Abstract
In nature, several abiotic stresses occur simultaneously, leading to retarded growth and biochemical changes in microalgae, including the commercial cyanobacterium, Arthrospira platensis. To gain more understanding of stress response, we investigated the integrative effects of nitrogen depletion and high temperature stress on physiological changes of A. platensis C1. The results revealed that photosynthetic activities of the stressed cells were markedly reduced by more than a half in comparison to the non-stressed cells. Moreover, a reduction of biomass was observed within 24 h after prolonged exposure to combined stress of nitrogen depletion and high temperature. The total protein contents, including phycocyanin (PC), in the stressed cells, decreased rapidly within 8 h of incubation. This finding was concomitant with the increase in carbohydrate content. However, the accumulation of carbohydrates in the nitrogen depletion-treated cells was greater than that in the cells under the combined stress. Furthermore, the levels of polysaccharides increased only under long-term incubation under nitrogen depletion but not under the combined stress. In addition, the combination of nitrogen depletion and high temperature stress resulted in an increase in the proportion of linoleic acid but a decrease in γ-linolenic acid within 24 h. These results suggest that the response of A. platensis to the combined stress was different from the responses of cells to individual stress. The PC degradation, the increased carbohydrates, and the alteration in fatty acids profiles were required for physiological response to combined nitrogen depletion and high temperature stress of A. platensis C1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cyanobacteria, including Arthrospira (Spirulina), have been studied and used in many biotechnological applications. Most systems for commercial production of microalgae and cyanobacteria are operated in open ponds, where they are frequently exposed to several stresses such as high temperature, high light intensity, acidity, alkalinity, and nutrient limitation. These stresses resulted in different alterations of microorganisms in growth ability and synthesis of cellular compositions (Vonshak 1997; Cheng and He 2014). In general, cyanobacteria can assimilate different forms of nitrogen such as nitrate, nitrite, urea, and ammonium to synthesize many biomolecules (Flores and Herrero 2005). However, severe depletion of nitrogen might lead to the damage of cellular functions (Collier and Grossman 1992; Krasikov et al. 2012). Furthermore, leakage of nitrogen to the atmosphere through denitrification may occur during cyanobacterial cultivation in outdoor ponds (Vonshak 1997). In plants, more than half of nitrogen fertilizers added to crops is lost through gaseous emission, denitrification, surface runoff, ammonia volatilization, and bacterial competition (Raun and Johnson 1999). Therefore, understanding stress responses in terms of the changes in cellular physiology and metabolic processes may bring an opportunity to increase growth and productivities of cyanobacteria under stress (Aikawa et al. 2012; Hasunuma et al. 2013).
Nitrogen limitation causes a decrease in growth, photosynthetic activity, and pigments in many microalgae and cyanobacteria such as Synechococcus sp. PCC 7942, Rhodomonas sp., Botryococcus braunii, and Synechocystis sp. PCC 6803 (Collier and Grossman 1992; Silva et al. 2009; Choi et al. 2011; Krasikov et al. 2012). Phycobilisome degradation is well known as a response mechanism to nitrogen depletion in cyanobacteria such as Synechococcus sp. PCC 7942, Synechocystis sp. PCC 6803, and A. platensis NIES-39 (Collier and Grossman 1992; Krasikov et al. 2012; Hasunuma et al. 2013). The breakdown of pigments in microalgae and plants triggers chlorosis, causing change in coloration of microalgae from blue-green to yellow (Collier and Grossman 1992) and seedling yellowing symptoms in plants (Scheible et al. 2004). Moreover, intracellular proteins are degraded to provide nitrogen for the synthesis of essential compounds in cellular metabolism, respiration, and growth under nitrogen depletion. The excess carbon from the degradation of intracellular proteins can be stored as lipids or carbohydrates (Scheible et al. 2004; Choi et al. 2011; Aikawa et al. 2012). For example, the green algae, B. braunii and Chlorella vulgaris, accumulate lipids as a carbon sink under nitrogen deficiency (Choi et al. 2011; Mujtaba et al. 2012). When high temperature stress was combined with nitrogen starvation, lipid accumulation of the freshwater green alga Scenedesmus obtusus XJ-15 could be highly induced up to 47.6 % dry weight (Xia et al. 2015). On the other hand, the microalga Rhodomonas sp. (Silva et al. 2009) and the cyanobacterium A. platensis NIES-39 (Aikawa et al. 2012) grown in a nitrogen-lacking medium preferred the accumulation of carbohydrates. In plants, the accumulation of sugars and starch is increased in nitrogen-deficient seedlings of Arabidopsis thaliana (Scheible et al. 2004). The increased glycogen in A. platensis NIES-39 under nitrogen depletion was synthesized with carbon derived from photosynthetic products and from the degradation of intracellular proteins through gluconeogenesis (Hasunuma et al. 2013). Correspondingly, proteomic study of Arthrospira sp. PCC 8005 revealed the up-regulation of proteins involved in carbohydrate synthesis and the down-regulation of proteins related to glycogen degradation and inorganic carbon fixation pathways under nitrogen depletion (Deschoenmaeker et al. 2014). These results indicated that the response mechanism to nitrogen depletion of Arthrospira sp. relates to the reversible pathway of glycogen synthesis and glycogenolysis. When considering the energetic requirements for the synthesis of lipids or carbohydrates, the energy required to synthesize a lipid is higher than that for a carbohydrate. Moreover, the decreased efficiency of carbon accumulation in lipids might be caused by a loss of reduced carbon during the conversion of pyruvate to acetyl-CoA (Subramanian et al. 2013). Therefore, the accumulation of either lipids or carbohydrates in living organisms in response to nitrogen depletion depends on the genetic characteristics of each organism.
Due to the change of global environment, temperature and light are two major factors considered to be the limiting factors of nutrient-sufficient cultivation in outdoor areas. Many studies have revealed that high light intensities and high or low temperatures reduce the photosynthetic activity of Arthrospira (Spirulina) spp., resulting in low biomass productivity of cells (Torzillo and Vonshak 1994; Vonshak and Novoplansky 2008; Vonshak et al. 2014). Moreover, raising temperature above optimal temperature caused an increase in respiration rate in A. platensis M2 (Torzillo and Vonshak 1994). In addition, the combination of high light intensity and high temperature caused a greater decrease in the photosynthetic activities, indicating that photosynthesis of cells was more sensitive to these combined stresses (Torzillo and Vonshak 1994). The increased ambient temperature affected not only the decrease in photosynthetic activity but also the changes in levels of biochemical compounds such as proteins, carbohydrates, pigments, and fatty acids of Arthrospira sp. (Chaiklahan et al. 2007; Panyakampol et al. 2015; Zhang and Liu 2015). Besides, previous studies on transcriptome (Panyakampol et al. 2015) and proteome (Hongsthong et al. 2009) under high temperature stress in A. platensis C1 revealed a correlation between high temperature and nitrogen assimilation. High temperature stress rapidly inhibited the transcript levels of genes encoding nitrate transporters, nitrite transporter, and nitrate reductase; however, it increased the transcript levels of gene encoding amino acid permease-associated region of A. platensis C1 (Panyakampol et al. 2015). It corresponded to the decrease in protein levels of nitrate reductase of A. platensis C1 after prolonged exposure to high temperature stress (Hongsthong et al. 2009). These findings pointed out a decreased efficiency of the nitrate- and nitrite-uptake system and nitrogen assimilation of A. platensis C1 under thermal stress, which might be referred to the conditions of nitrogen depletion concomitant with high temperature stress. The alteration of biochemical compounds under the combination of nitrogen depletion and high temperature stress also has been studied in some green algae (Xia et al. 2015). However, there is little knowledge on physiological and biochemical changes under the combined stress in the commercial cyanobacteria, including Arthrospira sp. Therefore, the aim of the current work was to investigate the physiological changes in A. platensis C1 under the combined stress of nitrogen depletion and high temperature. Since high temperature was an uncontrollable factor in outdoor cultivation of microalgae, we expect that the knowledge from changes in biomass and biochemical compositions under the combined stress should provide a better strategy for the nutrient management of microalgae outdoor cultivation to overcome low biomass productivity under nitrogen depletion and high temperature stress.
Materials and methods
Culture conditions and growth analysis
Cultures of Arthrospira platensis C1 or Arthrospira sp. PCC 9438 were grown at 35 °C under illumination at 100 μmol photons m−2 s−1 with continuous stirring in Zarrouk’s medium (Zarrouk 1966) until mid-log phase. Then, the cells were immediately transferred to nitrogen-depleted Zarrouk’s medium (excluding NaNO3) at normal temperature (35 °C), representing the nitrogen depletion conditions, and under high temperature stress (42 °C), representing the combined stress of nitrogen depletion and high temperature conditions. In this study, the culture of Arthrospira grown under these stress conditions was further incubated with the same light conditions for 24 h.
To analyze growth of A. platensis C1 in each designated time period under the stress conditions, the cell cultures were filtered through a GF/C filters and washed with an equal volume of acidified water (pH 4), prepared from 0.1 N H2SO4. Then, samples were dried at 80 °C until the weight of biomass was constant.
Photosynthetic activity measurement
The photosynthetic activity was measured as the rate of O2 evolution (moles of O2-evolved mg−1 chl h−1) at 35 °C using a Clark-type oxygen electrode (Vonshak et al. 1996). A cell concentration corresponding to 2.5 μg chl mL−1 was used.
Biochemical compounds measurements
Protein content was determined by Folin-Ciocalteau method according to the procedure of Lowry et al. (1951). The cell suspension was mixed with an equal volume of 1 N NaOH and boiled for 20 min. Then, 1 mL samples were mixed with 2.5 mL of reagent, containing 50 mL of 5 % Na2CO3, 1 mL of 1 % CuSO4.5H2O, and 1 mL of 2 % NaKC4H6O6.4H2O; after 10 min of incubation, 0.5 mL of twofold diluted Folin-Ciocalteau reagent (Merck, Germany) was added. After standing at room temperature for 30 min, the supernatant was measured absorbance at 750 nm. The obtained results were compared to standard solutions of known concentrations of bovine serum albumin (Amresco, USA).
Total carbohydrates as reducing sugars were assayed by the phenol sulphuric method as described by Dubois et al. (1956). One milliliter of cell suspension was mixed with 1 mL of 5 % w/v phenol solution and 5 mL of concentrated H2SO4. After incubating at room temperature for 30 min, the absorbance was measured at 490 nm. The standard solution was prepared from d-glucose (Merck, Germany). For the extraction of polysaccharides, freeze-dried cells were soaked with absolute ethanol (ratio 1:8 w/v) at 60 °C for 30 min to remove lipids. Dried-crude cells were re-suspended in water (ratio 1:45 w/v) and incubated at 90 °C for 2 h. Polysaccharides were precipitated by 20 % v/v of 1 % of cetyltrimethylammonium bromide overnight. After the samples were centrifuged at 10,000 rpm for 10 min, pellets were washed with saturated sodium acetate in 95 % ethanol, 95 % ethanol, and absolute ethanol, respectively (Chaiklahan et al. 2013).
For the extraction of lipids, 100 mg of freeze-dried cells were added with 3.75 mL of chloroform/methanol (ratio 1:2 v/v) and 1 mL of water followed by vigorous mixing at room temperature for 20 min. After adding 1.25 mL of chloroform and 0.25 mL of water, samples were centrifuged at 4000 rpm for 5 min. The lower phase containing lipids was added with 2.5 mL of methanol/water (ratio 10:9 v/v) followed by mixing and centrifuging as described above. Crude lipids in the lower phase were collected. The remaining upper and intermediate phases were pooled and added with an equal volume of chloroform to extract lipids. The extraction and separation steps were repeated two more times. The total collection of lipids was evaporated and weighed (Sato and Murata 1988).
Transmethylation for fatty acid analysis was performed according to the method of Lepage and Roy (1984). One hundred milligrams of freeze-dried cells was added to 100 μL of heptadecanoic acid (C17H33COOH), an internal standard (Sigma, USA) prepared by dissolving 100 mg of C17H34O2 in 10 mL of petroleum ether. Then, 2 mL of methanol-hydrochloric (ratio 95:5 v/v) was added and incubated at 80 °C for 1 h. After cooling, samples were added with 1 mL of water and 1 mL of hexane containing 0.01 % of butylated hydroxytoluene. The fatty acid methyl esters in the hexane layer were analyzed by gas chromatography GC-17A (Shimadzu, Kyoto, Japan). The capillary column used in the analysis was a fused silica glass column OMEGAWAX™ 250 (Supelco, USA) of 30 m length and 0.25 μm film thickness. Oven temperature and the injector temperature were set at 205 and 250 °C, respectively. The relative fatty acids content was determined by comparing their peak areas with the internal standard.
Pigment analysis
Chl a was extracted by methanolic extraction (Bennett and Bogorad 1973). Cell suspension was filtered through GF/C filters. Then, the filtered cells were re-suspended in 5 mL of absolute methanol and incubated at 70 °C for 2 min. Samples were cooled and centrifuged at 3500 rpm for 5 min. Absorbance of clear supernatant was measured at 665 nm.
Phycocyanin (PC) was extracted with 100 mM phosphate buffer (pH 7) as described by Boussiba and Richmond (1979). Freeze-dried cells were mixed with 5 mL of 100 mM phosphate buffer and incubated at 4 °C overnight. Then, samples were centrifuged at 3500 rpm for 10 min and the absorbance was measured at 620 nm.
Data analysis
All experiments were conducted with three independent biological replicates under nitrogen depletion and the combination of nitrogen depletion and high temperature stress. Statistical analysis of physiological and biochemical data among the culture conditions was performed on SPSS 16.0 package (Chicago, USA) using a one-way ANOVA and Duncan’s test (p < 0.05). Based on the statistical test results, effect analyses under nitrogen depletion (35 °C, N−) and combined stress (42 °C, N−) were performed and compared with the control (35 °C, N+). The results of figures and tables were represented by the averages and the standard deviations.
Results and discussion
Effects of the combination of nitrogen depletion and high temperature stress on growth and photosynthesis of A. platensis C1
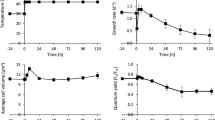
After A. platensis C1 was immediately transferred to nitrogen depletion and the combination of nitrogen depletion and high temperature stress, cell growth in terms of dry weight was investigated (Fig. 1). No significant differences between both conditions were observed in the first 12 h; however, afterwards, the dry weight under both conditions decreased significantly, while in the control it increased gradually (p < 0.05). During the first 12 h, the dry weight of A. platensis C1 under nitrogen depletion at 35 and 42 °C gradually increased to 0.61 ± 0.03 and 0.65 ± 0.07 g L−1, respectively, even though their growth was lower than that of the control (0.77 ± 0.04 g L−1) (Fig. 1). Further incubation for 24 h resulted in a decrease in cell dry weight to about half of the control. The decrease in dry weight of cells grown under the combination of high temperature stress and nitrogen depletion was consistent with that of cells grown under the individual stress of nitrogen depletion over 24 h (p > 0.05). Although the retarded growth of A. platensis C1 after exposure to nitrogen depletion at both 35 and 42 °C was observed, the stressed cells still grew in the first 12 h. This might be due to the availability of endogenous nitrogen for the synthesis of essential compounds to strive against the stress or to acclimate to stress, as found in many microalgae and cyanobacteria (Silva et al. 2009; Hasunuma et al. 2013; Deschoenmaeker et al. 2014).
Dry weight of A. platensis C1 after shifting the conditions to nitrogen depletion and the combined stress of nitrogen depletion and high temperature. Data in each time period are the average ± SD of three independent replicates. Culture conditions of the control and after shifting the conditions to nitrogen depletion and the combined stress of nitrogen depletion and high temperature are represented as 35 °C, N+ (black triangle); 35 °C, N− (white triangle); and 42 °C, N− (white circle), respectively
The poor growth of A. platensis C1 under nitrogen depletion at both normal and high temperature might reflect its photosynthetic activity. In other cyanobacteria, such as Synechocystis sp. PCC 6803 and Arthrospira sp., individual stress of either nitrogen depletion or high temperature caused rapid decline in photosynthetic activity (Krasikov et al. 2012; Panyakampol et al. 2015; Zhang and Liu 2015). Therefore, we investigated photosynthetic activities in the treated cells under nitrogen depletion combined with high temperature stress. The result in Fig. 2 showed that during the first 4 h of incubation, the O2 evolution of stressed cells was significantly lower than that of the control cells; they were continuously decreasing until 24 h of incubation (p < 0.05). The photosynthetic activities rapidly declined by approximately 26 and 18 % of the initial values under nitrogen depletion at 42 and 35 °C, respectively. Then, the photosynthetic activity of the combined stress-treated cells continuously decreased by more than half of the control within 8 h and dropped by 78 % at 24 h, whereas the photosynthetic activity of cells under nitrogen depletion at normal temperature at 24 h decreased to only half of the control. These results suggested that the combined stress of nitrogen depletion and high temperature had stronger negative effects on the activity of photosynthesis in the cells. Hence, the multiple stress factors greatly affected the photosynthetic activity in cells. As found in S. platensis M2, the combined stress of high light intensity and high temperature had a greater effect compared with the individual stress on the decrease in the photosynthetic activity (Torzillo and Vonshak 1994).
Photosynthetic activity of A. platensis C1 after shifting the conditions to nitrogen depletion and the combined stress of nitrogen depletion and high temperature. Data in each time period are the average ± SD of three independent replicates. Culture conditions of the control and after shifting the conditions to nitrogen depletion and the combined stress of nitrogen depletion and high temperature are represented as 35 °C, N+ (black triangle); 35 °C, N− (white triangle); and 42 °C, N− (white circle), respectively
In terms of Chl a content, no significant differences between both stress cases and the control were observed in the first 12 h (p < 0.05) (Fig. 3). These results might indicate the stability of Chl a in A. platensis C1 during short-term response to stresses of nitrogen depletion at 35 and 42 °C. When cells were further incubated under both stresses, Chl a content significantly gradually increased until the end of 24 h of incubation (p < 0.05). A previous study has also shown that Chl a content of A. platensis C1 decreased after prolonged exposure to high temperature at 43 °C for over 48 h (Chaiklahan et al. 2007). However, Chl a content of Synechocystis sp. PCC 6803 still remained constant after long-term exposure to nitrogen deprivation (Krasikov et al. 2012). Therefore, the different alterations of Chl a contents in cyanobacteria might depend on species, culture conditions and range of exposure time to stresses. Many studies have reported that either nitrogen depletion or high temperature stress cause reduction of photosynthetic activity. The reduction of photosynthetic activity of Synechocystis sp. PCC 6803 grown under nitrogen depletion resulted from the diminished carbon fixation activity (Krasikov et al. 2012). The change in photosynthetic activity under high temperature was related to alteration of membrane fluidity (Aminaka et al. 2006). Therefore, it is possible that nitrogen depletion and high temperature may affect other metabolic processes of A. platensis C1. To better elucidate the integrative effects of nitrogen depletion combined with high temperature stress, we further examined the changes in accumulation of biochemical compounds such as proteins, phycocyanins, carbohydrates, polysaccharides, lipids, and fatty acids of A. platensis C1 under these stress conditions.
Chl a content of A. platensis C1 after shifting the conditions to nitrogen depletion and the combined stress of nitrogen depletion and high temperature. Data in each time period are the average ± SD of three independent replicates. Culture conditions of the control and after shifting the conditions to nitrogen depletion and the combined stress of nitrogen depletion and high temperature are represented as 35 °C, N+ (black triangle); 35 °C, N− (white triangle); and 42 °C, N− (white circle), respectively
Protein and phycocyanin content decreased during exposure to nitrogen depletion and high temperature stress
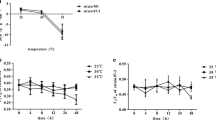
Limitation of nitrogen source causes the reduction of protein content. As shown in Fig. 4a, comparing with the control, no significant difference between both stress cases and the control were observed before 8 h; then, total proteins started to decrease (p < 0.05). This significant decrease reached 79 and 68 % when cells were subjected to nitrogen depletion for 12 and 24 h, respectively. A similar result was also found in cells grown under the conditions of the combined stress of nitrogen depletion and high temperature over 24 h (Fig. 4a). Moreover, a previous study has shown that the protein content of A. platensis C1 declined to approximately 72 % in comparison to the control, when cells were under high temperature stress (Panyakampol et al. 2015). These observations indicated that the combined stress of high temperature and nitrogen depletion, as well as individual stress, caused similar effect on the decrease in total protein content of A. platensis C1.
Alteration of a total proteins, b PC, and c the percentage of PC/total protein content of A. platensis C1 after shifting the conditions to nitrogen depletion and the combined stress of nitrogen depletion and high temperature. Data in each time period are the average ± SD of three independent replicates. Culture conditions of the control and after shifting the conditions to nitrogen depletion and the combined stress of nitrogen depletion and high temperature are represented as 35 °C, N+ (black triangle); 35 °C, N− (white triangle); and 42 °C, N− (white circle), respectively
Furthermore, we investigated the content of PC, a pigment-protein complex, in A. platensis C1 (Fig. 4b). Similarly to the analysis of total proteins, we observed a significant decline to approximately 69 % of the control at 8 h which further declined to 45 % at 24 h under the integrative effect of nitrogen depletion and high temperature stress (Fig. 4b). The changes in PC content grown under nitrogen depletion were similar to those of cells grown under the combined stress. After exposure to nitrogen depletion for 8 and 24 h, the PC content decreased to approximately 72 and 41 % of the control, respectively. This result was consistent with the study of Synechocystis sp. PCC 6803, whose PC started to degrade after 9 h and disappeared after 48 h of nitrogen depletion (Krasikov et al. 2012). Since the decrease in total protein of A. platensis C1 grown under nitrogen depletion at 35 and 42 °C was slower than the decrease of PC, we analyzed the PC/total proteins ratio to compare the rate of PC degradation under each stress condition. The results showed that a significant reduction of the PC/total proteins ratio could be observed at 24 h in the nitrogen-free medium grown cells at both normal and high temperature (Fig. 4c). The alteration of the PC/total proteins ratio in A. platensis C1 was quite similar to that of cells grown under both conditions (p > 0.05). We suggest that nitrogen depletion, rather than high temperature stress, had a marked effect on the reduction of PC in A. platensis C1.
The decrease in total protein content of other Arthrospira strains, such as A. platensis NIES-39 (Hasunuma et al. 2013) and Arthrospira sp. PCC 8005 (Deschoenmaeker et al. 2014) under nitrogen deficiency was related to the degradation of intracellular proteins to increase the generation of free amino acids for the synthesis of essential proteins and metabolites for coping with stress. In A. platensis C1, the results from Fig. 4b clearly showed that PC was a major intracellular nitrogen compound degraded under nitrogen depletion at both normal and high temperature. Like other cyanobacteria, the degradation of PC under nitrogen depletion was to provide nitrogen from intracellular proteins for growth, acclimation to stresses, and compensation for the damaged proteins (Collier and Grossman 1992). In Arthrospira sp. PCC 8005, nitrogen depletion inhibited the levels of several proteins playing a role in photosynthesis, Calvin cycle, and the synthesis of amino acids and sugars; however, it increased the levels of some proteins involved in glutamine synthesis and carbohydrate metabolism (Deschoenmaeker et al. 2014). High temperature stress resulted in a decrease in the protein levels of nitrate reductase, PC-α-phycocyanobilin lyase-related protein and several proteins involved in two-component system and desaturation process, but also caused the increase in the levels of heat-shock proteins and some signaling proteins (Hongsthong et al. 2009). Although total protein content of cells cultivated under nitrogen depletion and the combined stress of nitrogen depletion and high temperature decreased, cells might synthesize essential proteins to cope with stress conditions. The acclimation of Arthrospira sp. under the stresses was related to the induction or the inhibition of protein levels involved in the different processes.
Accumulation of carbohydrates under nitrogen depletion and high temperature stress
Changes in total carbohydrate content of A. platensis C1 were investigated in terms of reducing sugar content, as shown in Table 1. We observed a significant increase in carbohydrate content of A. platensis C1 grown in nitrogen-depleted medium which was up to 53 and 75 % compared to the control at 12 and 24 h, respectively (Table 1). Particularly, nitrogen depletion resulted in the increased polysaccharides of A. platensis C1 to 43 % at 24 h compared to the control (Fig. 5). Meanwhile, the induction of carbohydrate content in the cells treated with nitrogen depletion combined with high temperature stress was only 28 and 23 % within 12 and 24 h, respectively, when compared to the control. Unlike the cells grown under nitrogen depletion at normal temperature, the combined stress-treated cells did not show changes in polysaccharide content within 24 h. Therefore, our observation indicated that high temperature might retard the accumulation of total carbohydrates of cells within 24 h under nitrogen limitation conditions.
Effects of nitrogen depletion and the combined stress of nitrogen depletion and high temperature on the production of polysaccharides in A. platensis C1. Data are the average ± SD of three independent replicates. Culture conditions of the control and after shifting the conditions to nitrogen depletion and the combined stress of nitrogen depletion and high temperature are represented as 35 °C, N+; 35 °C, N−; and 42 °C, N−, respectively
Studies of Arthrospira sp. have revealed the response mechanisms to nitrogen depletion (Hasunuma et al. 2013; Deschoenmaeker et al. 2014) and high temperature stress (Panyakampol et al. 2015), which were associated with carbohydrate synthesis. Physiological responses to nitrogen depletion and high temperature stress in cyanobacteria might be caused by changes at molecular levels. At transcriptional responses, high temperature stress rapidly induced the transcript levels of a gene encoding phosphoenolpyruvate synthase involved in carbohydrate metabolism of A. platensis C1 (Panyakampol et al. 2015). In Arthrospira sp. PCC 8005, several proteins involved in carbohydrate synthesis were up-regulated after 24 h of incubation under nitrogen depletion (Deschoenmaeker et al. 2014). As found in many microalgae, the excess of carbon skeletons could be stored as storage molecules of intracellular energy and/or a carbon source, such as glycogen or lipids when cells grow in the absence of nitrogen (Choi et al. 2011; Aikawa et al. 2012). Correspondingly, Hasunuma et al. (2013) have proposed that A. platensis NIES-39 responded to nitrogen depletion by synthesizing carbohydrates as glycogen from the carbon derived from the degradation of intracellular proteins through gluconeogenesis. Furthermore, Grundel et al. (2012) reported that glycogen metabolism might be involved in maintaining redox homeostasis and acclimating to changes in photosynthetic activity. The high accumulation of polysaccharides may be suggested as a physiological strategy for coping with nitrogen starvation of Rhodomonas sp. (Silva et al. 2009). However, it is possible that the lower accumulation of intracellular carbohydrates in A. platensis C1 grown under nitrogen depletion at high temperature conditions, as compared to cells grown under nitrogen depletion at normal temperature conditions, might have other mechanisms for the synthesis of biochemical compounds. Several studies have suggested that cyanobacteria have the possibility to convert a two-carbon molecule of acetyl-CoA, an intermediate compound, into other compounds such as ethanol, isoprene, isobutanol, polyhydroxybutyrate, and exopolysaccharides (Moreno et al. 1998; Asada et al. 1999; Ducat et al. 2011).
Changes in lipid and fatty acid profiles under nitrogen depletion and high temperature stress
During the first 12 h, we observed a decrease in the lipid content of A. platensis C1 grown under the conditions of nitrogen depletion at both 35 and 42 °C (p < 0.05). However, the lipid contents in the treated cells were not significantly different compared with the control at 24 h (Table 2, p > 0.05). Moreover, the total fatty acids (% of dry weight) significantly decreased by approximately 28 and 27 % of the initial value after exposure to nitrogen depleted conditions at both 35 and 42 °C, respectively (Table 3, p < 0.05). When we considered the patterns of fatty acid profiles for 24 h, the integrative effects of nitrogen depletion and high temperature stress, but not nitrogen depletion at normal temperature, affected the decrease in the proportions of palmitoleic acid (C16:1) and γ-linolenic acid (C18:3) and the increase in the proportion of linoleic acid (C18:2). Correspondingly, the individual stress of high temperature had no effect on total lipid content in A. platensis C1 (Panyakampol et al. 2015); however, it had effects on total fatty acids and fatty acids composition (Chaiklahan et al. 2007). These results suggest that high temperature, rather than nitrogen depletion, was more likely to affect the alteration of fatty acid profiles in A. platensis C1. High temperature stress causes an increase in fluidity of membranes in cyanobacteria (Los and Murata 2004) which can lead to disintegration of the lipid bilayer and may result in the loss of functionality of photosynthetic machinery located on the thylakoid membrane. Deshnium et al. (2000) revealed that the mechanism of A. platensis C1 to cope with high temperature stress was the decrease in the fluidity of the membrane by inducing the rate of desD degradation which may suppress the desaturation of fatty acids from C18:2 to C18:3, resulting in the increase in the proportion of C18:2. Furthermore, the transcript levels of desD of A. platensis C1 rapidly decreased by approximately 1.5-fold in comparison with the control within the first 20 min of incubation at high temperature stress and returned to basal levels at 120 min (Panyakampol et al. 2015). Several studies have proposed that nitrogen depletion contributes to the accumulation various biochemical compounds in microalgae (Choi et al. 2011; Aikawa et al. 2012). Cyanobacteria, including A. platensis C1, preferred to accumulate carbohydrates rather than lipids to be used as a carbon source in response to nitrogen depletion (Aikawa et al. 2012; Hasunuma et al. 2013). However, some microalgae such as B. braunii and C. vulgaris, cultured in a medium with low nitrate concentration showed increases in lipid contents instead (Choi et al. 2011; Mujtaba et al. 2012). In particular, S. obtusus XJ-15 grown under the combination of nitrogen deficiency and high temperature stress showed the highest lipid content of up to 47.6 % dry weight and alterations of fatty acids by increasing the percentage of saturated fatty acids but decreasing the percentage of polyunsaturated fatty acids (Xia et al. 2015).
In conclusion, the combination of nitrogen depletion and high temperature stress showed stronger effects on the photosynthetic activity than the individual stress of nitrogen depletion. Although nitrogen depletion caused a decrease in protein content, including PC and an increase in carbohydrate contents, the accumulation of carbohydrates, especially the increase in the levels of polysaccharides, seemed to be growth temperature-dependent. In regard to fatty acid profiles, these were changed only under the combination of nitrogen depletion and high temperature stress. Although nitrogen depletion highly increased the production of carbohydrates and polysaccharides in A. platensis C1, the growth of stressed cells decreased at 24 h of incubation. In order to utilize carbohydrates as a source for pharmacological applications and the production of biofuels (Aikawa et al. 2012; Chaiklahan et al. 2013; Hasunuma et al. 2013), high productivity of carbohydrates under stress conditions should be considered concomitant with cell growth in further study. Moreover, nutrient management, as a factor that can be controlled, should be regarded as one of the essential requirements for the improvement of microalgal mass cultivation under stresses. Therefore, the knowledge on physiological responses could be applied for further improvement of the management of an outdoor cultivation of microalgae.
References
Aikawa S, Izumi Y, Matsuda F, Hasunuma T, Chang JS, Kondo A (2012) Synergistic enhancement of glycogen production in Arthrospira platensis by optimization of light intensity and nitrate supply. Bioresour Technol 108:211–215
Aminaka R, Taira Y, Kashino Y, Koike H, Satoh K (2006) Acclimation to the growth temperature and thermosensitivity of photosystem II in a mesophilic cyanobacterium, Synechocystis sp. PCC6803. Plant Cell Physiol 47:1612–1621
Asada Y, Miyake M, Miyake J, Kurane R, Tokiwa Y (1999) Photosynthetic accumulation of poly-(hydroxybutyrate) by cyanobacteria—the metabolism and potential for CO2 recycling. Int J Biol Macromol 25:37–42
Bennett A, Bogorad L (1973) Complementary chromatic adaptation in a filamentous blue-green alga. J Cell Biol 58:419–438
Boussiba S, Richmond AE (1979) Isolation and characterization of phycocyanins from the blue-green alga Spirulina platensis. Arch Microbiol 120:155–159
Chaiklahan R, Khonsarn N, Chirasuwan N, Ruengjitchatchawalya M, Bunnag B, Tanticharoen M (2007) Response of Spirulina platensis C1 to high temperature and high light intensity. Kasetsart J (Nat Sci) 41:123–129
Chaiklahan R, Chirasuwan N, Triratana P, Loha V, Tia S, Bunnag B (2013) Polysaccharide extraction from Spirulina sp. and its antioxidant capacity. Int J Biol Macromol 58:73–78
Cheng D, He Q (2014) Assessment of environmental stresses for enhanced microalgal biofuel production—an overview. Front Energy Res 2:1–8
Choi GG, Kim BK, Ahn CY, Oh HM (2011) Effect of nitrogen limitation on oleic acid biosynthesis in Botryococcus braunii. J Appl Phycol 23:1031–1037
Collier JL, Grossman AR (1992) Chlorosis induced by nutrient deprivation in Synechococcus sp. strain PCC 7942: not all bleaching is the same. J Bacteriol 174:4718–4726
Deschoenmaeker F, Facchini R, Leroy B, Badri H, Zhang CC, Wattiez R (2014) Proteomic and cellular views of Arthrospira sp. PCC 8005 adaptation to nitrogen depletion. Microbiology 160:1224–1236
Deshnium P, Paithoonrangsarid K, Suphatrakul A, Meesapyodsuk D, Tanticharoen M, Cheevadhanarak S (2000) Temperature-independent and -dependent expression of desaturase genes in filamentous cyanobacterium Spirulina platensis strain C1 (Arthrospira sp. PCC 9438). FEMS Microbiol Lett 184:207–213
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Ducat DC, Way JC, Silver PA (2011) Engineering cyanobacteria to generate high-value products. Trends Biotechnol 29:95–103
Flores E, Herrero A (2005) Nitrogen assimilation and nitrogen control in cyanobacteria. Biochem Soc Trans 33:164–167
Grundel M, Scheunemann R, Lockau W, Zilliges Y (2012) Impaired glycogen synthesis causes metabolic overflow reactions and affects stress responses in the cyanobacterium Synechocystis sp. PCC 6803. Microbiology 158:3032–3043
Hasunuma T, Kikuyama F, Matsuda M, Aikawa S, Izumi Y, Kondo A (2013) Dynamic metabolic profiling of cyanobacterial glycogen biosynthesis under conditions of nitrate depletion. J Exp Bot 64:2943–2954
Hongsthong A, Sirijuntarut M, Yutthanasirikul R, Senachak J, Kurdrid P, Cheevadhanarak S, Tanticharoen M (2009) Subcellular proteomic characterization of the high-temperature stress response of the cyanobacterium Spirulina platensis. Proteome Sci 7:1–19
Krasikov V, Aguirre von Wobeser E, Dekker HL, Huisman J, Matthijs HC (2012) Time-series resolution of gradual nitrogen starvation and its impact on photosynthesis in the cyanobacterium Synechocystis PCC 6803. Physiol Plant 145:426–439
Lepage G, Roy CC (1984) Improved recovery of fatty acid through direct transesterification without prior extraction or purification. J Lipid Res 25:1391–1396
Los DA, Murata N (2004) Membrane fluidity and its roles in the perception of environmental signals. Biochim Biophys Acta 1666:142–157
Lowry OH, Rosebrough NJ, Farr AL, Randall R (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Moreno J, Vargas MA, Olivares H, Rivas J, Guerrero MG (1998) Exopolysaccharide production by the cyanobacterium Anabaena sp. ATCC 33047 in batch and continuous culture. J Biotechnol 60:175–182
Mujtaba G, Choi W, Lee CG, Lee K (2012) Lipid production by Chlorella vulgaris after a shift from nutrient-rich to nitrogen starvation conditions. Bioresour Technol 123:279–283
Panyakampol J, Cheevadhanarak S, Sutheeworapong S et al (2015) Physiological and transcriptional responses to high temperature in Arthrospira (Spirulina) platensis C1. Plant Cell Physiol 56:481–496
Raun WR, Johnson GV (1999) Improving nitrogen use efficiency for cereal production. Agron J 91:357–363
Sato N, Murata N (1988) Membrane lipid. Methods Enzymol 167:251–259
Scheible WR, Morcuende R, Czechowski T et al (2004) Genome-wide reprogramming of primary and secondary metabolism, protein synthesis, cellular growth processes, and the regulatory infrastructure of Arabidopsis in response to nitrogen. Plant Physiol 136:2483–2499
Silva AF, Lourenco SO, Chaloub RM (2009) Effects of nitrogen starvation on the photosynthetic physiology of a tropical marine microalga Rhodomonas sp. (Cryptophyceae). Aquat Bot 91:291–297
Subramanian S, Barry AN, Pieris S, Sayre RT (2013) Comparative energetics and kinetics of autotrophic lipid and starch metabolism in chlorophytic microalgae: implications for biomass and biofuel production. Biotechnol Biofuels 6:150–162
Torzillo G, Vonshak A (1994) Effect of light and temperature on the photosynthetic activity of the cyanobacterium Spirulina platensis. Biomass Bioenerg 6:399–403
Vonshak A (ed) (1997) Spirulina platensis (Arthrospira): physiology, cell-biology and biotechnology. Taylor & Francis, London
Vonshak A, Novoplansky N (2008) Acclimation to low temperature of two Arthrospira platensis (cyanobacteria) strains involves down-regulation of PSII and improved resistance to photoinhibition. J Appl Phycol 44:1071–1079
Vonshak A, Chanawongse L, Bunnag B, Tanticharoen M (1996) Light acclimation and photoinhibition in three Spirulina platensis (cyanobacteria) isolates. J Appl Phycol 8:35–40
Vonshak A, Laorawat S, Bunnag B, Tanticharoen M (2014) The effect of light availability on the photosynthetic activity and productivity of outdoor cultures of Arthrospira platensis (Spirulina). J Appl Phycol 26:1309–1315
Xia L, Song S, Hu C (2015) High temperature enhances lipid accumulation in nitrogen-deprived Scenedesmus obtusus XJ-15. J Appl Phycol. doi:10.1007/s10811-015-0636-z
Zarrouk C (1966) Contribution à l’étude d’une cyanophycée. Influence de divers’ facteurs physiques et chimiques sur la croissance et la photosynthèse de Spirulina maxima, Ph.D. Thesis. Université de Paris, Paris
Zhang L, Liu J (2015) Effects of heat stress on photosynthetic electron transport in a marine cyanobacterium Arthrospira sp. J Appl Phycol. doi:10.1007/s10811-015-0615-4
Acknowledgments
This work was supported by the Cluster and Program Management Office, National Science and Technology Development Agency (no. P-11-00603 and no. P-12-04794 to K.P.). We would like to thank Nattayaporn Chirasuwan (Algal Biotechnology Laboratory, King Mongkut’s University of Technology Thonburi) for suggesting pigment and polysaccharides analysis, Chutimas Chotvijitjinda (School of Bioresources and Technology, King Mongkut’s University of Technology Thonburi) for technical support in growth analysis and Łukasz Szmelc and Craig Butler (School of Bioresources and Technology, King Mongkut’s University of Technology Thonburi) for proof-reading the manuscript. We are grateful to the Bioinformatics and Systems Biology HRD project, National Center for Genetic Engineering and Biotechnology and King Mongkut’s University of Technology Thonburi for providing a scholarship (no P-00-20121) to Jaruta Panyakampol.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Panyakampol, J., Cheevadhanarak, S., Senachak, J. et al. Different effects of the combined stress of nitrogen depletion and high temperature than an individual stress on the synthesis of biochemical compounds in Arthrospira platensis C1 (PCC 9438). J Appl Phycol 28, 2177–2186 (2016). https://doi.org/10.1007/s10811-015-0765-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-015-0765-4