Abstract
The influence of nitrogen (N) deficiency on the cell growth and intracellular lipid production of the alga Botryococcus braunii UTEX 572 was investigated. Biomass concentration and lipid content of B. braunii cultivated in modified Chu-13 medium containing 0.04, 0.37, and 3.66 mM nitrate were 0.23–0.38 g L−1 and 36–63% of dry cell weight, respectively. The specific growth rate of B. braunii reached a constant of 0.185 day−1 during cultivation with an initial nitrate feed of 3.66 mM. The maximum lipid content of B. braunii was 63% with 0.04 mM nitrate. However, the maximum lipid productivity of 0.019 g L−1 day−1 was achieved with 0.37 mM nitrate. The level of oleic acid, an important component of biodiesel, was higher at 86% of the total fatty acids under N-limited conditions (0.04 mM nitrate) compared to 69% under N-sufficient conditions (3.66 mM nitrate). Furthermore, expression of the stearoyl-ACP desaturase gene (sad) encoding a stearoyl-ACP desaturase involved in the synthesis of oleic acid was 2.6-fold higher under N-limited conditions than under N-sufficient conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the shortage of fossil fuels and their production of greenhouse gas carbon dioxide upon combustion, alternative fuels are now receiving considerable attention, especially biodiesel from microalgae (Chisti 2007). Microalgae have emerged as a particularly promising feedstock due to their widespread availability and higher lipid yields. As a photosynthetic organism, algae can fix atmospheric CO2, and burning algal hydrocarbons is not a net contributor to CO2, thereby benefiting environmental protection (Yang et al. 2004a). Previous studies have reported a high quality of biodiesel production from the heterotrophic microalgae Chlorella protothecoides (Xu et al. 2006). Furthermore, other good sources for liquid fuel production include autotrophic microalgae, such as Nitzschia communis (Dempster and Sommerfeld 1998), Botryococcus braunii (Rao et al. 2007), Dunaliella (Takagi et al. 2006), and the diatom Chaetoceros muelleri (McGinnis et al. 1997).
The green alga B. braunii is a colonial member of Trebouxiophyceae (Casadevall et al. 1985) and grows as a colony of individual cells held together by a colony matrix containing a mixture of liquid hydrocarbons and unusual lipids. This alga is widespread in freshwater and brackish lakes, reservoirs, and ponds. In 1976, a bloom of B. braunii appeared in the Darwin River reservoir, and despite a high liquid hydrocarbon content, the algal colonies still floated (Wake and Hillen 1980). Botryococcus braunii is also well known for its high content of fatty acid and has already been proposed as a renewable source of biodiesel for future energy requirements (Banerjee et al. 2002). Different strains of B. braunii produce different types of hydrocarbons; however, due to the absence of clear morphological differences between these strains, they have been classified into three chemical races—A, B, and L—depending on the type of hydrocarbons synthesized (Metzger and Largeau 2005). Race A produces long straight-chain odd-carbon-numbered hydrocarbons (mono- to tetra-unsaturated) from C23 to C33 derived from fatty acids, typically alkadienes, with the formula C n H2n − 2. Race B produces branched triterpenoids from C30 to C37 with the generalized formula C n H2n − 10, termed botryococcenes. Race L produces an acyclic tetraterpene hydrocarbon called lycopadiene, typically C40H78.
The lipids from microalgal sources have to be transesterified, leading to fatty acid methyl esters (FAMEs or biodiesel) that fulfill diesel characteristics (Miao and Wu 2006). Biodiesel is produced by a mono-alcoholic transesterification process. Triacylglycerols (TAGs) react with a mono-alcohol (usually methanol or ethanol) based on the catalysis of alkali, acids, or enzymes (Li et al. 2008b). Neutral lipids, mainly TAGs, are preferred over phospholipids or glycolipids due to their higher percentage of fatty acids and lack of phosphate. Among the many vegetable oils currently available, those with a high content of oleic acid are the most suitable due to the greater stability of their alkyl esters and their better characteristics as fuels (Knothe 2005).
Desaturases are known for their ability to catalyze the formation of unsaturated fatty acids. These enzymes act by introducing a double bond at a specific defined position in the hydrocarbon chain and are grouped into three types, where microalgal fatty acid desaturases are part of the family of acyl-ACP desaturases or plant-type desaturases (Tocher et al. 1998), which are membrane-bound enzymes (Mustardy et al. 1996). Acyl-ACP desaturases introduce double bonds into fatty acids that have been esterified to glycerolipids. The stearoyl-ACP desaturase (SAD) of microalgae converts stearic acid (18:0) into oleic acid (18:1). A quantitative real-time polymerase chain reaction (qRT-PCR) is among the best methods available for determining changes in gene expression due to its ability to quantify target genes rapidly and accurately, even those with very weak expression levels (Cook et al. 2009).
Accordingly, in this study, the cultivation of various initial nitrate concentrations to increase the lipid production and change the fatty acid composition of B. braunii was investigated. First, the effect of nitrogen concentration on the cell growth and lipid productivity was examined in a batch culture. Second, sad gene expression level in this alga under nitrogen-limited culture conditions was assessed using qRT-PCR.
Materials and methods
Botryococcus braunii UTEX 572 belonging to race A was used throughout this study and obtained from UTEX in the USA. A modified Chu-13 medium was also used, which contained the following (mg L−1): KNO3, 400; K2HPO4, 80; MgSO4⋅7H2O, 200; CaCl2⋅2H2O, 80; Fe-citrate, 20; and citric acid, 200 (Dayananda et al. 2007). Pre-cultures were carried out in 125-mL Erlenmeyer flasks in a shaking incubator: temperature 25°C; shaking at 100 rpm; light intensity 120 μmol photons m−2 s−1 with continuous light; inoculation every 3 weeks (10 mL culture into 30 mL fresh medium). For nitrogen starvation, the cells were washed three times with sterilized distilled water and suspended in a nitrogen-free Chu-13 medium. The growth curves were obtained from dry weight measurements and optical density measurements. The dry weight measurements were carried out based on the filtration of aliquots using an ash-free Whatman filter (GF/C), which was weighed after dried (100°C, 1 h), that had been thoroughly rinsed (distilled water 20 mL) and dried (100°C, 1 h). Meanwhile, optical density measurements were obtained as follows: When comparing the optical density at 680 nm with the dry weight for a large range of samples, the two sets of figures clearly exhibited a linear relationship (r 2 = 0.985), at least during the first stages of the batch culture: biomass (g/L) = OD680 nm × 0.495. Variations in the algal biomass were also determined from the OD680 nm. The mean biomass doubling time during the exponential stage of the batch culture was determined according to ln2/μ, μ is a specific growth rate. In addition, nitrogen content in the medium was checked during the experiment using a nitrogen (total) cell test kit (Merck 1.00613, Germany).
Fatty acid analysis
Total lipids were extracted using a modified version of the method of Bligh and Dyer (1959). The lipid fraction was extracted with chloroform/methanol (2:1, v/v), then isolated in the chloroform phase after adjusting the solvent ratio to 2:2:1 (chloroform/methanol/water, v/v/v). The chloroform phase was then removed by evaporation under a vacuum and the weight determined gravimetrically. The fatty acids were analyzed using the method of Lepage and Toy (1984). A sample aliquot (10 mg) was placed in a capped test tube and then saponified with 1 mL of a saturated KOH–CH3OH solution at 75°C for 10 min, followed by methanolysis with 5% HCl in methanol at 75°C for another 10 min. Finally, 2 mL of distilled water was used to extract fatty acids. All the samples were analyzed using a gas chromatograph (HP 5890A, USA) equipped with a flame ionization detector. The injector temperature was 250°C, and the separation was achieved on a 25-m × Φ 0.32-mm fused silica capillary column. The flow of the carrier gas (N2) was 2 mL min−1, and 1 μL of a methyl ester solution was injected for each analysis. The temperature program was as follows: The initial temperature of 80°C was maintained for 3 min, raised to 250°C at a rate of 10°C min−1, and then maintained at 250°C for 5 min. The components were identified by comparing their retention time and fragmentation pattern with established standards.
cDNA synthesis and PCR primer design
Culture samples (25 mL) were centrifuged at 3,500×g for 20 min. After removing the supernatant, each cell pellet was resuspended in 600 μL of 50 mM Tris–HCl (pH 8.0), 100 mM EDTA, and 130 μL of chloroform and incubated in ice for 5 min to eliminate any external RNases. The buffer was then removed by centrifugation at 12,000×g for 10 min at 4°C. Finally, the cell pellets were frozen in liquid nitrogen and kept at −70°C until isolating the RNA. The total RNA of B. braunii was extracted using a NucleoSpin RNA Plant Kit (Maxherey-Nagel, Germany) according to the manufacturer’s instructions and eluted in 30 μL of DEPC water. Total RNA concentration and purity were determined using a spectrophotometer (Nanodrop ND-1000, USA); each total RNA sample was assayed twice and an average value determined. The total RNA (1 μg) was reverse-transcribed to produce cDNA using a Sprint RT Complete First-Strand cDNA Synthesis Kit (Clontech, USA) with anchored-oligo (dT) 18 primers in a total reaction volume of 20 μL. For the reverse transcription, the mixture was incubated at 42°C for 1 h and finally heated at 75°C for 10 min to terminate the reaction. All the cDNA samples were diluted 100-fold with DEPC water. From an alignment of SAD amino acid sequences from GenBank Accession no. ABP57425 (Haematococcus pluvialis), ACX71635 (Muriella zofingiensis), and EDP04705 (Chlamydomonas reinhardtii), two consensus amino acid sequence blocks, EENRHGD and MPAHLMD, were identified, corresponding to amino acid positions 170–176 and 299–305, respectively, in ABP57425. The degenerate primers ACP-D2f (5′-GAG GAR AAC CGY CAC GGC GAC-3′) and ACP-D7r (5′-TBC ATG AGG TGC GCS GGC AT-3′) were then synthesized based on the sequences of these two blocks. This also included predicted melting temperatures (T m) of 58–60°C, primer lengths of 20–21 nucleotides, guanine–cytosine contents of 40–57%, and PCR amplicon lengths of about 400 bp. All the primers were custom-ordered from a commercial supplier (Genotech, Korea).
qRT-PCR conditions
qRT-PCRs were performed by a DNA Engine Choromo4 thermocycler (Bio-Rad, CA, USA) using the iTaqTM SYBR® Green Supermix with ROX (Bio-Rad). The reactions were performed in triplicate and included 10 μL of the SYBR Green Supermix with ROX, 7 μL of sterile water, 1 μL of the cDNA template, and 1 μL of the 10 μM forward and reverse gene-specific primers in a final volume of 20 μL. The reactions were incubated at 95°C for 3 min to activate the FastStart Taq DNA polymerase, followed by 45 cycles at 95°C for 30 s, 61°C for 30 s, and 72°C for 30 s. The specificity of the PCR reaction was confirmed using a heat dissociation protocol (from 65°C to 95°C) following the final PCR cycle. This ensured that the resulting fluorescence originated from a single PCR product and did not represent primer dimers formed during the PCR or a non-specific product. The amplification of a single product of the expected size was verified by gel electrophoresis on a 1.0% agarose gel (Sigma-Aldrich, USA) and ethidium bromide staining. CFX Manager Software version 1.0 (Bio-Rad) was used to collect the fluorescence data. A threshold cycle (C t) value was determined from each amplification plot. The PCR efficiencies were calculated using the equation E = 10−1/slope − 1 on a standard curve generated using a tenfold dilution series of one sample over five dilution points measured in triplicate, according to the method of Pfaffl (2001).
Results
Effect of initial nitrate concentration on growth of B. braunii
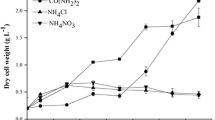
The growth profiles of B. braunii with different nitrate concentrations in the modified Chu-13 medium are shown in Fig. 1. After 10 days of cultivation, a higher initial nitrate concentration in the medium was found to produce an increased biomass concentration, where the highest biomass concentration was 0.38 g L−1 when using an initial nitrate feed of 3.66 mM, which also produced a specific growth rate of 0.185 day−1 (Table 1). Meanwhile, the lowest growth rate of 0.090 day−1 was measured with an initial nitrate feed of 0.04 mM. Furthermore, the average doubling time was 3.8 and 7.7 days with an initial nitrate feed of 3.66 and 0.04 mM, respectively (Table 1). At the end of the cultivation, 100% and 99% of the nitrate had been removed from the media with an initial nitrate feed of 0.37 and 3.66 mM, respectively (Fig. 1). For the medium with 0.37 mM nitrate, no nitrate was detected after day 6.
In the medium with 0.04 mM nitrate, the color of the algal cells turned yellow and the chlorophyll-a content in the algal cells was very low (Fig. 2). However, chlorophyll-a concentration slowly increased after day 6. The cultures with 0.04 and 0.37 mM nitrate concentrations turned yellow-red and mucoid on day 10, and the supernatants were brown and turbid after centrifugation. This was probably because the medium nitrate was all used up and the nitrogen-limited conditions allowed carotenoids and exopolysaccharides to accumulate.
Effect of initial nitrate concentration on lipid production of B. braunii
The lipid content increased from 23% to 63% of the biomass after 10 days of cultivation with an initial nitrate feed of 0.04 mM in the Chu-13 medium (Fig. 3). Also, the lipid content with 0.04 mM nitrate was two times higher than that with 3.66 mM nitrate. Thus, the experimental data indicated that increasing the nitrogen concentration in the nutrient medium led to a decrease of the lipid content in the cells. Notwithstanding, lipid productivity was highest with 0.37 mM nitrate (Table 1). As such, while the highest total lipid content was 627.2 mg g−1 when cultivated with an initial nitrate feed of 0.04 mM, the highest lipid yield was 0.19 g L−1 when cultivated with an initial nitrate feed of 0.37 mM. Therefore, even though the cells had a high lipid content, the biomass concentration was low when nitrogen was limited (initial nitrate concentration below 0.37 mM). Consequently, in this study, an initial nitrate feed of 0.37 mM was identified as the critical concentration to achieve a high growth rate and high lipid productivity of 0.019 g L−1 day−1 (Table 1).
Fatty acid composition with different initial nitrate concentrations
Oleic acid (C18:1) was the major fatty acid in all the samples, and the proportion of oleic acid increased with culture time (Fig. 4). During cultivation, palmitic acid (C16:0) decreased while oleic acid increased. With 3.66 mM nitrate, linoleic acid (C18:2) and linolenic acid (C18:3) showed no decrease during cultivation. However, with 0.04 and 0.37 mM nitrate, linoleic acid did decrease during cultivation. Oleic acid increased with a lower nitrate concentration. The highest content of oleic acid was 84% with 0.04 mM nitrate on day 10.
Stearoyl-ACP desaturase gene transcription as response to nitrogen-limited conditions
The stearoyl-ACP desaturase gene (sad) encodes a stearoyl-ACP desaturase involved in the synthesis of oleic acid. A transcriptional analysis of sad was performed on cells cultured with various nitrate concentrations. Using a real-time PCR relative quantification (qRT-PCR), the expression level of sad was measured and the “Pfaffl analysis method” was applied (Pfaffl 2001) using a kinetic PCR efficiency correction. The qRT-PCR efficiency (E) and slope were 0.916 and −3.54, respectively (data not shown). As shown in Fig. 5, qRT-PCR revealed that the sad transcript of B. braunii changed in response to nitrogen depletion in the culture medium. There was also an increase in sad transcription in the nitrogen-deficient cells when compared with the control cells, which coincided with the time when the cells reached nitrogen starvation conditions, after day 6 (Fig. 1). In the nitrogen-limited cultures, the percentage of oleic acid in the fatty acids increased sharply until day 6, then increased slowly thereafter (Figs. 3 and 6). Notwithstanding, the percentage of oleic acid continued to increase slowly throughout the culture period. When compared with the nitrogen-sufficient conditions (3.66 mM nitrate), sad gene expression was almost 2.6 times higher under the nitrogen-limited conditions (0.04 mM nitrate), representing a clear up-regulation of the gene under the nitrogen-limited conditions.
Discussion
The growth of B. braunii limited by nitrogen (a fourfold reduction of nitrate concentration in the medium) was slower and the final biomass was only about half that of the control at day 20 of cultivation (Zhila et al. 2005). Previous results have also shown that 4 mM nitrate in a modified Chu-13 medium is a growth-limiting nutrient (Yang et al. 2004b). However, on day 10, the culture with 3.66 mM nitrate was still green and the supernatant was clear as there was still enough nitrogen in the medium to support algal growth (Fig. 1). Therefore, it seems that the culture with 3.66 mM nitrate was under N-sufficient condition but the others with 0.04 and 0.37 mM nitrate were under N-limited condition during the cultivation period in this study.
An initial nitrate feed of 0.37 mM was identified as the critical concentration to achieve a high growth rate and high lipid productivity of 0.019 g L−1 day−1 (Table 1). These results are consistent with previous reports; for example, the total lipid content of Neochloris oleoabundans and Chlorella sp. increases by a factor of 2 at a low nitrogen concentration (Hsieh and Wu 2009; Li et al. 2008a). Furthermore, nitrogen limitation in Isochrysis galbana has been reported to increase the intracellular content of fatty acid and active diacylglycerol acyltransferase, which converts fatty acid to triglyceride (Sukenik and Wahnon 1991). This could explain why a low nitrate concentration raised the total lipid content. Furthermore, the results showed that different nitrate concentrations influenced cell growth and lipid production.
The pattern of fatty acids in B. braunii is very typical for Chlorococcales (Ahlgren et al. 1992), being very rich in palmitic acid, oleic acid, and linolenic acid. As seen in Fig. 4, oleic acid was the major fatty acid produced with all the nitrate concentrations and throughout the cultivation, followed by palmitic acid and linolenic acid. However, these results differ with those for cyanobacteria where the proportion of palmitic acid is usually >43% of the total fatty acids. For example, the major fatty acids in Synechocystis sp. PCC 6803 are palmitic acid, Palmitoleic acid (C16:1), stearic acid, and oleic acid. A previous report also showed that oleic acid is a precursor for n-alkadienes (Banerjee et al. 2002), and the algal strains used in the present study have been reported to belong to race A (Dayananda et al. 2007).
The data obtained for the cultures of B. braunii grown on the Chu-13 medium were generally compatible with previous observations on the aging of nitrogen-replete cultures (Khozin-Goldberg et al. 2002), which are characterized by an enhanced proportion of oleic acid and total fatty acid (TFA) content. The increase in oleic acid proportion during aging is believed to be, at least partially, the result of a shift from ω-3 to ω-6 unsaturated fatty acid in lipids that ensues after the transfer from the logarithmic to the stationary phase, as previously observed in many microalgae (Khozin-Goldberg et al. 2002). It has also already been shown that the TFA content and composition both change during culture growth; however, nitrogen starvation induces a larger increase in the fatty acid (FA) content than the culture growth itself (Khozin-Goldberg et al. 2002). TAGs are the main lipidic reservoir and usually accumulate due to low growth rates related with nutrient deficiency (Lombardi and Wangersky 1995). Carbon incorporation is forced and, because of nitrogen deficiency, carbon skeletons are not incorporated into proteins to allow cell growth; therefore, the lipidic synthesis pathway is taken as a carbon sink. In this study, the total phospholipids were correlated with growth. Therefore, it is possible that the total phospholipid contents are related with carbon availability, besides nitrogen, and that light has an indirect effect through the photosynthetic capacity to assimilate CO2, which is finally reflected in the growth rate, rather than a direct effect via the structures supporting photosynthesis. A strong negative correlation exists between TAGs and the total phospholipids. Both lipid classes have common precursors, and the common intermediates are likely to be channeled towards TAG synthesis if phospholipid synthesis is hindered. Thus, B. braunii, which produces large amounts of hydrocarbons, exhibited a faster biomass doubling time than algae like Chlorella, yet using less energy.
Contrary to glyco- or phospholipids, which play an important structural role in cells, TAG cellular accumulation corresponds to a shift of metabolism under unfavorable environmental conditions. Nitrogen starvation is already known to trigger TAG accumulation to the detriment of cell division. Lipid productivity depends on both the lipid content in the cells and biomass accumulation, yet the optimal culture conditions for these two factors are different, making it difficult to optimize lipid productivity. Under nitrogen starvation, many algal species have been reported to accumulate lipids (mostly TAG) which generally contain saturated and monounsaturated FAs (Chu et al. 1997). Decreasing the nitrogen concentration in the medium causes quite a number of algal species to accumulate neutral lipids, principally TAGs (Thompson 1996), accompanied by an increase in the content of saturated FAs and a decrease in polyenoic FAs (Alonso et al. 2000). In addition, under nitrogen deficiency, chlorophyll-a concentration tends to decrease while carotenoids increase (Geider et al. 1998).
When decreasing the nitrogen concentration in the Chu-13 medium, changes in the sad expression were not larger, yet the production of oleic acid was increased. Therefore, this is the first example of an increase in the transcription of the essential sad expression and correlative oleic acid synthesis, suggesting that nitrogen deficiency can trigger oleic acid synthesis. A similar behavior was previously observed in Microcystis aeruginosa when studying the synthesis of microcystin that acts as a toxic material (Sevilla et al. 2008).
References
Ahlgren G, Gustafsson I-B, Boberg M (1992) Fatty acid content and chemical composition of freshwater microalgae. J Phycol 28:37–50
Alonso DL, Belarbi E-H, Ferńandez-Sevilla JM, Rodriguez-Ruiz J, Grima EM (2000) Acyl lipid composition variation related to culture age and nitrogen concentration in continuous culture of the microalga Phaeodactylum tricornutum. Phytochemistry 54:461–471
Banerjee A, Sharma R, Chisti Y, Banerjee UC (2002) Botryococcus braunii: a renewable source of hydrocarbons and other chemicals. Crit Rev Biotechnol 22:245–279
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Casadevall E, Dif D, Largeau C, Gudin C, Chaumont D, Desanti O (1985) Studies on batch and continuous cultures of Botryococcus braunii: hydrocarbon production in relation to physiological state, cell ultrastructure, and phosphate nutrition. Biotechnol Bioeng 27:286–295
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306
Chu WL, Phang SM, Goh SH (1997) Environmental effects on growth and biochemical composition of Nitzschia inconspicua Grunow. J Appl Phycol 8:389–396
Cook NL, Vink R, Donkin JJ, van den Heuvel C (2009) Validation of reference genes for normalization of real-time quantitative RT-PCR data in traumatic brain injury. J Neurosci Res 87:34–41
Dayananda C, Sarada R, Rani MU, Shamala TR, Ravishankar GA (2007) Autotrophic cultivation of Botryococcus braunii for the production of hydrocarbons and exopolysaccharides in various media. Biomass Bioenerg 31:87–93
Dempster TA, Sommerfeld MR (1998) Effects of environmental conditions on growth and lipid accumulation in Nitzschia communis (Bacillariophyceae). J Phycol 34:712–721
Geider RJ, MacIntyre HL, Graziano LM, McKay RML (1998) Responses of the photosynthetic apparatus of Dunaliella tertiolecta (Chlorophyceae) to nitrogen and phosphorus limitation. Eur J Phycol 33:315–332
Hsieh CH, Wu WT (2009) Cultivation of microalgae for oil production with a cultivation strategy of urea limitation. Bioresour Technol 100:3921–3926
Khozin-Goldberg I, Didi-Cohen S, Shayakhmetova I, Cohen Z (2002) Biosynthesis of eicosapentaenoic acid (EPA) in the fresh water eustigmatophyte Monodus subterraneus. J Phycol 38:745–756
Knothe G (2005) Dependence of biodiesel fuel properties on the structure of fatty acid alkyl esters. Fuel Proc Technol 86:1059–1070
Lepage G, Toy CC (1984) Improved recovery of fatty acid through direct transesterification without prior extraction or purification. J Lipid Res 25:1391–1396
Li Y, Horsman M, Wang B, Wu N, Lan CQ (2008a) Effects of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Appl Microbiol Biotechnol 81:629–636
Li Y, Horsman M, Wu N, Lan CQ, Dubois-Calero N (2008b) Biofuels from microalgae. Biotechnol Prog 24:815–820
Lombardi AT, Wangersky PJ (1995) Particulate lipid class composition of three marine phytoplankters Chaetoceros gracilis, Isochrysis galbana (Tahiti) and Dunaliella tertiolecta grown in batch culture. Hydrobiologia 306:1–6
McGinnis KM, Dempster TA, Sommerfeld MR (1997) Characterization of the growth and lipid content of the diatom Chaetoceros muelleri. J Appl Phycol 9:19–24
Metzger P, Largeau C (2005) Botryococcus braunii: a rich source for hydrocarbons and related ether lipids. Appl Microbiol Biotechnol 66:486–496
Miao X, Wu Q (2006) Biodiesel production from heterotrophic microalgal oil. Bioresour Technol 97:841–846
Mustardy L, Los DA, Gombos Z, Murata N (1996) Immunocytochemical localization of acyl-lipid desaturases in cyanobacterial cells: evidence that both thylakoid membranes and cytoplasmic membranes are sites of lipid desaturation. Proc Natl Acad Sci USA 93:10524–10527
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45
Rao AR, Dayananda C, Sarada R, Shamala TR, Ravishankar GA (2007) Effect of salinity on growth of green alga Botryococcus braunii and its constituents. Bioresour Technol 98:560–564
Sevilla E, Martin-Luna B, Vela L, Bes MT, Fillat MF, Peleato ML (2008) Iron availability affects mcyD expression and microcystin-LR synthesis in Microcystis aeruginosa PCC7806. Environ Microbiol 10:2476–2483
Sukenik A, Wahnon R (1991) Biochemical quality of marine unicellular algae with special emphasis on lipid composition. I. Isochrysis galbana. Aquaculture 97:61–72
Takagi M, Karseno, Yoshida T (2006) Effect of salt concentration on intracellular accumulation of lipids and triacylglyceride in marine microalgae Dunaliella cells. J Biosci Bioeng 101:223–226
Thompson GAJ (1996) Lipids and membrane function in green algae. Biochim Biophys Acta 1302:17–45
Tocher DR, Leaver MJ, Hodgson PA (1998) Recent advances in the biochemistry and molecular biology of fatty acyl desaturases. Prog Lipid Res 37:73–117
Wake LV, Hillen LW (1980) Study of a “bloom” of the oil-rich alga Botryococcus braunii in the Darwin River reservoir. Biotechnol Bioeng 22:1637–1656
Xu H, Miao X, Wu Q (2006) High quality biodiesel production from a microalga Chlorella protothecoides by heterotrophic growth in fermenters. J Biotechnol 126:499–507
Yang S, Wang J, Cong W, Cai Z, Ouyang F (2004a) Effect of bisulfite and sulfite on the microalga Botryococcus braunii. Enzyme Microb Technol 35:46–50
Yang S, Wang J, Cong W, Cai Z, Ouyang F (2004b) Utilization of nitrite as a nitrogen source by Botryococcus braunii. Biotechnol Lett 26:239–243
Zhila NO, Kalacheva GS, Volova TG (2005) Influence of nitrogen deficiency on biochemical composition of the green alga Botryococcus. J Appl Phycol 17:309–315
Acknowledgments
This study was supported by grants from the Carbon Dioxide Reduction and Sequestration Research Center, a 21st Century Frontier Program funded by the Korean Ministry of Education, Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Choi, GG., Kim, BH., Ahn, CY. et al. Effect of nitrogen limitation on oleic acid biosynthesis in Botryococcus braunii . J Appl Phycol 23, 1031–1037 (2011). https://doi.org/10.1007/s10811-010-9636-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-010-9636-1