMCF, Franco TT (2009) Effect of light cycles (night/day) on CO2 fixation and biomass production by microalgae in photobioreactors. Chem Eng Process 48:306–310. https://doi.org/10.1016/j.cep.2008.04.007
Zhang L, Cheng J, Pei H, Pan J, Jiang L, Hou Q, Han F (2018) Cultivation of microalgae using anaerobically digested effluent from kitchen waste as a nutrient source for biodiesel production. Renew Energy 115:276–287. https://doi.org/10.1016/j.renene.2017.08.034
Costa JAV, Radmann EM, Cerqueira VS, Santos OC, Calheiros MN (2006) Fatty acids profile the microalgae Chlorella vulgaris and Chlorella minutissima grown under different conditions. J Alim Nutr Araraquara 17:429–436
Ramos MJ, Fernández CM, Casas A, Rodríguez L, Pérez A (2009) Influence of fatty acid composition of raw materials on biodiesel properties. Bioresour Technol 100:261–268. https://doi.org/10.1016/j.biortech.2008.06.039
Ge S, Qiu S, Tremblay D, Viner K, Champagne P, Jessop PG (2018) Centrate wastewater treatment with Chlorella vulgaris: simultaneous enhancement of nutrient removal, biomass and lipid production. CEJ 18537, PII S1385-8947(18)30259-6. Chem Eng J. https://doi.org/10.1016/j.cej.2018.02.058
Gai C, Liu Z, Han G, Peng N, Fan A (2015) Combustion behavior and kinetics of low-lipid microalgae via thermogravimetric analysis. Bioresour Technol 181:148–154. https://doi.org/10.1016/j.biortech.2015.01.045
Li F, Srivatsa SC, Batchelor W, Bhattacharya S (2017) A study on growth and pyrolysis characteristics of microalgae using thermogravimetric analysis-infrared spectroscopy and synchrotron Fourier transform infrared spectroscopy. Bioresour Technol 229:1–10. https://doi.org/10.1016/j.biortech.2017.01.005
Bataller BG, Capareda SC (2018) A rapid and non-destructive method for quantifying biomolecules in Spirulina platensis via Fourier transform infrared – attenuated total reflectance spectroscopy. Algal Res 32:341–352. https://doi.org/10.1016/j.algal.2018.04.023
Acknowledgements
The authors would like to thank the FAPESB — Research Support Foundation of the State of Bahia, Brazil (Project RED0001/2020), the FAPESP — Research Support Foundation of the State of São Paulo, Brazil (Processes 2019/26571-0) the CNPq—National Council for Scientific and Technological Development, Brazil (Processes CNPq 44039/2019-3; 309955/2022-0 and 134194/2019-5 and INCT-MIDAS CNPq – 465594/2014-0) and CAPES – Coordination for the Improvement of Higher Education Personnel, Brazil (CAPES PRINT – UFBA) for financial support. The authors thank the Janice Izabel Druzian, professor at the Federal University of Bahia (in memoriam), for all the teachings provided – our eternal gratitude.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Jesus Silva, J.S., Silva, D.A., Oliveira, M.B.P.P. et al. Luminosity and Chemical Stress Improve the Production of Biomass and Biomolecules from Chlorella vulgaris Cultivated in Produced Water. Bioenerg. Res. 16, 2465–2478 (2023). https://doi.org/10.1007/s12155-023-10596-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-023-10596-5