Abstract
The availability of clean water is insufficient to meet our needs because of both the rapid population growth and the advancement of technology. Heavy metals introduced into the water as a result of various activities cause major problems and create an unfavorable scenario in terms of sustainability. In this study, a specially designed electrodialysis cell was used to remove chromium (VI) and nickel (II) ions from effluents. The compartments were divided by Ionac MC 3470 cation exchange and Ionac MA 3475 anion exchange membranes. The cathode and anode were made of carbon fiber and stainless steel, respectively. The effects of voltage, initial pH, time, Na2SO4 concentration, feed flow rate, and metal ion concentration on metal removal efficiency, energy consumption, current efficiency, current density, and flux were investigated. The optimum values for 97.9 ± 1% removal of 50 mg/L Cr (VI) ions in 90 min are voltage 25 V, pH = 3, Na2SO4 addition 0.1 g and feed flow rate 40.3 mL/min, as observed. At the end of this period, the concentration was calculated as 1.05 mg/L, the energy consumption was 38.57 ± 0.01 Wh/L, the current efficiency was 28.56 ± 1.5%, and the flux was calculated as 10.87 × 10−5 ± 0.15 mol/m2s. Optimal values were observed as 20 V, pH = 3, Na2SO4 addition of 0.1 g, and feed flow rate (Qf) = 40.3 mL/min for 92.3 ± 1% removal of 50 mg/L Ni(II) ions in 90 min. The concentration of nickel ions at the end of this period was 3.85 mg/L, the energy consumption was 32.14 ± 0.01 Wh/L, the current efficiency was 95.11 ± 1.5%, and the flux was calculated to be 37.71 × 10−5 ± 0.15 mol/m2s. Effective removal of Cr (VI) and Ni (II) ions from dilute wastewater can be achieved using a cost-effective ED cell in electrodialysis, with reasonable energy consumption and high current efficiency under optimal process conditions.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Water plays an important role in all aspects of our lives, particularly in domestic, drinking water, and industrial activities. The United Nations has designated March 22 as World Water Day in order to raise awareness about the importance of water. Water scarcity not only affects developing or underdeveloped countries, but it also causes diseases. Although existing water resources are insufficient, they cannot meet the demand for water [1]. The waters are in grave danger due to the release of a wide range of pollutants into the environment, including heavy metals, pesticides, and dyes. These pollutants, which are spread through industrial or domestic waste, contaminate water, poison living things, and harm the ecosystem [2].
Metals with a specific gravity of 4–5 or more are classified as heavy metals. Mercury, zinc, chromium, nickel, copper, iron, lead, tin, gold, tungsten, and platinum are some of them [3]. Heavy metals are emitted to the environment by natural means, such as volcanic eruptions, or as a result of human-induced activities, especially metal plating and mining [4, 5]. As a result of interaction with heavy metals, problems such as cancer, stomach disorders, skin disorders, and visual impairment not only occur, but also lead to further death. Heavy metal pollution causes serious damage to the entire ecosystem [6]. Nickel, one of the heavy metals, is a metallic element found in nature. Due to their chemical and physical properties, their compounds are widely used in industry. It is used in zinc castings, silver refineries, and batteries, and is also a versatile finishing process with a wide range of applications such as nickel electroplating, engineering, and decorative [7]. On the other hand, nickel is a carcinogenic metal and also causes pulmonary and reproductive toxicity with prolonged and repeated exposure [8, 9]. Chromium, another heavy metal, is among the indispensable metals due to its use in the production of stainless steel, and therefore it has widespread use. It is also used in applications such as chromium pigment synthesis, paint, leather tanning, electroplating, petroleum product synthesis, textiles, alloying, and ceramics [10]. Therefore, it is found in the relevant factory wastewater, and since Cr (VI) is included in the group of carcinogenic chemicals, it should definitely be removed [11, 12]. Chromium in wastewater is very dangerous from an environmental point of view. Cr (VI) accumulation in living tissues can also cause serious problems in the liver and kidneys [10]. It can cause skin irritation, eye diseases, and respiratory problems in humans. Because wastewater is a major source of this pollutant, WHO has limited maximum allowable levels of chromium (VI) and nickel (II) at 0.05 mg/L and 2 mg/L, respectively [13]. As a result, reducing high metal concentrations in wastewater to allowable limits before releasing it into the environment is highly desirable.
There are various methods such as ion exchange, adsorption, chemical precipitation and electrocoagulation to eliminate or reduce heavy metals in effluents. However, these processes also have their own shortcomings. For example, a secondary contamination, resin encrustation, may occur in the ion exchange process. Adsorption is a widely used technique for the removal of heavy metals from wastewater due to its simplicity and affordability [14]. However, this method has several disadvantages that should be considered. One of the main drawbacks of adsorption is the frequent need for adsorbent material regeneration or replacement, which can be both costly and time-consuming. Moreover, adsorbents that are not properly regenerated are transformed into secondary pollutants. In the chemical precipitation process, the desired metal may be precipitated at a lower level than it should be due to the precipitation of other metals [15]. Electrocoagulation, one of the most widely used electrochemical techniques for metal removal from wastewater, is a process in which coagulants are produced in situ by the dissolution of Fe or Al anodes. These coagulants adsorb the pollutants and the resulting sludge either settles to the bottom or is carried to the surface by hydrogen gas released at the cathode. The main disadvantage of this process, which requires simple equipment, is the formation of metal-containing sludge. Electrodialysis (ED) is a membrane separation process that uses electrical potential difference as the driving force and operates according to the principles of dialysis and electrolysis [16]. A simple ED cell consists of anion and cation exchange membranes arranged between an anode and a cathode. When a solution containing dissolved ions is pumped into the cell and a direct current is applied between the two electrodes, selective transport of ions across the membranes takes place. The electric field applied across the membranes drives the migration of ions towards the oppositely charged electrode. Anions pass through the anion exchange membrane into the anode compartment and cations pass through the cation exchange membrane into the cathode compartment. The electrical potential gradient across the membrane causes a concentration gradient of the ions, leading to the separation of the ions [17]. As a result of these transport events, ion concentration increases in some compartments and decreases in others. The compartments where the ion concentration decreases are called dilute compartments while the compartments where the ion concentration increases are called concentrated compartments. The ion separation performance of the ED process is influenced by the properties of the ion exchange membrane, the process conditions and the physicochemical properties of the ions to be separated [18]. As for the process conditions, current density in the ED cell, pH, conductivity of the solutions, and ion concentration are important. The ED process, which shows a fast and effective separation performance, is mainly used for the removal of various ions from water and wastewater. It has been used for salinity removal of brackish water [19, 20], removal of iron ions from brackish water [21], removal of Cu and Ni from synthetic and real electroplating wastewater [18, 22, 23, 24, 25], treatment of white carbon black wastewater [26], Cr(VI) removal from effluents [27], acid recovery from waste streams [28] and separation of Li, Ni, Mn, and Co from waste Li-ion batteries [29]. The use of electrodialysis for metal recovery, especially in the electroplating industry, has the potential to provide near-zero emissions as well as resource recovery [30].
The novel aspect of our work is based on the development of a cost-effective electrodialysis module for the removal of Cr(VI) and Ni(II) from aqueous solutions using inexpensive and readily available materials as an alternative to commercial cells at laboratory scale. In the experiments, the effects of voltage, pH, sodium sulfate as a supporting electrolyte, flow rate and metal concentration were investigated. To our present knowledge, there is no comparative study in the literature for Cr and Ni removal in the same ED cell. In addition, while in most electrodialysis studies only the removal efficiency (RE) is evaluated, in this study the optimal values of the variables will be determined by considering current efficiency(CE), energy consumption(EC) and molar flux.
2 Material and method
In this study, the ED process, which is one of the electrochemical membrane separation methods, was used to remove Cr (VI) and Ni (II) ions from model solutions.
2.1 Experimental setup
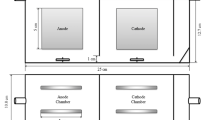
Figure 1 depicts the experimental system, which includes a specially designed ED cell, anolyte and catholyte (electrode rinsing water), and feed (metal effluent) solution tanks and recirculation lines, peristaltic pumps, and a DC power supply. Experiments were carried out in batch recirculation mode.
2.2 Electrodialysis cell
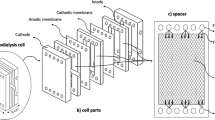
Our main motivation in this study is to develop an alternative ED module to commercial ones at laboratory scale using cheap and readily available materials and to determine the Cr(VI) and Ni (II) removal performance of this cell. The AutoCAD program was used to design the compartments of the ED cell used in the experiments. The components of the ED cell, consisting of teflon and polyethylene plates, were cut at a local company using a water jet with a tolerance of ± 0.1 mm. The solution flow chambers and seals were cut from Teflon and silicone sheets, respectively. The internal edges of these flow chambers are also curved to reduce dead zones. Thus, there was no need to use flow enhancers in the chambers. The cell's components were assembled in the laboratory. Featuring a filter press design, our ED module is significantly less expensive than other laboratory scale commercial modules. The module has an inlet and an outlet for anolyte, catholyte, and feed solutions. In other words, there are six liquid inlet and outlet compartments in total. The two outer frames are made of polyethylene, the inner frames through which the solution passes are made of teflon, and the gaskets that keep each part separate from each other and prevent leakage are made of silicone material. The parts of the cell are given in Fig. 2.
2.3 Ion exchange membranes
Ionac MA 3475 anion exchange membrane and Ionac MC 3470 cation exchange membrane, which are heterogeneous membranes, were used in three compartment ED cell. The properties of the membranes are given in Table 1. The membranes were preconditioned in distilled water at 80 °C for about 30 min. In addition, after each experimental run, the ED cell was rinsed with 0.01 M sulfuric acid and distilled water.
2.4 Instruments and chemicals
The feed solution and electrolyte liquids were circulated through the cell using two peristaltic pumps (Heidolph). The DC power supply can deliver up to 30 V/3 A. A Mettler Toledo pH meter was used to adjust the pH of the solution. Metal analysis was performed using Aquamate’s ultraviolet–visible spectrophotometer. Metal ion supplies included nickel (II) nitrate hexahydrate (Ni (NO3)2.6H2O) and potassium dichromate (K2Cr2O7). As a conductivity enhancer and supporting electrolyte, sodium sulfate (Na2SO4) was used. Diphenylcarbazide (C13H14N4O) and diethyldithiocarbamic acid (C5H10NNaS2.3H2O, sodium salt trihydrate) were used as indicators in the UV spectrophotometric analysis of Cr (VI) and Ni (II) ions, respectively. The pH of the solution was adjusted using sulfuric acid (H2SO4) and sodium hydroxide (NaOH). All chemicals are provided by Merck.
2.5 Experimental conditions and metal analysis
The ED cell is divided into a dilute compartment and two electrolyte compartments by a pair of anion and cation exchange membranes. As the electrolyte solution, distilled water with pH adjusted to 3 with H2SO4 was used and Na2SO4 was added to 7 mM. The speed of the pump that provides the flow of the electrolyte solution is 20 rpm (33.58 mL/min). The feed solution given to the dilute chamber contains metal ions, and the pH, metal concentration, and amount of supporting electrolyte vary between experiments. The effects of pH (2–4.59 for chromium and 2–5.67 for nickel), the addition of supporting electrolyte (3.5–21.1 mM), feed flow rate (40–50 mL/min) and voltage (5–30 V) on the removal efficiencies, energy consumption, and current efficiency were investigated using effluent containing metal ions at a concentration of 50 mg/L (separately). The effect of metal ion concentrations in the range of 50–200 mg/L on the determined optimum values of the parameters was also investigated. In addition, in order to determine the limiting current density, the voltage values were gradually increased, and the current values passing through the cell were investigated. The removal of both metals was observed for 90 min, and a sample was taken every 15 min. Cr(VI) analysis was performed by diphenylcarbazide at 540 nm wavelength, and nickel analysis was determined by diethyldithiocarbamic acid at 395 nm by a spectrophotometric method as shown in Fig. 3 [32].
2.6 Batch recirculation operation in ED cell
The model solutions of Cr (VI) and Ni (II) ions were separately prepared from K2Cr2O7 and nickel (II) nitrate hexahydrate (Ni(NO3)2.6H2O), respectively. The volume of feed solution was 100 mL in each experiment. The ED system was studied in batch recirculation mode at constant potential (potentiostatic). Corresponding current values were recorded under constant voltage with an externally connected power supply. Another method, the galvanostatic mode, could also be used. However, with the galvanostatic method, it may not be possible to develop high voltages at very low concentrations. Therefore, the potentiostatic method is more convenient and reliable [33]. When electric current is supplied to the ED cell through a DC power supply, nickel and dichromate ions in the middle chamber pass through the cation and anion exchange membranes, respectively, and are directed to the cathode or anode (Figs. 4 and 5).
Electrolysis of water takes place at the anode, and hydrogen ions and oxygen gas are released [34]:
(E0 = 1,299 V; 25 °C, 1 atm).
At the cathode, H+ ions are reduced and hydrogen gas is evaluated:
(E0 = 0 V; 25 °C, 1 atm).
These reactions take place at the anode and cathode, as well as during the cathodic reduction of nickel ions [35]:
(E0 = 0,24 V; 25 °C, 1 atm).
2.7 Theorical calculation equations
The removal efficiency, energy consumption, and current efficiency were calculated to see how the experimental parameters affect the performance of the ED system in quantitative terms. The removal efficiency (RE) is a parameter that determines how much of the heavy metal in the wastewater that enters the ED cell is removed at the end of the experiment.
Here, Ci is the initial feed concentration (mol/m3), Cd is the outlet concentration in the dilute solution at t time (mol/m3).
The energy consumed (EC) in an electrodialysis cell is proportional to the applied volts, time, the intensity of the current, and the volumetric amount used. The experiment’s time is measured in minutes, and the applied current is measured in amps.
(W = Current x Voltage).
Where W is the power (watt), t is the time (hour), and V is the volume (L).
Current efficiency (CE) is an important parameter in determining the ED system's optimal range of applicability. The current efficiency also shows how effectively the ions are transported by the applied current during the ED process [36].
Here, z is the ion charge, F is Faraday constant (96.485 As/mol), Qf is the dilute flow rate (m3/s), n is the cell pair number, I is the current (A).
The amount of matter (electrical, molar, or volumetric) passing through a given area in a given time is referred to as flux. Flux is another critical parameter for comparing ED cell efficiency [37]:
Here, J is the molar flux (mol/m2s) and A is the effective membrane area (m2).
3 Results and discussion
In the study, it was first investigated whether the limiting current density was reached for both metal ions in the operating range of the power supply. Then, the effects of applied potential, flow rate, supporting electrolyte concentration, pH, and metal ion concentrations in the feed on removal percentage (RE), energy consumption (EC), and current efficiency (CE) were examined, and the most suitable treatment conditions were tried to be determined. In all experiments, distilled water with a pH of 3 containing 7 mM Na2SO4 was circulated in the electrode chambers with a peristaltic pump at a rate of 33.6 mL/min.
3.1 Limiting current density
The limiting current density is one of the important parameters of the electrodialysis system principle [20]. It provides information about the amount of current, and electrical resistance. Since the number of ions is already high at the initial stage of the experiment, the amount of current begins to increase with the voltage applied to the system [38], and ion migration takes place. The situation where ion migration does not occur anymore is the point when the system reaches the limiting current density. In this case, even if the voltage continues to rise, there is no more ion migration and the current becomes stable.
To determine the limiting current density, a voltage of 5 to 30 V was applied separately to Cr (VI) and Ni (II) solutions at 50 mg/L and adjusted to pH 3. Every 5 min, the voltage was increased by two units. At each voltage increase, current values were recorded. Even with minor fluctuations, the limit current value could not be reached in this experiment, which was carried out up to 30 V. The reason for this can be seen in Fig. 6, which shows that the graph is nearly linear. We can say that the current–voltage curves are in the ohmic region based on the linearity of the graphs [33]. As a result, the current density does not reach the limiting current density for this ED cell in the 0–30 V range.
3.2 The effect of applied voltage
The driving force of the electrodialysis process is the electrical potential difference. The effect of voltage was investigated at 5, 10, 15, 20, 25, and 30 V for the removal of 50 mg/L Cr (VI) and Ni (II) ions. Although there were minor fluctuations, the removal efficiency of Cr (VI) and Ni (II) ions increased as the voltage increased. However, there is a decrease of 25 V. It is observed in Fig. 10 that the optimum voltage value for Cr (VI) removal is 25 V. In Fig. 7, it is observed that the removal efficiency of nickel ions is high at 20 and 25 V. This is expected because the ionic charge in the solution is already high.
The number of ions transported across the membrane is proportional to the current density or electric current. As the voltage increases, the current density also increases. Increasing current density also increases the number of transported ions, i.e., the flux. Furthermore, Moshtarikhah et al. [39] in their study on ion transport in membranes showed in their study on ion transport in membranes that increasing current density increases the membrane pore diameter, the number of active pores and consequently the conductivity of the membrane. Therefore, it is expected that metal ion removal will increase with time and with increasing voltage [18, 40]. Furthermore, the effect of the electrical potential can vary depending on the ions, depending on properties like charge, hydrated radius, or ionic mobility. It has been discovered that the ED cell's selectivity for both ions decreases after a certain voltage value. Table 2 gives the quantity and percentage change according to voltage transitions. It is seen that the amount of metal ion removed and percentage change for both Ni(II) and Cr(VI) is the highest at the transition from 10 to 15 V. In addition, there was a negative change when the voltage increased from 25 to 30 V. According to these results, it can be stated that the highest voltage that can be applied in this cell for both ions is 25 V.
When considering energy consumption, there is a consistent increase in voltage. Low energy consumption and high removal efficiency are required in separation systems in terms of cost. As a result, 25 V for Cr(VI) removal and 20 V for Ni(II) removal satisfy both requirements. The energy consumed increased with the voltage value as the current increased. Many studies, including those by Mustafa et al. [41], Abou-Shady et al. [42], Ben Sik Ali et al. [21], and Gherasim et al. [33], show that as voltage increases, so does energy consumption.
Aside from low energy consumption, high current efficiency is also critical. High current efficiency was observed at low voltage values for the removal of Cr (VI) and Ni(II). The current efficiency decreases as the voltage increases. A similar situation was observed in the study by Mustafa et al. [41]. At 5 and 10 V, the highest current efficiency was observed (Fig. 7). Concentration polarization could be the cause of the significant decrease in current efficiency after 10 V. Contamination in the ED system is possible because OH− ions have the ability to combine with cations in the chamber as a result of electrodialysis [43]. While the resistance of ion exchange membranes is slightly low, the resistance is high in the dilute chamber with low ion concentration [44]. As the voltage value increases, the ions are transported faster due to the increasing current. As time progresses, metal ions are depleted in the dilute chamber and the electrical resistance of the chamber increases. Thus, lower current efficiency is obtained at high voltage values compared to low voltages [45]. In the removal of nickel ions, current efficiency values exceeding 100% were obtained. This demonstrates that the electrodialysis method works in nickel ion removal not only with the potential difference driving force but also with the concentration difference control (Fig. 7). Therefore, low voltage is better for high current efficiency and low energy consumption, while high voltage is better for achieving high metal removal. Hence, a trade-off is necessary to achieve optimum process performance [41].
It is expected that the amount of flux will increase due to the ion transfer to the anode and cathode compartments as the flux increases for both ions as the voltage value increases. Since the limiting current density was not reached, no decrease in flux was observed. Because when the limiting current density is reached, an increase in voltage or increase in current will not make sense, since there are no ions to be transported in the medium. The variation of flux for different voltage values is given in Fig. 8. According to this, it was observed that the voltage and flux with time increased remarkably, especially in the range of 5–15 V.
However, it was observed that the flux values were close to each other, especially in the 20–30 V range, at the 90th minute for both nickel and chromium separately. The molar flux for Ni(II) in the designed ED cell was approximately four times that of Cr(VI). First of all, we can attribute the increased flux in both, due to the increase in voltage, to the increase in driving force due to ion migration. The reason for the different performances of the two ions and the difference between their fluxes may be due to the difference in the hydrated radii. Each ion has its own unique ionic and hydrated radius size. This affects the ion migration and the flux. Ions with large hydrated radii also have high fluxes. In other words, the ion migration rate is higher. We can attribute this difference to Ye et al. [46].
3.3 The effects of supporting electrolyte concentration
In the electrodialysis method, conductivity-increasing chemicals can be used in order to make the ion transition more convenient and faster in undesirable situations such as increased resistance due to membrane contamination or the effect of competing ions. These can be salts such as sodium sulfate, sodium perchlorate, sodium nitrate, and sodium chloride. Wu et al. [47] investigated the ionic conductivity of sodium sulfate, sodium nitrate, and sodium perchlorate salts for some molar concentrations at 25 °C. According to the findings, sodium sulfate has a high conductivity at low concentrations. Electrical conductivity is directly proportional to ionic strength. The higher the ionic strength of a substance, the more electrically conductive it is, accelerating the passage of ions. Accordingly, while the ionic strength of 1 molar NaCl salt is 1, the ionic strength of 1 molar Na2SO4 salt is 3. In other words, the electrical conductivity of sodium sulfate salt is also better than NaCl [48]. In this study, sodium sulfate was used as a supporting electrolyte. The effect of sodium sulfate was investigated for the removal of Cr (VI) and Ni (II) ions at a 50 mg/L concentration. As can be seen in Fig. 9, when the sodium sulfate amount in both ions increases from 3.5 mM to 7 mM, the removal increases, but there appears to be a decrease in the removal from 7 to 21.1 mM.
Due to the presence of sodium sulfate in solution, sodium and sulfate ions are also present in the environment, and the current in the cell is carried not only by metal ions but also by sodium and sulfate ions. Therefore, excessive use of the supporting electrolyte will reduce metal ion transport. In addition, in the study of Wu et al. [47], it was determined that the rate of increase in conductivity decreased after 2 M sodium sulfate. When the removal percentage, current efficiency, and energy consumption are evaluated together, it is seen that the addition of 7 mM Na2SO4 is sufficient for the effective removal of both metal ions. This value corresponds to a very low amount of 1 g per 1 L waste stream. Not many studies have been observed on chemicals used either as electrolytes or as conductivity enhancers. It is understood from the current values that the sodium sulfate chemical increases the conductivity to a great extent in the studies carried out using electrolytes and synthetic wastewater, but it has been observed that its use at high dosages does not have a great effect. Apart from this, the effect of using chemicals other than sodium sulfate can be investigated to provide clearer data. In a study, the effect of the use of NaCl, Na2SO4, H2SO4, and NaNO3 chemicals as electrolytes was investigated [47]. It has been observed that sodium sulfate separates nitrate more effectively than the other three in the selected processing time and provides lower energy consumption [49].
3.4 The effect of pH of feed solution
It is seen from Fig. 10 that the removal increases with the increase of the pH value from 2 to 3, but decreases at values after 3. Maximum removal was achieved at pH 3 for both metal ions. The difference in the amount of removal with a pH change may be caused by the hydrogen ions. The amount of acid used to lower the original pH of the solution from 4.59 to 3.0 is much less than the amount of acid added to lower the pH from 3.0 to 2. Apart from this, the presence of dichromate species can also affect the system. There are different types of chromium (VI), and they are affected by certain pH values and redox reactions. Chromium (VI) species are given in Fig. 11 [50]. Chromium (VI) in aqueous solutions exists as chromate (CrO42−), dichromate (Cr2O72−), hydrogen chromate (HCrO4−), dihydrogen chromate (chromic acid, H2CrO4), hydrogen dichromate (HCr2O7−), trichromate (Cr3O102−) and tetrachromate (Cr4O132−). Hydrogen dichromate (HCr2O7−), trichromate (Cr3O102−) and tetrachromate (Cr4O132−) are found in solutions where the chromium concentration is greater than 1 M and the pH value is less than zero. Dichromate (Cr2O72−) and hydrogen chromate (HCrO4−) can be found together in their aqueous solutions according to the pH range, this range is between pH = 2 and 6 according to Tandon et al. [51] and between pH = 0.75 and 6.45 according to Pourbaix [50]. Accordingly, we can attribute the decrease and increase in the removal of chromium (VI) ions, which is maximum at pH = 3, up to pH = 4.59, to the existence of different chromium (VI) forms, as seen in Fig. 11. Therefore, hydrogen chromate ions can also be found in the medium in the pH 3–4.59 range. This can be explained by the following equations:
Potential–pH balance diagram of chromium (VI) species (adapted from Pourbaix [50])
When the sulfuric acid placed in the feed solution is dissolved in water, a hydronium ion is released, as shown in Eq. (8). In the acid medium, the dichromate ion dissolves in the hydronium ion, and chromic acid (H2CrO4) is released as given in Eq. (9) [52]. The equilibrium reaction of chromic acid takes place in two steps, as shown in Eqs. (10) and (11). The pKa values of the equilibrium reactions are also given [53]. The calculation of the pH value according to the Henderson-Hasselbalch equation is given in Eq. (12) for Eq. (10) [54]. According to the equation, the ratio of the concentration of the products to the concentration of the reactants should be a maximum of 1 or a minimum of − 1 [55]. Consequently, in this case, the pH value of Eq. (10) is close to 2. The low pH value also represents the abundance of hydrogen dichromate ions in the medium. This confirms the pH range mentioned by Pourbaix [50] and Tandon et al. [51]. In other words, the dichromate ion and the hydrogen chromate ion exist together in the same pH range. The presence of hydrogen chromate may be responsible for the decrease and increase of the dichromate ion in analyses performed with feed solution samples at varying pH values. To explain in more detail, the diphenylcarbazide method only forms complexes with hexavalent chromium, regardless of the chromium (VI) type. Chromium (VI) combines with 1,5-diphenylcarbazide in the presence of acid to form a Cr (III)-diphenylcarbazone complex, and a purple-colored solution is formed. This only happens in the presence of chromium (VI) [32]. As a result, another reason why the removal efficiency, which is high at pH = 3, decreases at pH = 4 and increases slightly at pH = 4.59, may be hydrogen chromate, another chromium (VI) type in the medium.
A similar situation can be described for the nickel ion. For each pH value, the amount of removal increased over time. Energy consumption and current efficiency also increased over time for each pH value. Because the pH value changes with time for each pH value, the ion mobility in the medium affects the energy used and the current efficiency. It was observed that energy consumption increased with decreasing pH values. At low pH values, conductivity is high due to the hydrogen ions in the medium. The excess of conductivity caused the current to increase. Increasing the current under constant voltage also increased the amount of energy used. At pH 2, the lowest current efficiency values were obtained for both metal ions. The reason for this is that acidity increases as pH decreases; that is, the amount of hydrogen ions increases, and this ion uses the majority of the current [56]. Furthermore, the presence of H+ and SO42− ions in the medium as a result of the sulfuric acid added to the solution at low pH values, and thus the high current values, is another reason for the decrease in current efficiency for both metal ions. The pH = 3 value shows the highest removal efficiency. Even though the energy consumption is low at pH values of 4 and 5.67, pH = 3 was observed to be the most appropriate value due to the high difference in removal efficiency. The removal decreased after reaching the optimum value of pH = 3. There are more hydrogen ions at lower pH values. Therefore, the removal is less at low pH, as the Ni(II) ions in the middle are in competition with the hydrogen ions [57]. In addition, since the mobility of hydrogen ions (3.756) is much higher than that of metal ions (Ni(II) = 0.267; Cr(III) = 0.240; Cu(II) = 0.288), it is easier for them to pass through the membrane as the pH decreases [58]. Bernardes et al.[8], emphasized that in the tests in which they evaluated the pH values of synthetic wastes by purifying them in the ED cell, the separation of nickel ions was less due to their competition with hydrogen at low pH values. In addition, in the case of nickel precipitation in wastewater with a pH value of 5, agitation with a magnetic stirrer is recommended.
It has been observed that the nickel ion is present in certain concentrations at the cathode (especially 48 mg/L at pH 3). When the pH values of the cathode chamber were measured to look at the pH parameter, values of 10 and above were observed. In the basic medium, OH− ion is released by the reduction of the water molecule at the cathode. Nickel (II) ions, which pass to the cathode, combine with hydroxide and precipitate [35]. The nickel ion can react with the hydroxide ion formed in the basic medium and form precipitation, and as a result, nickel (II) hydroxide can be formed instead of nickel reduction. Instead of being reduced, nickel can be combined with the hydroxide (OH−) ion if its solubility exceeds the limits. The potential pH balance diagram for the nickel-water system at 25 ℃, given in Fig. 12, shows that the nickel ion exists in the range of 6–7 and the chemical Ni(OH)2 exists in the pH range of 9–12 [50].
3.5 The effect of flow rate to the feed solution
Because the difference in removal efficiency for both flow rates in nickel ion is small, the energy consumption is also small. However, in chromium, a higher energy consumption was obtained at a lower flow rate. The removal efficiency for both ions decreased with the increase in flow rate (Fig. 13).
Flow rate refers to the residence time of the solution in the compartment. As the flow rate increases, the retention time of the ions will decrease, so the liquid will come out as it doesn’t undergo ion transfer between the membranes. Therefore, ions that cannot be separated cause a decrease in removal efficiency [21, 59, 60]. The diffusion of ions in water can also affect this situation. The diffusivity of nickel in water is 0.661 × 10−9 m2/s, and the diffusivity of chromate in water is 1.132 × 10−9 m2/s [58]. If the diffusion of the dichromate ion in water is low, like that of the chromate ion, the following interpretation can be made. In connection with the expression of the residence time of the solution in the cell, we can say that low diffusion reduces the transfer to the concentrated compartment. On the contrary, ions with high diffusion (such as nitrate and fluoride) can take advantage of the residence time in the membrane [61]. The increase in current density along with the decrease in current with the increase in speed shows that the concentration polarization phenomenon is slightly reduced [33].
The energy consumption increased with the increase in flow rate due to the increasing current density in nickel removal. In contrast, chromium removal decreased as current density decreased. While the current efficiency decreased with the increase in flow rate for nickel ions, it increased for chromium ions. In this case, it may be due to the increase in ion transfer being more or less than the increase in current density [33].
Flow rate is one of the important parameters in the electrodialysis method. While it can reduce the concentration polarization phenomenon caused by the accumulation of substances in the membranes and increase the efficiency, it can also negatively affect the removal efficiency by reducing the retention time in the membranes. As a result, selecting the appropriate flow rate is critical. The flow rate varies according to the characteristics of each ion. Since the mobility of chromium (VI) (dichromate) ions is higher than that of nickel, it has been observed that the removal efficiency of chromium is higher at low velocities. Based on this interpretation, as the flow rate increased, the removal efficiency of chromium was lower than that of nickel.
3.6 The effect of initial metal concentration in the feed solution
As the concentration increases, a decrease in the removal efficiency and an increase in the current efficiency and energy consumption are observed. The reason for this can be explained by the abundance of Cr (VI) ions in the medium.
When the feed concentration increases, there are more metal ions in the medium and thus the percentage of current transport increases, leading to an increase in current efficiency [41]. The experiment is performed for 90 min, and it is seen that the high concentration affects the efficiency. The increase in concentration also indicates that the conductivity increased, but despite this, the removal efficiency decreased. Concentration polarization can also help to reduce removal efficiency. The limited separation capacity of the membranes can also affect this situation. Because as the concentration increases, excess ions can create resistance and cause pollution, and in this case, the ED cell will have an adverse effect, resulting in accumulation and stratification in the membranes, and the removal efficiency will decrease (see Fig. 14) [60]. Furthermore, as concentration increased, the formation of counter-ion competition increased. Due to the abundance of ions in the medium as well as the presence of other ions, using counter-ion transport instead of the ions that need to be removed may have reduced the removal efficiency. Li et al. [56], in their study that the removal efficiency decreased due to the presence of counterions. In Nataraj et al.'s [44] study of chromium (VI) removal by the electrodialysis method with a batch recycle, it is seen that the removal decreases with increasing concentration values. In other words, the removal efficiency is much higher at low concentrations, regardless of nickel and chromium. Similar results has been reported by Ben Sik Ali et al. [21] for iron removal by electrodialysis.
Although the energy consumed is low at 120 mg/L of concentration for both ions, the energy used increases with the increase in concentration. As the concentration increases, the medium becomes dense with ions. In addition, the current density increased with the concentration. In this case, it is expected to spend more energy than necessary to remove these ions. In many studies, an increase in energy consumption was observed with an increase in concentration [33, 62].
The current efficiency increased as the concentrations of both ions increased. The decrease in the electrical resistance of the solutions may have increased the current efficiency. As seen in Fig. 15, the molar flux increased gradually for both Cr(VI) and Ni(II) as the metal ion concentration in the feed solution increased from 50 mg/L to 150 mg/L. The result shows that the increase in molar flux has a positive effect on the current efficiency [33].
Chromium, which has high ion mobility, adhered less to the membrane and moved away from the medium. Nickel, on the other hand, acted in the opposite direction. Chromium and nickel ions are used in different concentrations in many areas of industry. For this reason, research was carried out using a feed solution at concentrations of 50, 100, 120, and 150 mg/L for both ions. It was observed that the removal efficiency decreased with increasing concentration. At high concentrations, the removal of ions is problematic due to either excess ions in the medium (more conductivity) or concentration polarization, but the efficiency decreases. In addition, due to the abundance of ions in the medium, the time is kept for a long time to ensure removal, and besides, energy consumption increases. This is undesirable in both laboratory scale systems and industrial activities. As a result, our ED cell with one membrane pair seems to be more suitable for concentrations of 50 mg/L and lower in terms of complete removal.
In Table 3, some studies on Ni(II) and Cr(VI) removal by electrodialysis in the literature are compared with the results of this study. The results obtained in this study are similar to other studies in terms of metal removal efficiency. In addition, both more membrane pairs and commercial ED cells were used in most of the studies in the literature.
Since the Ni concentration was higher in our study, the removal time was longer than in Min's study [18], which included 5 membrane pairs and used a commercial ED module. For Cr(VI), the reason for the longer removal time than Dos Santos' study [27] can be explained by the lower number of membrane pairs. The active membrane surface area can be easily increased by adding more membrane pairs to the ED cell we designed. In addition, stainless steel and carbon fiber electrodes are more economical than Pt or Ti containing electrodes.
4 Conclusion
Nickel and chromium ions are the leading heavy metals that cause serious environmental problems. In this study, the performance of our designed and assembled ED module for the removal of nickel and chromium ions from water was investigated. The effects of parameters such as applied voltage, treatment time, metal concentration, pH value, and feed solution circulation rate on removal efficiency, energy consumption, current efficiency and molar flux were investigated and the best operating conditions were determined. pH is very effective in removing metal ions and lowering energy consumption. It is more appropriate to operate the ED cell at an optimum pH value of 3 for the removal of chromium (VI) and nickel (II) ions. It is understood from the current values that the sodium sulfate increases the conductivity to a great extent in the studies carried out using electrolytes and synthetic wastewater, but it has been observed that its use at high dosages does not have a great effect. Flow rate is one of the important parameters in the electrodialysis method. While it can reduce the concentration polarization phenomenon caused by the accumulation of substances in the membranes and increase the efficiency, it can also negatively affect the removal efficiency by reducing the retention time in the membranes. Chromium and nickel ions are used in different concentrations in many areas of industry. It was observed that the removal efficiency decreased with increasing concentration. For both Cr(VI) and Ni(II), the increase in voltage and NaSO4 concentration had an increasing effect on energy consumption, while the increase in pH had a decreasing effect. Flow rate and metal concentrations are the least effective parameters in terms of energy consumption. In order to obtain high current efficiency, it is necessary to work with high metal concentration, low voltage and low supporting electrolyte addition. It was determined that the increase in voltage, metal concentration and flow rate increases the molar flux. In this study, a cost-effective ED module was prepared using cheap and readily available materials, and a removal performance close to that of commercial cells was obtained for both Cr(VI) and Ni(II) with only one pair of ion exchange membranes. The most important advantage of the designed cell is that it is a disassembled system and thus the membrane and electrodes are easy to adapt to the system. Thus, different membranes and electrodes can be tested. The number of membranes and flow chambers can be easily increased. These features of our ED cell facilitate its use for laboratory purposes.
Change history
15 July 2023
The ORCID of first author “Senem Kırmızı” is corrected.
References
Nataraj SK (2022) Emerging Materials and Technologies, CRC Press, p.1
Lichtfouse E, Muthu SS, Khadir A (2022) Inorganic – Organic Composites for Water and Wastewater Treatment. Springer p.2
Akitsu T (2019) Environmental Science: Society, Nature and Technology. Pan Standford Publishing Pte.Ltd. CRC: p.153
Baby R, Saifullah B, Hussein MZ (2019) Carbon nanomaterials for the treatment of heavy metal contaminated water and environmental remediation. Nanoscale Res Lett 14(1):1–17
Sonone SS, Sankhla MS, Jadhav SV, Kumar R (2020) Water contamination by heavy metals and their toxic effect on aquaculture and human health through food chain. Lett Appl NanoBiosci 10(2):2148–2166
Saxena G, Purchase D, Mulla SI, Saratale GD, Bharagava RN (2019) Phytoremediation of heavy metal-contaminated sites: Eco-environmental concerns, field studies, sustainability ıssues, and future prospects. Reviews of Environmental Contamination and Toxicology: 1–61
Büyükpınar Ç, Yazıcı E, Nevim S, Komesli OT, Sezgin B (2021) Determination of nickel in daphne tea extract and lake water samples by flame atomic absorption spectrophotometry with a zirconium-coated T-shaped slotted quartz tube-atom trap and photochemical vapor generation sample introduction. Environ Monit Assess. https://doi.org/10.1007/s10661-021-09430-2
Bernardes AM, Rodrigues MAS, Ferreira JZ (2014) Electrodialysis and Water Reuse, Springer-Verlag Berlin Heidelberg. p.25, 26, 68, 133–135, 137
Rizvi A, Parveen S, Khan S, Naseem I (2020) Nickel toxicology with reference to male molecular reproductive physiology. Reprod Biol 20:3–8
Bui XT, Chimchaisri C, Fujioka T, Varjani S (2019) Water and Wastewater Treatment Technologies Springer: p.82, 369–370, 510
Manahan SE (2010) Environmental Chemistry. Ninth Edition. CRC Press: p.497–498, 688
Peng H, Guo J (2020) Removal of chromium from wastewater by membrane filtration, chemical precipitation, ion exchange, adsorption electrocoagulation, electrochemical reduction, electrodialysis, electrodeionization, photocatalysis and nanotechnology: a review. Environ Chem Lett 18:2055–2068
Mahmud HN, Huq AK, Binti Yahya R (2016) The removal of heavy metal ions from wastewater/aqueous solution using polypyrrole-based adsorbents: a review. RSC Adv 6:14778
Liu X, Zhang Y, Liu Y, Zhang T (2022) Magnetic red mud/chitosan based bionanocomposites for adsorption of Cr(VI) from aqueous solutions: synthesis, characterization and adsorption kinetics. Polym Bull. https://doi.org/10.1007/s00289-022-04137-x
Ferreira LC, Ferreira LC, Cardoso VL, Filho UC (2019) Mn (II) remowal from water using emulsion liquid membrane composed of chelating agents and biosurfactant produced in loco. J Water Proces Eng 29:10792
Sedighi M, Behvand Usefi MM, Ismail AF, Ghasemi M (2023) Environmental sustainability and ions removal through electrodialysis desalination: Operating conditions and process parameters. Desalination. https://doi.org/10.1016/j.desal.2022.116319
Arana Juve J, Christensen FMS, Wang Y, Wei Z (2022) Electrodialysis for metal removal and recovery: A review. Chem Eng J 435:134857
Min KJ, Choi SY, Jang D, Lee J, Park KY (2019) Separation of metals from electroplating wastewater using electrodialysis. Energy Sources, Part A: Recovery, Util Environ Eff 41(20):2471–2480. https://doi.org/10.1080/15567036.2019.1568629
Zhao J, Chen Q, Wang J (2022) Novel ecofriendly cation exchange membranes for low-cost electrodialysis of brackish water: desalination and antiscaling performance. J Membr Sci. https://doi.org/10.1016/j.memsci.2022.120908
Ben Sik Ali M, Mnif A, Hamrouni B (2018) Modelling of the limiting current density of an electrodialysis process by response surface methodology. Ionics 24:617–628
Ben Sik Ali M, Ennigrou DJ, Hamrouni B (2013) Iron removal from brackish water by electrodialysis. Environ Technol 34(17):2521–2529. https://doi.org/10.1080/09593330.2013.777081
Min KJ, Kim JH, Oh EJ, Ryu JH, Park KY (2021) Flow velocity and cell pair number effect on current efficiency in plating wastewater treatment through electrodialysis. Environ Eng Res 26(2):190502
Wang C, Li T, Yu G, Deng S (2021) Removal of low concentrations of nickel ions in electroplating wastewater by combination of electrodialysis and electrodeposition. Chemosphere. https://doi.org/10.1016/j.chemosphere.2020.128208
Benvenuti T, Krapf RS, Rodrigues MAS, Bernardes AM, Zoppas-Ferreira J (2014) Recovery of nickel and water from nickel electroplating wastewater by electrodialysis. Sep Purif Technol 129:106–112. https://doi.org/10.1016/j.seppur.2014.04.002
Aytaç E, Altin S (2021) Considering the ion types while evaluating the performance criteria in electrodialysis systems. Environ Eng Manag J 20(1):89–90
Wang X, Zhou X, Ma S, Wang Z, Wang E, Li Z (2022) White carbon black wastewater treatment by electrodialysis: Salt separation, silicon sol transporting and wastewater recycling. J Environ Chem Eng. https://doi.org/10.1016/j.jece.2022.107856
Dos Santos CSL, Miranda Reis MH, Cardoso VL, De Resende MM (2019) Electrodialysis for removal of chromium (VI) from effluent: analysis of concentrated solution saturation. J Environ Chem Eng. https://doi.org/10.1016/j.jece.2019.103380
Yuzer B, Aydin MI, Yildiz H, Hasançebi B, Selcuk H, Kadmi Y (2022) Optimal performance of electrodialysis process for the recovery of acid wastes in wastewater: practicing circular economy in aluminum finishing industry. Chem Eng J. https://doi.org/10.1016/j.cej.2022.134755
Chan KH, Malik M, Azimi G (2022) Separation of lithium, nickel, manganese, and cobalt from waste lithium-ion batteries using electrodialysis. Resour, Conserv Recycl. https://doi.org/10.1016/j.resconrec.2021.106076
Benvenuti T, Siqueira Rodrigues MA, Bernardes AM, Zoppas-Ferreira J (2017) Closing the loop in the electroplating industry by electrodialysis. J Clean Prod 155:130–138. https://doi.org/10.1016/j.jclepro.2016.05.139
Karabacakoğlu B, Tezakıl F, Güvenç A (2014) Removal of hardness by electrodialysis using homogeneous and heterogeneous ion exchange membranes. Desalin Water Treat 54(1):8–14
Sandell EB (1978) Colorimetric Determination of Traces of Metals, Chemical Analysis a Series of Monographs on Analytical Chemistry and Its Application. Fourth Edition New York Wiley: p.191–195, 46
Gherasim CV, Krivcik J, Mikulasek P (2014) Investigation of batch electrodialysis process for removal of lead ions from aqueous solutions. Chem Eng J 256:324–334
Shen PK, Wang CY, Jiang SP, Sun X, Zhang J (2016) Electrochemical Energy: Advanced Materials and Technologies CRC Press: p.590
Brett CMA, Brett AMO (1993) Electrochemistry: Principles, Methods and Applications, Oxford University Press: p.130–131, 334
Sadrzadeh M, Mohammadi T (2009) Treatment of sea water using electrodialysis: Current efficiency evaluation. Desalination 249:279–285
Arar Ö, Yüksel Ü, Kabay N, Yüksel M (2014) Demineralization of geothermal water reverse osmosis (RO) permeate by electrodeionization (EDI) with mixed bed configuration. Desalination 342:23–28
Silva V, Poiesz E, Van Der Heijden P (2013) Industrial wastewater desalination using electrodialysis: evaluation and plant design. J Appl Electrochem 43(11):1057–1067. https://doi.org/10.1007/s10800-013-0551-4
Moshtarikhah S, Oppers NAW, de Groot MT, Keurentjes JTF, Schouten JC, van der Schaaf J (2017) Nernst-Planck modeling of multicomponent ion transport in a Nafion membrane at high current density. J Appl Electrochem 47(1):51–62. https://doi.org/10.1007/s10800-016-1017-2
Mulder M (1991) Basic Principles of Membrane Technology, Kluwer Academic Publishers: p.271, 273
Mustafa J, Al-Marzouqi AH, El-Naas MH, Ghasem N (2021) Electrodialysis based waste utilization methodology for the desalination industry. Desalination 520. https://doi.org/10.1016/j.desal.2021.115327
Abou-Shady A, Peng C, Almeria OJ, Xu H (2012) Effect on pH on separation of Pb (II) and NO3- from aqueous solutions using electrodialysis. Desalination 285:46–53
AWWA (1995) Electrodialysis and Electrodialysis Reversal, American Water Works Association p.1, 7, 16, 22–24
Beier SP (2014) Electrically Driven Membrane Processes, Bookboon: p.9–11,18,19,23–25
Nataraj SK, Hosamani KM, Aminabhavi TM (2007) Potential application of an electrodialysis pilot plant containing ion-exchange membranes in chromium removal. Desalination 217:181–190
Ye ZL (2019) Fractionating various nutrient ions for resource recovery from swine wastewater using simultaneous anionic and cationic selective electrodialysis. Water Research: 424–434
Wu W, Shabhag S, Chang J, Rutt A, Whitacre JF (2015) Relating electrolyte concentration to performance and stability for NaTi2(PO4)/NaO,44MnO2 aqueous sodium-ion batteries. J Electrochem Soc 162(6):A803–A808
Van Oss CJ (2008) The Properties of Water and Their Role in Colloidal and Biological Systems. Interface Science and Technology. pp. 31–48
Mohammadi R, Ramasamy DL, Sillanpӓӓ M (2021) Enhancement of nitrate removal and recovery from municipal wastewater through single- and multi-batch electrodialysis: Process optimisation and energy consuption. Desalination 498:114726
Pourbaix M (1974) Atlas of Electrochemical Equilibria in Aqueous Solutions. NACE International Cebelcor, Second English Edition: p.258–259, 261–262, 333
Tandon RK, Crisp PT, Ellis J, Baker RS (1984) Effect of pH on chromium (VI) species in solution. Talanta 31(3):227–228
Anonymous (2021) Chromic acid, https://en.wikipedia.org/wiki/Chromic_acid, date of access: 08.04.2021
Sanchez-Hachair A, Hofmann A (2018) Hexavalent chromium quantification in solution: comparingdirect UVvisible spectrometry with 1,5-diphenylcarbazidecolorimetry. C R Chim 21:890–896
Symons R, Chatterji R, Whenan K, Horvath, R, Thomas PS (2019) Blood gas analysis and instrumentation. In: Narayan, R., (ed.) Encyclopedia of Biomedical Engineering, Elsevier, New York, https://doi.org/10.1016/B978-0-12-801238-3.10894-3
Anonymous (2021) pH and pKa Relationship: The Henderson-Hasselbalch Equation, https://www.thoughtco.com/the-ph-and-pka-relationship-603643, date of access: 08.04.2021.7
Gering KL, Scamehorn JF (1988) Use of electrodialysis to remove heavy metals from water. Sep Sci Technol 23(14–15):2231–2267
Li C, Ramasamy DL, Sillanpӓӓ Repo E (2021) Separation and concentration of rare earth elements from watewater using electrodialysis technology. Sep Purif Technol 254:117442
Kreysa G, Ota KI, Savinell R (2014) Encyclopedia of Applied Electrochemistry, Springer Science+Business Media, New York: p.1126
Sadrzadeh M, Razmi A, Mohammadi T (2007) Separation of monovalent, divalent and trivalent ions from wastewater at various operating conditions using electrodialysis. Desalination 205:53–61
Sadrzadeh M, Mohammadi T (2008) Sea water desalination using electrodialysis. Desalination 221:440–447
Aliaskari M, Schӓfer AI (2021) Nitrate, arsenic and fluoride removal by electrodialysis from brackish groundwater. Water Res 190:116683
Kabay N, Arar Ö, Samatya S, Yüksel Ü, Yüksel M (2008) Separation of fluoride from aqueous solution by electrodialysis: Effect of process parameters and other ionic species. J Hazard Mater 153:107–113
Author information
Authors and Affiliations
Contributions
Both authors Senem Kırmızı and Belgin Karabacakoğlu contributed equally.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kırmızı, S., Karabacakoğlu, B. Performance of electrodialysis for Ni(II) and Cr(VI) removal from effluents: effect of process parameters on removal efficiency, energy consumption and current efficiency. J Appl Electrochem 53, 2039–2055 (2023). https://doi.org/10.1007/s10800-023-01894-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-023-01894-z