Abstract
The rhodophyte Gracilaria lemaneiformis is an important macroalga of great economic value, and is cultured on the southeastern coast of China. In this study, two G. lemaneiformis strains, 981 and 07–2, were cultured at three temperatures (25, 30 and 35 °C) for 48 h. Analysis of the physiological parameters of the two strains under the heat treatments showed that the specific growth rates (SRGs) and photochemical efficiencies (Fv/Fm) were decreased in both strains with increasing temperatures. The decrease in the Fv/Fm in strain 07–2 was less than that in strain 981. However, the phycobiliprotein (PB) and soluble protein (SP) concentrations in strain 981 were decreased at 35 °C throughout the experiment. The PB and SP concentrations in strain 07–2 exhibited initial decreases and then recovered under the heat treatments (25, 30 and 35 °C). These findings suggest that the heat tolerance of strain 07–2, but not that of strain 981, is probably promoted by heat stress. Furthermore, proteomics analysis of the two strains at 35 °C was conducted using 2-DE. Thirteen proteins were identified and classified into eight categories, including photosynthesis, energy metabolism, protein-folding catalysis, transcription, molecular chaperoning, and unknown function. The western blotting results demonstrated that cytosolic HSP70 expression was increased at 35 °C in strain 07–2 and was stable in strain 981. These results indicate that this cytosolic heat shock protein plays an important role in ameliorating damage caused by heat stress and that strain 07–2 is more heat-resistant than strain 981.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gracilaria lemaneiformis (Bory de Saint-Vincent) Greville, which can produce high-quality agar, is an important cultivar in China (Zhou et al. 2006). It is widely distributed from the eastern to the southern coasts of China, but it only grows well at seawater temperatures between 12 and 21 °C. The cultivar will decompose when the water temperature exceeds 23 °C (Gu et al. 2012). The production of G. lemaneiformis is limited by high temperatures during the summer, especially in the south China coast. Thus, strain 981 was been bred from the wild-type strain, and this strain can tolerate high water temperatures exceeding 25 °C and grow quickly, allowing for an increase in the cultivation scale on the south coast (Yu and Yang 2008). Strain 07–2 was subsequently bred from strain 981, and it can grow faster and produce higher yields than strain 981. It can also tolerate high temperatures (Meng et al. 2009). Researchers have investigated the response of G. lemaneiformis to heat shock for years. A comparison of the antioxidant activities of three G. lemaneiformis strains (strain 07–2, strain 981 and wild-type) has also been performed at three temperatures (24, 28 and 32 °C) (Lu et al. 2010). The physiological response of two G. lemaneiformis species were investigated at 23, 27 and 31 °C (Guo et al. 2011). In recent years, molecular techniques have been used to examine the heat shock mechanism employed by G. lemaneiformis. Quantitative mRNA analyses of cytoplasmic hsp70 and endoplasmic hsp70 of G. lemaneiformis under heat stress at 28 and 32 °C were also conducted (Gu et al. 2012). However, comparative proteomics analysis of G. lemaneiformis in response to high-temperature stress has not yet been performed.
Two-dimensional electrophoresis (2-DE) is a universal tool used to examine critical proteins, including those functioning in metabolic pathways (Zhao et al. 2013). This method is very useful for separating proteins in mixtures, such as those involved in glycolysis, amino acid biosynthesis and stress responses, on the basis of the isoelectric point (pI) in the first dimension and the molecular weight (MW) in the second dimension (Brauner et al. 2014). A distinct advantage of 2-DE technology is that it can be used to detect different forms of proteins that have been translated from mRNA or have undergone proteolytic processing (Henzel et al. 1993). In addition, 2-DE can be used to separate proteins that have undergone post-translational modification. Proteomics allows for the direct observation of changes in G. lemaneiformis protein expression, and is useful for studying physiological responses at the molecular level. Therefore, we employed 2-DE in this study to investigate the proteome of G. lemaneiformis. Analysis of 2-DE spot patterns can be further performed to identify additional potential shock-response proteins.
In this work, cellular physiology was assessed through measurements of algal growth, maximum photochemical efficiency, and the concentrations of phycobiliproteins and soluble proteins. Furthermore, to elucidate the mechanism underlying the heat-stress response in G. lemaneiformis, two G. lemaneiformis strains (981 and 07–2) were investigated, including comparative 2-DE analysis of the two strains under three heat treatments (25, 30 and 35 °C). The findings of this study may be used to facilitate the breeding of new heat-tolerant strains that are able to adapt to higher temperatures, which is especially important considering that both of the current strains are unable to survive in the South China Sea during the summer season.
Materials and methods
The two strains (981 and 07–2) of G. lemaneiformis used in this study were obtained from Nan’ao Island cultivation field (116.5°E, 23.3°N), Shantou, Guangdong, China. We domesticated the two strains for 1 week under a constant temperature in a light incubator under the following conditions: a temperature of 20 ± 1 °C, light intensity of 50 μmol photons m−2s−1, and photoperiod of 12:12 h LD.
Samples (approx. 3 g wet weight) of the two G. lemaneiformis strains were cultured continuously in 1 L filtered natural seawater with a salinity of 30 % and a pH of 8.0. The experiment was conducted at three temperatures: 25, 30, and 35 °C. The heat treatments lasted for 48 h and were performed with three replicates each. The specific growth rates (SGRs) were measured at 0 and 48 h. The maximum photochemical efficiency of PSII, the phycobiliprotein and soluble protein concentrations were determined at 0, 4, 8, 12, 24 and 48 h.
Growth rates and morphological observations
After the G. lemaneiformis samples were treated for 48 h at the three temperatures, changes in seaweed morphology were evaluated. The specific growth rates of the G. lemaneiformis strains were calculated according to the following formula:
where W o is the fresh weight (g) at time 0, W t is the weight at time t, and t is the interval (d) between the two measurements.
Maximum effective quantum yield of photosystem II
All the treatments were followed by measurements of chlorophyll fluorescence using a pulse amplitude-modulated (PAM) fluorometer (Imaging-PAM; Walz, Germany). The youngest growth branches were detached from the same individual and cut into small segments. Then, the maximum effective quantum yield of photosystem II (PSII) was determined as Fv/Fm, where Fv = (Fm-F0), and F0 which was measured after the cultivar after dark adaption for 15 min (Wang et al. 2010). Saturating actinic light pulses were employed to gain maximum fluorescence Fm in the dark-adapted cultivar.
Phycobiliprotein
Approximately 0.5 g of algal material was dried using a vacuum freeze dryer, ground to a powder with liquid nitrogen and extracted at 0–4 °C in 50 mM phosphate buffer at pH 5.5. The homogenates were centrifuged at 2000g for 20 min. The phycobiliprotein levels [phycoerythrin (PE), phycocyanin (PC) and allophycocyanin (APC)] were determined by UV–VIS spectrophotometry using the following equations (Kursar et al. 1983):
Soluble protein
The supernatant from the above phycobiliprotein extraction was used to determine the soluble protein concentration using a Coomassie brilliant blue kit purchased from Jiancheng Biotech (Nanjing, China) according to the manufacturer’s instructions. A 50-μL aliquot of the supernatant was measured by UV–VIS spectrophotometry at 595 nm.
Protein extraction and 2-DE separation
Protein extraction was performed according to an improved protocol (Lee and Lo 2008). Approximately 1 g fresh weight of G. lemaneiformis was ground rapidly in liquid nitrogen. Approximately 3 mL Trizol reagent was added to the algal powder, and the mixture was sonicated (for a total of 5 min, with short pulses of 5 s) on ice. Subsequently, 600 μL chloroform was added to the Trizol lysate before shaking vigorously for 15 s. The mixture was allowed to stand for 5 min at room temperature before being centrifuged at 12,000 × g for 15 min at 4 °C. The top colorless layer was removed. Ethanol (1 mL) was added to resuspend the reddish bottom layer, and the mixture was centrifuged at 2000 × g for 5 min at 4 °C. The supernatant was transferred to a new tube, and 6 mL isopropanol was added. The mixture was allowed to stand for at least 20–40 min at 4 °C for precipitation of proteins. It was then centrifuged at 9000 × g for 20 min at 4 °C. The precipitate was briefly washed three times with 95 % ethanol and was then allowed to air-dry. Finally, it was stored at −80 °C for first-dimension IEF.
The protein precipitate was dissolved in a solubilization buffer containing 7 M urea, 2 M thiourea, 4 % CHAPS, 65 mM DTT and 0.2 % Bio-Lyte. Traces of bromophenol blue were added and mixed vigorously for 5–10 min. Protein quantification was performed using a Coomassie brilliant blue kit. Samples were applied by in-gel rehydration. A total of 200 μL solubilization buffer containing 800 μg protein sample was incubated with dry IPG strips (pH 4–7; 11 cm; Bio-Rad, USA) at 20 °C for 14 h. First-dimension separation was conducted at 20 °C with an isoelectric focusing instrument (Bio-Rad, USA). Voltage was applied according to the following progression: 3 h at 250 V, 4 h at 1000 V, 5 h at 6000 V and 70,000 Vh at 6000 V. The gel strip was then equilibrated with equilibration buffer (50 mM Tris, pH 8.8, 6 M urea, 20 % glycerol, 2 % SDS and 2 % DTT) for 15 min. Next, the gel strip was placed in fresh equilibration buffer containing 2.5 % iodoacetamine (instead of DTT) for another 15 min. The strip was placed on top of a 12 % SDS-PAGE gel and sealed into place with 0.5 % agarose. Electrophoresis was carried out at 80 V for 30 min and then at 120 V until the bromophenol blue dye reached the end of the gel. After electrophoresis, the gel was stained with Coomassie blue (R250). The stained gels were scanned, and the images were analyzed using PDQuest 8.0 (Bio-Rad, USA).
Western blotting
Approximately 300 μg of extracted protein was separated by SDS-PAGE and electrophoretically transferred onto a PVDF membrane using a dual mini-electroblot system (Precision Instruments, India) at 1 mA cm−2, as previously described. The membranes were reacted with a rabbit polyclonal anti-rat Hsp70/ heat shock protein 70 cytoplasmic antibody (Agrisera, Sweden) for 1 h and washed three times in TBST wash buffer. After washing, the membranes were reacted with Goat radish peroxidase-conjugated anti-rabbit IgG (H+L) (Thermo, USA) for 1 h and washed three times in TBST wash buffer. Signals were detected using an enhanced Chemiluminescence Plus Western Blotting Detection system (Beyotime) according to the manufacturer’s instructions. Quantitative densitometric analysis of the immunoblot bands was conducted using Quantity One Software (Bio-Rad).
Data statistics and analysis
Data were analyzed by independent sample t test. Calculation and statistical analyses were performed with SPSS 13.0 for windows. A value of p = 0.05 was considered statistically significant. The relative abundance of each protein spot was estimated by the volume percentage of total spot densities in the gel. The result of each protein spot was analyzed using Student’s t test with p = 0.05 indicating statistical significance. All data were calculated with three biological replicates and reported as the means ± SD.
Results
SGR and maximum photochemical efficiency in Gracilaria under heat stress
Gracilaria lemaneiformis was cultured at 25, 30 or 35 °C for 48 h. The algae grew well at 25 °C (Fig. 1a). At 30 °C, the SGRs of strains 981 (1.92 %) and 07–2 (2.35 %) immediately decreased to 0.45 % (p > 0.05) and 1.36 % (p < 0.05) of the values at 25 °C, respectively. Moreover, these SGRs dropped to −9.59 % (p < 0.01) and −8.55 % (p < 0.01), respectively, of the values at 25 °C at the highest temperature (35 °C). After treatment at 35 °C for 48 h, the frond of strain 981 withered and changed from a yellow to a dark red color, and became fragile (data not shown). These results indicate that high-temperature stress may be detrimental to algal growth.
Physiological indecies of two different Gracilaria lemaneiformis strains, 981 and 07–2, under high temperatures for 48 h: a SGRs of strains 981 and 07–2 at three different temperatures; the lines with solid circles represent strain 981, and those with solid squares indicate strain 07–2; b photochemical efficiency (Fv/Fm) of strain 981 at three different temperatures; the lines with solid circles indicate the 25 °C treatment; those with solid squares depict the 30 °C treatment; and those with solid triangles represent the 35 °C treatment; c photochemical efficiency (Fv/Fm) of strain 07–2 at three different temperatures. Bars mean ± SD
The maximum photochemical efficiency (Fv/Fm values) of strain 981 did not significantly differ from that of strain 07–2 or the control at 0 h (p > 0.05) when the strains were cultured at 25 °C and at 30 °C for 48 h. However, when the temperature was increased to 35 °C, the Fv/Fm values of the two algal strains decreased after 24 h of culture (p < 0.05). The Fv/Fm of strain 981 dropped by 29 % (p < 0.05), and that of strain 07–2 slightly decreased by 1 % (p < 0.05) under the same conditions (Fig. 1b, c). The results demonstrated that the maximum photochemical efficiency of strain 07–2 was less affected by heat stress than that of strain 981.
Phycobiliprotein concentrations
The concentrations of phycobiliproteins, including phycoerythrin (PE), phycocyanin (PC) and allophycocyanin (APC), were analyzed (Fig. 2). The PE concentration in strain 981 showed no significant change during culture for 48 h at two temperatures (25 and 30 °C) (p > 0.05). When the temperature reached 35 °C, its concentration in strain 981 decreased by 64 % compared with that at 0 h (p < 0.01) (Fig. 2a). In contrast, the PE concentrations in strain 07–2 at the three different temperatures decreased by 60.4 % during the first 8 h (p < 0.01) and then increased by 55.2 %, reaching nearly the same level as that at 0 h (Fig. 2b).
The effects of high-temperature stress on the phycobiliprotein concentrations in two different G. lemaneiformis strains, 981 and 07–2; the lines with solid circles indicate the 25 °C treatment; those with solid squares represent the 30 °C treatment; and those with solid triangles depict the 35 °C treatment; PE, PC and APC represent phycoerythrin, phycocyanin and allophycocyanin, respectively; a, b the PE concentrations in strains 981 and 07–2, respectively; c, d the PC concentrations in strains 981 and 07–2, respectively; e, f: the APC concentrations in strains 981 and 07–2, respectively. DW dry weight. Bars mean ± SD
The changes in the PC concentrations in the two strains were similar to the changes in the PE concentrations (Fig. 2c, d). Under high-temperature stress at 35 °C, the PC concentration in strain 981 decreased by 56.9 % over 48 h (p < 0.01), but that in strain 981 was stable for 48 h under heat stress at two temperatures (25 °C and 30 °C) (p > 0.05). However, the PC concentrations in strain 07–2 at the three temperatures decreased by 70.5 % during the first 8 h (p < 0.01) and then increased to a similar level as that at 0 h (Fig. 2d).
During the first 8 h at 25 °C, the APC concentration in strain 981 increased (p < 0.01) (Fig. 2e). After that, it decreased at the three temperatures to 0.45 mg g−1 at 12 h compared with 0.64 mg g−1 at 0 h (p < 0.01). Then, it increased to 0.95 mg g−1 at 48 h at two temperatures (25 and 30 °C). Nevertheless, it remained at 0.45 mg g−1 from 12 to 48 h at 35 °C. The APC concentration in strain 07–2 decreased by 90 % during the first 8 h (p < 0 .01) and then quickly increased by 91 %, rising above the level observed at 0 h (Fig. 2f).
The phycobiliprotein concentrations in strain 07–2 initially decreased under heat stress but then recovered to their original levels. However, the phycobiliprotein concentrations in strain 981 decreased and did not recover. These results indicates that the recovery of phycobiliproteins in strain 07–2 (but not in strain 981) could have allowed for maintenance of the light-harvesting capability of strain 07–2 under these conditions.
Total soluble protein concentration
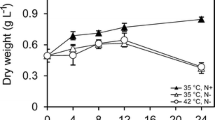
The soluble protein (SP) concentrations in strains 981 and 07–2 were investigated (Fig. 3). The phycobiliproteins contents took 15.1 and 21.3 % of soluble proteins, in strains 981 and 07–2, respectively. During the heat stress experiment, the SP concentrations in strain 981 at two temperatures (25 and 30 °C) did not significantly differ from those in the control at 0 h (p > 0.05). However, at 35 °C, the SP concentration in strain 981 decreased by 76.4 % compared with that in the control (p < 0.01). Under heat stress at the three different temperatures, the SP concentration in strain 07–2 exhibited an initial decrease (44.6 %) during the first 8 h at 30 °C and then increased to a maximum value of 68.69 mg g−1 at 24 h, which was higher than the 45.5 mg g−1 observed at 0 h (p < 0.01) (Fig. 3b). At 35 °C, the SP concentration in strain 07–2 decreased by 46.8 % compared with that in the control (Fig. 3b). These results demonstrated that the concentrations of SPs in both strains were influenced by heat stress but that the SP concentration in strain 07–2 finally decreased less than that in strain 981.
The effects of high-temperature stress on the SP concentrations in two different G. lemaneiformis strains, 981 and 07–2; the lines with solid circles represent the 25 °C treatment; those with solid squares depict the 30 °C treatment; and those with solid triangles indicate the 35 °C treatment. SP indicates soluble protein; a the SP concentration in strain 981; b the SP concentration in strain 07–2. DW dry weight. Bars mean ± SD
Proteomics analysis of the two strains under heat stress
Protein expression in strains 981 and 07–2 under 35 °C heat stress for 8 and 24 h was compared with that in the control at 0 h (Fig. 4). We found that the distribution of the protein spots in the 2-DE gels for strain 07–2 was very similar to the protein spot distribution for strain 981 (Fig. 4). Twenty-five distinct spots were detected by PDQuest using Student’s t test (p < 0.05). However, a big challenge is that there is little information in databases on proteins and genes for proteomics research in Gracilaria as a non-model organism. In this study, over a half of the detected spots can be matched to peptides or genes sequences from homologous species. Therefore, a total of 14 spots were identified by MALDI-TOF-TOF MS and by searching the NCBI database (Table 1), which were marked on six 2-DE gel images (Fig. 4) and classified into five categories according to the GO database.
Time-course results of 2-DE gels for two G. lemaneiformis strains, 981 and 07–2, exposed to 35 °C heat stress; a strain 981 at 0 h; b strain 981 at 8 h; c strain 981 at 24 h; d strain 07–2 at 0 h; e strain 07–2 at 8 h; and f strain 07–2 at 24 h. All the gels were run in duplicate. Protein extracts (800 μg) were applied to linear pH 4–7 IPG strips, with 12 % linear vertical SDS-PAGE as the second dimension. The arrows with numbers on the 2-DE gel images indicate the proteins identified by MALDI-TOF and LC-MS. The molecular mass marker (M) and pI are displayed on the left hand sides and above the gels, respectively
Proteins identified that are involved in carbon metabolism included alpha-1,4-glucan, lyase isozyme 1 (spot 1), phosphoglycerate kinase (spot 14) and triosephosphate isomerase (spot 16). Proteins with involvement in energy metabolism included the ATP synthase CF1 beta and alpha subunits (spots 9, 10 and 11). One protein involved in photosynthesis was identified: oxygen evolving enhancer 1 precursor in photosynthetic system II (spot 15). Proteins related to heat stress resistance included HSP82 (spot 2) and HSP70 and cytosolic HSP70 (spots 3 and 7). Additional proteins identified included disulfide isomerase 1, involved in protein folding (spot 5), elongation factor Tu, involved in transcription (spot 13), and cyclophilin-like protein, classified as a molecular function protein (spot 25). An unknown protein was also detected (spot 26).
Generally, heat shock and cyclophilin-like proteins were identified by 2-DE as the heat-responsive proteins in the two strains under 35 °C heat stress (Table 1). Our results showed that this protein was up-regulated by 1.7-fold at 8 h (p < 0.05) and was up-regulated by 2.3-fold at 24 h (p < 0.05) in strain 981 compared with the control strain 981 at 0 h (Fig. 5a). Cyclophilin-like protein is a type of stress-responsive protein. Cyclophilin-like protein was up-regulated by 2.9-fold in strain 07–2 at 24 h (p < 0.05) compared with the control at 0 h (Fig. 5b), but there was no significant difference in its expression at 8 h (p > 0.05). Under 35 °C heat stress, cyclophilin expression in strain 981 increased at both 8 and 24 h, but it was only up-regulated at 24 h in strain 07–2. These results indicated that the cyclophilin protein was expressed in response to heat stress in strains 981 and 07–2.
The differential expression (fold change >1.5 or <−1.5) of heat-responsive proteins (HRPs) in two strains compared with the respective control at 0 h under 35 °C heat stress, as determined by 2-DE. Asterisk indicates statistical significance; a the differential expression in terms of relative abundance of two heat shock proteins and cyclophilin-like protein in strain 981; hite bars indicate HSP82; lack bars represent cytosolic HSP70; and grey bars depict cyclophilin-like protein (± SD); b the differential expression of two heat shock proteins and cyclophilin-like protein in strain 07–2
Heat shock proteins (HSP82, HSP70 and cytosolic HSP70) show different responses to heat stress and are classified into two families: HSP90 and HSP70. HSP70 was evaluated by MALDI-TOF-TOF MS and a BLAST search to identify homologous sequences in the NCBI database. Part of the HSP70 protein sequence was found to have homology to chloroplast HSP70, but it lacked a chloroplast-targeting signal peptide sequence; therefore, its homology should be further investigated. The differences in the relative abundances of two heat shock proteins (HSP82 and cytosolic HSP70) in response to heat stress were also assessed in the two algal strains (Fig. 5a, b). The relative abundance of HSP82 in strain 981 increased by 2.9-fold at 24 h (p = 0.05), but it did not increase in abundance in strain 07–2 (Fig. 5a). The relative abundance of cytosolic HSP70 did not significantly change in strain 981 (p > 0.05), but it was up-regulated by 1.6-fold at 8 h in strain 07–2 (p = 0.01) and even increased by 2.5-fold at 24 h (p = 0.05) compared with the control at 0 h (Fig. 5b). Western blotting (WB) verification of the relative abundances of cytosolic Hsp70 in the two strains under high-temperature stress indicated that this protein was induced by heat stress (Fig. 6a–c). At 8, 12 and 24 h, the relative abundance of cytosolic HSP70 in strain 981 increased by 1.70-fold, 1.86-fold and 1.76-fold, respectively, compared with the control at 0 h (p <0.01). The relative abundance of this protein in strain 07–2 at 8, 12 and 24 h changed by 1.66-fold, 1.69-fold and 2.34-fold, respectively, compared with the control at 0 h (p < 0.01), in accordance with the expression pattern observed on 2-DE. These results showed that the expression of this protein in strain 07–2 was higher than that in strain 981, indicating that it played an important role in resistance to heat stress in strain 07–2.
Time-course results of western blotting of HSP70 in the two strains exposed to 35 °C. Asterisk indicates statistical significance. All gels were run in triplicate. The molecular mass marker (M) and pI are displayed on the lefthand sides and above the gels, respectively; a expression of cytosolic HSP70 protein in strain 981 at 0, 8, 12 and 24 h; b expression of cytosolic HSP70 protein in strain 07–2 at 0, 8, 12 and 24 h; c the differential expression in terms of relative abundance (fold change >1.5 or <−1.5, p > 0.05) of cytosolic HSP70 proteins of the two strains (981 and 07–2) were assessed at 8, 12 and 24 h under 35 °C heat stress, compared with the cytosolic HSP70 proteins of strains 981 and 07–2 at 0 h, respectively. White bars represent cytosolic HSP70 protein in strain 981, and black bars depict cytosolic HSP70 in strain 07–2 (± SD)
Discussion
Physiological and biochemical responses of G. lemaneiformis to heat stress
In this study, two G. lemaneiformis strains were exposed to high temperatures (25, 30 and 35 °C). Severe heat stress can lead to destruction of the algal frond and even algal death, and we found that the two G. lemaneiformis strains exhibited different responses to heat stress. These differences were detected through a survey of physiological photosynthesis in the cultivars. The Fv/Fm in macro-algae is influenced by environmental conditions (Enríquez and Borowitzka 2010). Activation of electron transfer depends on light harvesting by phycobiliprotein complexes, then the light energy conversion into chemical energy for carbon fixation later. Therefore, Fv/Fm reflects the electron transfer efficiency of the reaction center of PSII. Wang et al. (2010) and Xie et al. (2010) demonstrated that the young and new branches had the lowest Fv/Fm due to having the lowest levels of photosynthetic activity. Therefore, the electron transfer system of G. lemaneiformis is important for the survival of this alga at high temperatures. Zhang et al. (2012) have demonstrated that the Fv/Fm of the red macroalga Pyropis (Porphyra) yezoensis is significantly decreased under high-temperature stress. In our study, the temperature was increased up to the higher limit (35 °C), which results in a decrease in Fv/Fm in strain 981. However, the Fv/Fm of strain 07–2 decreased much more slowly than that of strain 981 under 35 °C heat stress, indicating that the light harvesting and electron transfer processes of the reaction center in PSII in strain 07–2 were more heat-resistant than in strain 981.
The light energy was first transferred in the photosystem II by PE to PC, then to APC, and finally to chlorophyll a. In this research, the phycobiliprotein concentrations in strain 07–2 decreased earlier and increased later at three high temperatures, indicating that the light-harvesting complexes had an early depression and the electron transfers from PE to APC were reversibly depressed under heat stress. Previous studies have shown that electron transfer from PE to PC is inhibited by heat stress in Grateloupia turuturu exposed to a temperature of 40 °C for 12 h, because the light-harvesting complexes can be inactivated by heat shock (Havaux 1993; Srivastava et al. 1997; Wu et al. 2002). However, Haque has claimed that the activities of the light-harvesting proteins in PSII and oxygen-evolving complex reversibly decrease during the early stage of heat treatment (Haque et al. 2014). At the transcriptional level, the expression of the PE gene was susceptible to change under heat stress (Ding et al. 2015). At the translational level, the degradation of linker protein of the phycobilisome disturbed the stability of PE in Pyropia haitanensis, whereas the upregulation of the PC and APC subunits were likely compensatory for the loss of light harvest and electron transfer (Xu et al. 2014). In our study, at three high temperatures, the phycobiliprotein concentrations in strain 981 essentially declined in at least the first 8 h, indicating that the light-harvesting complexes in strain 981 (which was insensitive to heat stress) probably adapted early to heat stress when compared to the strain 07–2. In 2013, a research study reported that the chlorophyll a content showed instability at different temperatures (20 and 30 °C), although the PE became low when compared to 10 °C (Ye et al. 2013). The Fv/Fm of the 07–2 strain remained stable under heat stress, when the phycobilisomes declined in this case, indicating that the capability of energy transfer in strain 07–2 was maintained under heat stress.
Contrary to the sharp decrease of phycobiliproteins in strain 981 at 35 °C after 24 h of heat stress, the reversible increase of phycobiliproteins in strain 07–2 after the decline suggested the recovery of phycobiliproteins in strain 07–2, which maintained the activities of proteins in photosynthesis. However, the heat stress caused a decrease in photosynthetic activity in strain 981 without recovery. Heat stress does not directly cause severe damage to PSII, but it can inhibit its repair and cause damage to the photosynthetic reaction center, due to the generation of reactive oxygen species (ROS) (Yordanov et al. 2000; Allakhverdiev et al. 2008). ROS can lead to the autocatalytic peroxidation of membrane lipids. Lu et al. (2013) demonstrated that the activities of enzymes in the antioxidant process, such as glutathione peroxidase, glutathione reductase and superoxide dismutase, increased in accordance with the changes of transcripts in thermotolerant strains of G. lemaneiformis exposed to high temperatures in strain 981 and not in the wild strain. In our study, the transfer of electrons from the recovered phycobiliproteins to the photosynthetic reaction center in strain 07–2 can be a stressful burden to the decreasing photochemical efficiency, suggesting that the reaction center strongly suffers from damage by the production of ROS at the later phase of heat stress. However, photosynthesis of strain 981 suffered from severe inactivation at 35 °C. Fu et al. (2014) claimed that the increased SOD activity was correlated with increments of tolerance to heat shock and detected that SOD activity increased in strain 981 in the short term. Nevertheless, Lu et al. (2010) showed that strain 07–2 had a higher total antioxidant activity level than strain 981 at 32 °C. Therefore, although we did not measure the activities of antioxidants, the dismissal of the over-oxidative damage strain 07–2 was supposed to be intensively provoked by the disorganization of PSII, probably when the phycobiliproteins concentrations decreased. However, enzymes in the antioxidant process in both strains 981 and 07–2 response to heat stress have rarely been reported and require further investigation.
Under heat stress at 35 °C, the soluble protein concentrations in the two strains decreased from 24 to 48 h, suggesting that this high temperature likely inhibited protein synthesis, and may have even led to protein degradation over time. In 2010, Gonzalez and Barrett (2010) reported that severe heat stress causes an increase in cell membrane penetrability in algae and the leakage of cytoplasm, resulting in long-term damage to the cell structure. However, the increase in the SP concentration observed in strain 07–2 at 24 h in our study indicates that this strain probably responds to high temperatures over the short-term by producing soluble proteins. Exposure of the alga to short-term heat stress has been previously shown to lead to the increased production of soluble proteins, resulting in increases in infiltration and the number of functional proteins, thereby maintaining and stimulating the necessary level of proteins, including phycobiliproteins (Xiao and Xiu 1997). In our study, the fluctuation of the SP concentration in strain 07–2 indicated that this strain was more sensitive to heat stress than strain 981 during the early stage of heat treatment. However, finally, the SP in strain 07–2 remained at a higher concentration than did strain 981, indicating that the adaptive response of soluble protein in strain 07–2 to high-temperature stress helped it to survive over the long term. Generally, the photosynthesis of strain 07–2 showed greater capability to heat stress and helped the alga adapt to high temperatures, so these findings provide important information for the culture of strain 07–2 in the South China Sea.
Stress-response proteins
Many proteomic studies showed that important stress-related proteins could be detected by 2-DE, especially the inducible heat shock proteins under heat stress (Lee et al. 2007). In a study of the proteomic profile analysis of Pyropia haitanensis under heat stress, proteins were classified into a series of functional categories, including photosynthesis, energy metabolism, redox homeostasis and translation or transcription regulation, and small HSPs with low molecular weight responded to high temperatures (Xu et al. 2014). In our study, several enzymes were identified from the 2-DE spots that were involved in metabolic pathways that function in response to heat stress in G. lemaneiformis, including enzymes with roles in carbon metabolism, energy metabolism, photosynthesis and heat resistance. We found 4 types of molecular chaperone proteins, including cyclophilin-like protein, HSP82, HSP70 and cytosolic HSP70 by 2-DE. Cyclophilin-like protein is widely distributed in plant cell nuclei, cytoplasm, chloroplasts and other organelles (Romano et al. 2004), but it has rarely been reported in algae. It belongs to the cyclophilin family and has peptidyl-prolyl cis-trans isomerase enzymatic activity. In addition, Thao demonstrated that the expression of the cyclophilin protein increased in celery in response to salt and heat stress (Thao et al. 2007). The increase of cyclophilin-like protein in both strains 981 and 07–2 under heat stress suggests that it may promote protein folding and metabolic regulation in G. lemaneiformis.
Heat-responsive proteins are universal in the proteomes of prokaryotic and eukaryotic organisms (Gupta et al. 2010). Here, we focused on the role of heat shock protein in the heat stress response in both strains. Molecular chaperones participate in protein folding and in the prevention of protein denaturation (Tripp et al. 2009). In G. lemaneiformis, HSP82 was up-regulated by 2.9-fold at 35 °C in strain 981, indicating that this protein was induced by heat stress. Breusegem et al. (1994) demonstrated that HSP82 mRNA was the most highly expressed within 120 min after rice was transitioned to 42 °C . However, Lindquist and Craig (1988) claimed that overproduction of the HSP82 protein did not protect cells from being killed by heat, even though cells cannot survive at high temperatures with this protein . In our study, HSP82 expression in strain 981 was significantly increased under the high-temperature stress until 48 h, but it became fragile following exposure to 35 °C for 48 h, as demonstrated by the marked decreases in all of the physiological indexes examined after 24 h. These findings suggest that HSP82 probably plays a supporting role in protection of G. lemaneiformis under heat stress.
The change in HSP82 expression is in great contrast with that of the heat-inducible protein HSP70. Gu et al. (2012) have compared the transcription levels of cytosolic HSP70 at 32 and 28 °C and have shown that its expression increases earlier and to a greater extent at the higher temperature. In this study, cytosolic HSP70 expression was up-regulated by 1.6-fold at 8 h and by 2.5-fold at 24 h in strain 07–2 compared with that in the control at 0 h, consistent with the western blotting results. Further, Cytosolic HSP70 expression was consistently upregulated in strain 981 at the translational level when compared with that in strain 07–2 over time. This result suggested that cytosolic HSP70 in strain 981 aided in the resistance of G. lemaneiformis to high temperatures at the early phase of heat shock, according to the sharp decline of the physiological indices of strain 981 after 24 h, but not in its survival under severe heat stress. Fu found that the expression of the hsp70 gene in strain 981 reached more than a 1.6-fold change than the control within 4 h under heat treatment (Fu et al. 2014). These findings suggested that HSP70 in strain 981 probably responded to heat stress in several hours in the early phase of heat stress when compared to strain 07–2. At the transcriptional level, five genes of hsp70, which were identified from the red macroalgae Pyropia haitanensis, significantly increased after 6 or 12 h under 29 °C, including two cytoplasmic hsp70s and three different hsp70s in other cellular organelles (Ji et al. 2015). However, the cytosolic HSP70 protein level was higher in strain 07–2 than in strain 981 throughout our experiment, but the members in the HSP70 family should be investigated in different cellular organelles under heat shock. Additionally, in a study of comparative calmodulin genes in strains 981 and 07–2 under heat shock, the results showed that the camodulin-1 gene in strain 981 played a key role in signal transduction, but did not show any advantages for the expression of camodulin-1 gene in strain 07–2 under 32 °C heat shock (Liu et al. 2015). It is important to note that there should be an efficient signal-transduction pathway in stain 07–2, which is likely to conduct the adaptive response to heat stress in thermorelated gene expressions. These findings implied that high temperatures strongly induced the translation of cytosolic HSP70 in strain 07–2 of G. lemaneiformis; therefore, this strain was more resistant to heat than strain 981. In our study, cytosolic HSP70 was found to play a key role as a molecular chaperone in protein folding and to aid in ameliorating the damage caused by heat stress, thereby retaining cell homeostasis.
Two-DE protocol for G. lemaneiformis proteomics research
Three main factors influence the results of 2-DE gels in proteomics research of macroalgae under stress, including the quality of the proteins extracted from samples, the pH range of the immobilized pH gradient (IPG) strip used in first-dimension isoelectric focusing electrophoresis and the separating gel concentration used in second-dimension electrophoresis (Nagai et al. 2008). In this study, the quality of proteins extracted from samples and the loading quality in isoelectric focusing are critical to 2-DE. It was difficult to extract total proteins from the G. lemaneiformis because it contained a low level of proteins, although it was rich in agar and polyphenol contaminants. Therefore, we used of Trizol reagent for isolating algal proteins. A pH of 4–7 and an 11-cm-long strip were employed to separate proteins according to their individual isoelectric points (pIs) and a 12 % gel concentration was used to separate the proteins of G. lemaneiformis according to their molecular masses. In this study, the stained gels with Coomassie blue (R250) were probably employed to detect proteins, whereas the higher sensitivity method of silver staining should be used to further detect proteins. However, an effective protocol for preparing and loading protein samples was established for high-quality 2-DE gel analysis of the G. lemaneiformis proteome. Distinct protein spots were detected in a single 2-DE gel. This study provides an applicable 2-DE/MS workflow for proteomic analysis of G. lemaneiformis.
References
Allakhverdiev S, Kreslavski V, Klimov V, Los D, Carpentier R, Mohanty P (2008) Heat stress: an overview of molecular responses in photosynthesis. Photosynth Res 98:541–550
Brauner JM, Groemer TW, Stroebel A, Grosse HS, Oberstein T, Wiltfang J, Kornhuber J, Maler JM (2014) Spot quantification in two dimensional gel electrophoresis image analysis: comparison of different approaches and presentation of a novel compound fitting algorithm. BMC Bioinformatics 15:2405–2412
Breusegem FV, Dekeyser R, Garcia AB, Claes B, Gielen J, Van Montagu M, Caplan AB (1994) Heat-inducible rice hsp82 and hsp70 are not always co-regulated. Planta 193:57–66
Ding Y, Sun H, Zhang R, Yang Q, Liu Y, Zang X, Zhang X (2015) Selection of reference gene from Gracilaria lemaneiformis under temperature stress. J Appl Phycol 27:1365–1372
Enríquez S, Borowitzka MA (2010) The use of the fluorescence signal in studies of seagrasses and macroalgae. In: Suggett DJ, Prásil O, Borowitzka MA (eds) Chlorophyll a fluorescence in aquatic sciences: methods and applications. Springer, Dordrecht, pp 187–208
Fu F, Sui ZH, Zhou W, Wang JG, Chang LP, Ci SF (2014) UV-irradiation mutation of tetraspores of Gracilariopsis lemaneiformis and screening of thermotolerant strains. J Appl Phycol 26:647–656
Gonzalez ME, Barrett DM (2010) Thermal, high pressure, and electric field processing effects on plant cell membrane integrity and relevance to fruit and vegetable quality. J Food Sci 75:R121–R130
Gu Y, Zhang X, Lu N, Zang X, Zhang X, Li G (2012) Cloning and transcription analysis of hsp70-1 and hsp70-2 of Gracilaria lemaneiformis under heat shock. Aquaculture 358:284–291
Guo C, Chen WZ, Cao HB, Wu WT, Jin YL (2011) The comparison of physiological response of different Gracilaria lemaneiformis species to heat stress. J South China Fish Sci 3:14–19 (Chinese edition)
Gupta SC, Sharma A, Mishra M, Mishra RK, Chowdhuri DK (2010) Heat shock proteins in toxicology: how close and how far? Life Sci 86:377–384
Haque MS, Kjaer KH, Rosenqvist E, Sharma DK, Ottosen C-O (2014) Heat stress and recovery of photosystem II efficiency in wheat (Triticum aestivum) cultivars acclimated to different growth temperatures. Environ Exp Bot 99:1–8
Havaux M (1993) Characterization of thermal damage to the photosynthetic electron transport system in potato leaves. Plant Sci 94:19–33
Henzel WJ, Billeci TM, Stults JT, Wong SC, Grimley C, Watanabe C (1993) Identifying proteins from two-dimensional gels by molecular mass searching of peptide fragments in protein sequence databases. Proc Natl Acad Sci U S A 90:5011–5015
Ji D, Li B, Xu Y, Chen C, Xie C (2015) Cloning and quantitative analysis of five heat shock protein 70 genes from Pyropia haitanensis. J Appl Phycol 27:499–509
Kursar TA, van der Meer J, Alberte RS (1983) Light-harvesting system of the red alga Gracilaria tikvahiae I. Biochemical analyses of pigment mutations. Plant Physiol 73:353–360
Lee FWF, Lo SCL (2008) The use of Trizol reagent (phenol/guanidine isothiocyanate) for producing high quality two-dimensional gel electrophoretograms (2-DE) of dinoflagellates. J Microbiol Methods 73:26–32
Lee DG, Ahsan N, Lee SH, Kang KY, Bahk JD, Lee IJ, Lee BH (2007) A proteomic approach in analyzing heat‐responsive proteins in rice leaves. Proteomics 7:3369–3383
Lindquist S, Craig E (1988) The heat-shock proteins. Annu Rev Genet 22:631–677
Liu Y, Zhang X, Sun H, Yang Q, Zang X, Zhang X, Tan Y (2015) Cloning and transcription analysis of six members of the calmodulin family in Gracilaria lemaneiformis under heat shock. J Appl Phycol. doi:10.1007/s10811-015-0575-8:1-9
Lu N, Zang X, Zhang X, Chen W, Zhang X, Gu Y, Li Q, Zhang L (2010) Comparison of antioxidant activities of different strains of Gracilaria lemaneiformis under high-temperature stress. J Wuhan Univ (Nat Sci Edn) 5:016
Lu N, Ding Y, Zang XN, Zhang XC, Chen H, Mu XS (2013) Molecular cloning and expression analysis of glutathione peroxidase and glutathione reductase from Gracilaria lemaneiformis under heat stress. J Appl Phycol 25:1925–1931
Meng L, Xu D, Chen WZ, Zhang XC (2009) Selection and characterization of a new strain of Gracilaria lemaneiformis. Period Ocean Univ China 39:94–98
Nagai K, Yotsukura N, Ikegami H, Kimura H, Morimoto K (2008) Protein extraction for 2‐DE from the lamina of Ecklonia kurome (Laminariales): recalcitrant tissue containing high levels of viscous polysaccharides. Electrophoresis 29:672–681
Romano PG, Horton P, Gray JE (2004) The Arabidopsis cyclophilin gene family. Plant Physiol 134:1268–1282
Srivastava A, Guissé B, Greppin H, Strasser RJ (1997) Regulation of antenna structure and electron transport in Photosystem II of Pisum sativum under elevated temperature probed by the fast polyphasic chlorophyll a fluorescence transient: OKJIP. Biochim Biophys Acta Bioenerg 1320:95–106
Thao NP, Chen L, Nakashima A, Hara SJ, Umemura K, Takahashi A, Shirasu K, Kawasaki T, Shimamoto K (2007) RAR1 and HSP90 form a complex with Rac/Rop GTPase and function in innate-immune responses in rice. Plant Cell Online 19:4035–4045
Tripp J, Mishra SK, Scharf KD (2009) Functional dissection of the cytosolic chaperone network in tomato mesophyll protoplasts. Plant Cell Environ 32:123–133
Wang Z, Wang G, Niu J, Wang W, Peng G (2010) Optimization of conditions for tetraspore release and assessment of photosynthetic activities for different generation branches of Gracilaria lemaneiformis Bory. Chin J Oceanol Limnol 28:738–748
Wu BG, Han ZG, Zang RB (2002) Effects of heat stress in marine red and green algae by chlorophyll fluorescence method. J Jinan Univ (Nat Sci) 23:108–112 (Chinese edition with English abstract)
Xiao RT, Xiu GF (1997) Relationship between light, temperature and growth, development of conchocelis of Porphyra haitanensis. Oceanol Limnol Sin 28:475–482
Xie X, Wang G, Pan G, Gao S, Xu P, Zhu J (2010) Variations in morphology and PSII photosynthetic capabilities during the early development of tetraspores of Gracilaria vermiculophylla (Ohmi) Papenfuss (Gracilariales, Rhodophyta). BMC Dev Biol 10(1):43
Xu Y, Chen C, Ji D, Hang N, Xie C (2014) Proteomic profile analysis of Pyropia haitanensis in response to high-temperature stress. J Appl Phycol 26:607–618
Ye CP, Zhang MC, Zhao JG, Yang YF, Zuo Y (2013) Photosynthetic response of the macroalga, Gracilaria lemaneiformis (Rhodophyta), to various N and P levels at different temperatures. Int Rev Hydrobiol 98:245–252
Yordanov I, Velikova V, Tsonev T (2000) Plant responses to drought, acclimation and stress tolerance. Photosynthetica 38:171–186
Yu J, Yang YF (2008) Physiological and biochemical response of seaweed Gracilaria lemaneiformis to concentration changes of N and P. J Exp Mar Biol Ecol 367:142–148
Zhang T, Shen Z, Xu P, Zhu J, Lu Q, Shen Y, Wang Y, Yao C, Li J, Wu Y, Jiang H (2012) Analysis of photosynthetic pigments and chlorophyll fluorescence characteristics of different strains of Porphyra yezoensis. J Appl Phycol 24:881–886
Zhao YP, Zhou W, Zhao PP, Cai JL, Zhu DL, Pan GH, Wang GC (2013) A protein extraction method suitable for two-dimensional electrophoresis analysis of Porphyra yezoensi. Mar Sci 37:19–24 (in Chinese with English Abstract)
Zhou Y, Yang H, Hu H, Liu Y, Mao Y, Zhou H, Xu X, Zhang F (2006) Bioremediation potential of the macroalga Gracilaria lemaneiformis (Rhodophyta) integrated into fed fish culture in coastal waters of north China. Aquaculture 252:264–276
Acknowledgments
This research was supported by the Research Funds for the National High Technology Research and Development Program of China (Grant No. 2012AA10A411), Guangdong Province Comprehensive Strategic Cooperation Project of the Chinese Academy of Science (Grant No. 2011A090100040) and the National Natural Science Foundation of China (Grant No. 31000189).
Author information
Authors and Affiliations
Corresponding author
Additional information
Yang Wang is the first author of this work.
Rights and permissions
About this article
Cite this article
Wang, Y., Feng, Y., Wang, H. et al. Physiological and proteomic analyses of two Gracilaria lemaneiformis strains in response to high-temperature stress. J Appl Phycol 28, 1847–1858 (2016). https://doi.org/10.1007/s10811-015-0723-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-015-0723-1