Abstract
Gold can be extracted from gold plating baths, printed circuit boards, and goldsmithing wastes by sodium hypochlorite leaching. However, the leach liquor should be purified to remove other metallic ions and recover the gold from the mixture. While most metals form cations in solutions, gold occurs in anionic complexes. This property makes it possible to separate gold from other ions by the electrodialysis method. In this research, a three-compartment batch electrodialysis setup consisting of an anion-exchange membrane and a cation-exchange membrane is developed on a laboratory scale for this purpose. The impact of three parameters, namely applied voltage, Au concentration in the feed solution, and flow rate, is investigated in gold recovery using the response surface methodology. The results indicate that voltage has the highest and Au concentration in the feed solution has the lowest impact on gold recovery by electrodialysis. The optimal condition to achieve the highest percentage of Au recovery (95%) was found to be a voltage of 32.51 V, 4.1 ppm gold concentration in the feed solution, and a flow rate of 0.32 L/min. Electrodialysis offers an innovative, environmentally friendly route for gold recovery and helps to reduce the loss of valuable metal ions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gold-coated particles are widely used in various industries due to their excellent electrical and thermal conductivity, coupled with high corrosion resistance (Habashi 2005). Electrochemically deposited gold particles have numerous functions in different areas, such as the electronics industry (to produce connectors, printed circuit boards and etc.), the aerospace industry, dentistry, decorative purposes, and jewelry (Lambrechts and Lall 2021; SI et al. 2020). The electroplating process is considered one of the main methods of Au-coating gold particles, while cyanide-based gold reagents are the most common electrolytes (Shacham-Diamand et al. 2015). Given the considerable economic value of gold, the metal-finishing industry uses almost every gram of gold in the plating bath to avoid waste (Hannapel and Richter 2004). During the plating process, using an insufficient amount of solution or low discharge of the plating solutions into the plating bath may lead to a significant loss of metal ions (Meng et al. 2020). Even if great care is taken to make optimal use of gold in the plating bath, the bath may reach a point where it loses its initial ability to produce the desired deposits (Van Grieken et al. 2005). In such cases, the efficiency of the bath decreases and, eventually, it must be discarded. In the wastewater, which contains 6 to 8% of the total initial gold, Au occurs in the form of [Au (CN)2]− and [Au (CN)4]− complexes (Wilkinson 1986); where [Au (CN)2]− is the more common ion in electrodeposition (Reid 1973). Removing plating solutions from the process leads to significant financial loss, especially when using precious metals (Heights 1983).
Formation of a layer of gold in the gold plating tanks and cables is a common phenomenon. Removing this layer is essential to avoid decreasing the process efficiency and increasing gold consumption. The gold layer can be removed with cyanide or hypochlorite/chloride-based reagents. When CN− is used for gold plating washing, wastewater containing significant concentrations of gold-cyanide complexes is generated (Johnson 2015). Treating this wastewater is necessary not only to recycle the Au, but also to remove its toxic cyanide content (Van Grieken et al. 2005). However, using conventional oxidation technologies for cyanide degradation is extremely difficult due to the high stability of these complexes (Sousa et al. 2021). Hypochlorite/chloride-based reagents are suitable alternatives for gold dissolution. The gold makes a stable complex with Cl based on Eq. 1.
The wastewaters from the washing of gold plating baths contain a mixture of Au and other metals. Recycling gold from this wastewater is important from economic and environmental points of view. The separation of Au ions from other metallic ions is important to recycle gold. Membranes can be employed for gold ions separation in hypochlorite/chloride-based solutions. Membranes have been used to separate ions from solutions and to purify water for decades (Han et al. 2019). However, they are known to be ineffective in dealing with metallic ions due to membrane fouling and the metal precipitations formed on the membrane surface (Wang et al. 2019). Extensive research has been carried out to solve this problem, resulting in methods such as chemical modification of membrane surfaces and synthesis of new membranes (Bazrgar Bajestani et al. 2020; Mubita et al. 2020).
Electrodialysis (ED), a relatively new method introduced by (Chaudhary et al. 2000), increases the separation efficiency of membranes and mitigates their disadvantages. In this electrochemical membrane separation technique, an external electric field is applied as a driving force for ions to transfer through selective ion-exchange membranes from one solution to another (Song and Zhao 2018). Although the main target of the ED process is water desalination, it is also regarded as a highly promising technology in hydrometallurgical processes and may be implemented to remove ions from solutions (Gmar and Chagnes 2019). Another application of ED is in the enrichment of ions in cathodic or anodic solutions based on the charge of the available ions in the solutions and the depletion of ions in the feed solution (Golubenko and Yaroslavtsev 2020). In other words, ED is an efficient method of separating ions from wastewater and metallurgical leach liquors.
ED has several advantages over conventional activated carbon extraction methods and selective precipitation, such as minimal energy expenditure (Chan et al. 2022), low waste rejection (Generous et al. 2021), and high modularity (Seyedhakimi et al. 2018). This method is economical and environmentally friendly because additional reagents are not required in the ED process, which will lower the severe environmental impacts and decrease operational costs (Kavitha et al. 2022; Vineyard et al. 2020). Moreover, since the system's primary operational cost is electricity usage, the process would be favorable in countries using low-price industrial electricity like Iran, Qatar, Russia, and Kyrgyzstan (Tonner and Tonner 2004). Consequently, it can be used to concentrate and purify metallic solutions (Afifah et al. 2018). However, ED is associated with a number of limitations, including limited selectivity in solutions containing a variety of metallic ions with similar valences (Zafari et al. 2022). Few papers have addressed electromembrane techniques or attempted to identify factors that influence the separation of ions from multi-metallic mixtures (Babilas and Dydo 2018).
In the present study, we investigate the possibility of recovering gold from solutions with a 3-compartment ED cell with the goal of minimizing the loss of gold and reducing waste generation through a technically and economically feasible process. The central objective of this study is to develop an electrochemical method of recovering gold from electroplating baths. We investigate the effects of three parameters including applied voltage (25 to 35 V), gold concentration in the feed solution (2 to 5 ppm) and circulating solution flow rate (0.24 to 0.48 L/min) on the recovery of gold from hypochlorite solutions by ED. The results of this research can be used to improve processes of separating and recycling gold ions from wastewater. The low investment cost and remarkable safety of ED make it an ideal technique, both in small- and large-scale plants. This manuscript's results are also important from an environmental point of view. The current manuscript investigates gold recycling to avoid Au release in nature. Releasing heavy metals (like Au) in the environment can cause severe environmental impacts and affect human and animal health.
Materials and methods
Leach liquor solution preparation
In order to synthesize the gold plating wastewater, the leach liquor was prepared by dissolving high-purity metallic gold in a leaching reagent containing 2% sodium hypochlorite, 1% hydrochloric acid and 1 g/L sodium chloride (Merck-Germany). The initial rate of gold dissolution was extremely rapid. Nearly, 90% of the gold was dissolved in only 30 min, according to reaction 1 (Yen and Pindred 1989).
The leaching experiment was carried out in a 500-mL flask, installed inside a temperature-controlled water bath (IKA, Germany), raising the leach solution temperature to about 55 °C with an agitation rate of 500 rpm. The obtained 300 mL leach liquor with a gold concentration of 365 ppm was used for all ED experiments after dilution. The gold content of solutions was determined with atomic absorption spectroscopy (AAS, Varian 220, Australia).
Electrodialysis cell
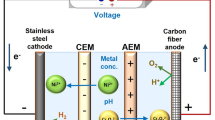
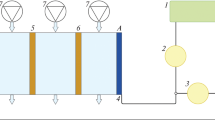
A three-compartment ED cell was developed and made to carry out the gold separation experiments. The cell diameters were 7.5 × 16 × 9 cm3, as shown in Fig. 1a. The dimensions of the cathodic and anodic solutions compartment were 5 × 12 × 1 cm3, and the feed solution compartment volume was 60 cm3.
The cathodic and anodic solutions were separated from the feed solution by the cathodic and anodic solid membranes (Fig. 1b). The solid cation and anion exchange membranes were Ionsep-AM-C and Ionsep-AM-A models, respectively (Ionsep, Zhejiang, China). These membranes were selected due to their high permselectivity, low electric resistance, broad pH range resistance and high mechanical strength (Table 1). The mixed metal oxide (MMO)-coated titanium plates (DSA® plate electrode, Ionsep, China) with a surface area of 5 × 12 cm2 were used as electrodes. The distance between the electrodes and their membrane was 1 cm. As shown in Fig. 1c, the spaces between the membranes (the deionized and the concentrated flow paths) were filled with inert (electrically non-conducting) net-like spacers, which mainly provided the stack with mechanical support and specified the geometry of the flow channel between the membranes. These spacers made of low-density polypropylene were placed between the membranes in the membrane stack to create independent flow branches. Using plate flow spacers increases the fluid flow, which can decrease the processing time. Another function of the spacer net is to make a turbulent flow in the solution. The solution flowing through the net-filled channel follows the so-called zig-zag flow, leading to increased mass transfer toward the membrane surface. Therefore, concentration polarization phenomena that might occur in the diffusion layer at the membrane surface were minimized since the fluid undergoes irregular fluctuations or mixing, in contrast to laminar flow, in which the fluid moves in smooth paths or layers. In addition, the precipitation of fine particles and ions can block the flow passes in the membrane surface. Using spacers reduces the blocking of flow passes (Tanaka 2015).
The solid membranes were soaked in water for 24 h before cell assembly. This action prevents the membranes from deforming, caused by its expansion after contact with the solution inside the cell. The direct electric current was supplied to the plane electrodes with a DC power supply (Tetaelectronic PS1203, Iran). Voltage was measured by a digital voltmeter. Semi-transparent silicone connection hoses and 4 containers were used for transmission and storing solutions. One-way pump and flow control valves were also used to circulate and control the cell flow rate.
Gold recovery experiments
The effect of three different parameters, including applied voltage, feed solution concentration and circulating solutions flow rate, were investigated on gold recovery. These factors were chosen because they are technically controllable on laboratory and industrial scales. The central composite design (CCD) method combined with response surface methodology (RSM) was employed to study the effect of the three mentioned parameters and statistical modeling of results. The response surface methodology is an optimization method determining the relationship between several variables with one or several response variables. CCD investigates the effect of parameters in 5 levels, including two factorial points (high level and low level), two axial points (plus and minus the alpha) and one central point (center of low and high levels) (Sina et al. 2022). The high and low levels for gold concentration in feed solution were 2 and 5 ppm, as actual values of the plating process wastewater effluents for voltage were 25 and 35 V and for flow rate were 0.24 and 0.48 L/min. The studied voltage and flow rate ranges were selected based on preliminary experiment results and previous studies (Kang et al. 2017; Korolev et al. 2018). The center point experiment was repeated 6 times to study the experiment’s repeatability and calculation of errors. The detailed experimental conditions are listed in Table 2.
1 L of the solution was fed into the cell compartments after preparing the feed solution by diluting the gold leach liquor to operate the experiments. The solution flow rate in the ED system for each experiment was adjusted based on the specific values in the design of the experiments. The specified voltage was applied after adjusting the flow rate, which is the beginning of the process. ED tests were continued for 4 h. The approximate time of experiments was selected based on preliminary experiments. The gold ion-free leaching solution was circulated as an electrolyte at the rear part of the cell, with the previously mentioned volume, to decrease the cell temperature. During this process, the solutions were constantly circulated inside the device (shown in Fig. 2) and passed through the desired membranes under the applied voltage.
The feed solution exchanged some of its ionic content in each cycle inside the cell in a batch process; therefore, the anodic and cathodic solutions get rich in ions. At the end of the process, when the feed solution attained a definite value, the solution was withdrawn from the cathode compartment and gold concentration was measured with atomic absorption spectroscopy (AAS). The results showed that the change in the volume of working solutions during the ED process was negligible. Also, the pH value of each compartment and current intensity changes was measured regularly during the experiments.
The gold recovery efficiency is calculated based on Eq. 2:
where C denotes the metal concentration (in g/l) in the cathode compartment, and C0 represents the feed solution's metal concentration (in g/l).
Results and discussion
Statistical modeling and process optimization
Electrodialysis is a membrane method to separate the ions in the aqueous environment. By using this method, selected ions (Au in our research) will migrate to the cathodic chamber, and a pure solution with a high concentration of gold ions (in the form of \(\left[ {{\text{AuCl}}_{4} } \right]^{ - }\) complexes) will be provided. This solution can then be used to produce pure gold with metal production methods, such as electrowinning or cementation.
Statistical analysis is an efficient technique to study parameters' effects and interactions. In addition, it can be used to predict a response based on investigated factors. Among statistical analysis methods, analysis of variance (ANOVA) is a robust tool for predicting a response based on independent variables. Design expert 7 (DX7) was employed for the statistical modeling of the obtained data. The following second-order polynomial (quadratic) equation was proposed based on ANOVA for predicting the gold recovery based on the investigated parameters and their interactions:
where RAu is a gold recovery (%), A is voltage (V), B is feed concentration (ppm), and C is flowrate (L/min). The statistical indexes for the model are listed in Table 3. With a p value lower than 0.05, the model was significant. The p value shows that there is only a 3.69% chance that the model occurs due to noise. Adequate precision is a statistical index that shows the ratio of the effect of investigated parameters (signal) to not-investigated parameters (noise) (Rezaei et al. 2022). The model’s adequate precision is 5.39, which shows that the selected parameters were highly effective in the process.
As mentioned, a test at the center points was repeated six times (Table 1) to study the repeatability and calculate the standard error. The population standard deviation for gold recoveries in repeated tests was 1.28%. The low quantity of standard deviation shows a high degree of experiment repeatability (Rafiee et al. 2021). The sample standard deviation was divided by the sample size’s square root (number of repeating tests) to calculate the standard error. The standard error of 0.52% also shows the high repeatability of experiments.
The optimal condition for the highest gold recovery was obtained at voltage 32.51 V, gold concentration of 4.1 ppm and flow rate of 0.32 L/min. This condition was suggested by Design Expert 7 software based on Eq. 3. According to the software calculations, the gold recovery reaches 96% at optimal conditions. A test was carried out at the optimal conditions to validate the optimization results. The validation experiment results showed that the gold recovery reached 94.9% at the actual test.
The kinetics of gold (III) transportation from the feed solution and metal accumulation in the cathodic solution is shown in Fig. 3. More than 94.5% of gold (III) was transported from the feed solution at optimal condition into the cathodic solution within 4 h of ED separation. It has been found that without electric field application, only 29% of gold (III) was transferred across the membrane from the feed solution.
Parameter’s influence
The effect of each parameter was comprehensively investigated. The ANOVA was employed for this purpose. ANOVA is a method that is mainly used to determine the critical parameters affecting the response by analyzing the total variance of the appropriate components and measuring the effect between each of them. The F-ratio represents the mean square error ratio to the residual error and is usually used to determine the effect of a parameter on the results. The higher the F-value, the greater the effect of that parameter. The lower the p value of a parameter, the greater the importance and impact of that parameter on the results (Ghassa et al. 2021). Therefore, in determining the recovery efficiency, the parameters such as applied electrical voltage, input current flow, and finally, the feed concentration were of operational importance, respectively.
The main reaction that takes place with the application of the electric current is the electrolysis of water during the ED process. Reactions for anode and cathode are shown in Eqs. 4 and 5.
As can be seen from Eqs. (2) and (3), while anode reactions produce acid, cathode reactions produce a base. The pH change was related to the anode and cathode reactions and the ionic composition change in the solutions (as shown in Fig. 4). A reasonable explanation for the increase of pH in the anodic solution could be the production of OH− ions in the cathode compartment. Also, the production of H+ ions in the anode compartment decreased the cathodic solution pH. The results showed that the process pH range does not affect membrane efficiency.
Voltage
The electrolyte solution continuously rotated in the vicinity of the electrodes to exchange the heat produced at the electrodes in the ED system. This solution does not contain valuable ions and has no role in the ion exchange process. However, the current flowing through the device was a function of the voltage applied by the rectifier, and either of these two factors can be considered. Due to safety issues, the ED unit’s operation at constant voltage (potentiostatic mode) was preferable to a constant current state (galvanostatic mode). During the ED process, the ions content and, consequently, the solution’s electrical conductivity in the feed region decrease. As a result, the electrical resistance of the ED unit increased. In the case of the galvanostatic mode, this causes an uncontrollable increase in voltage applied to the ED unit and, in some cases, causes damage to the electrical current source. Besides, the membrane stack heats up in many cases due to the heat generated by the solution’s resistance and impaired function. For the reasons mentioned above, the voltage was controlled by the rectifier to be constant during tests.
According to the ANOVA table (Table 3), the voltage had the lowest p value (0.0554) and highest F-value (4.7). As a result, this parameter had the highest effect on gold recovery with the ED technique, which means that the electric field represented the main driving force of the process. The applied electric field induced migration movement of all ions dissolved in the process solutions and contained in the membranes so that the cations move toward the cathode, while the anions move toward the anode.
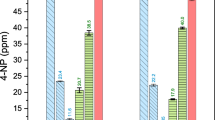
Figure 5 demonstrates the effect of changes in the electric voltage in the operating range of 25 to 35 V (at a flow rate of 0.36 L/min and a feed concentration of 3.5 ppm) on gold recovery. As can be seen, by increasing the electric current above 32 V, the process efficiency decreases. The reason for this can be considered as the thermal flow created by this increase in electrical voltage in the electrodes and its transfer to the whole system, thus reducing the permeability performance of ion-exchange membranes (Sadyrbaeva 2012; Tanaka 2015). Figure 5 clearly shows the importance of considering the heat flow generated in the electrodes of the ED system and shows the existence of a limitation in the use of the applied voltage in this system. It is always expected that due to the significant effect of the voltage on the ED process, increasing its value will improve the performance of the process, but increasing the voltage reduces the efficiency due to the effect on the system temperature as a harmful factor (Benneker et al. 2018), which is consistent for all scales due to the effects on the electrolyte and membrane efficiency (Vizserálek et al. 2014).
The current density changes during the test with optimum conditions are shown in Fig. 6. Regardless of the initial current (which depends on the applied voltage and, to some extent, the concentration of soluble ions), the general trend of changes in the passing current was decreasing. The current was reduced by passing of time due to an increase in the resistance of the medium resulting from the reduction in concentration of existing ions and increasing of the temperature of the solution (Strathmann 2010).
Gold concentration in the feed
The metal concentration is a controllable parameter in the pilot and industrial scales. As the ANOVA table shows, with the highest p value (0.2235) and lowest F-value (1.68), the gold concentration in feed solution had the lowest effect among all investigated parameters. The effect of gold concentration in feed solution (at a flow rate of 0.36 L/min and a voltage of 30 V) is depicted in Fig. 7. The gold recovery increased by increasing in gold concentration from 2 to 3.5 ppm. The gold recovery was in direct relation with Au concentration in solution because the current intensity at a constant voltage increased by increasing the ions concentration. Therefore, higher current intensity stimulates the movement of ions and reduces the resistance of the device.
It should be noted that the gold concentration range in this research was selected based on the actual values from industrial plating process wastewater effluents that were between 2 and 5 ppm (Sadyrbaeva 2012). Although gold concentration has little effect in this range, it may significantly affect the process in the higher ranges. Generally, the ED system shows inefficiency in dealing with a high concentration of ions available in feed solution due to precipitations that may form on the membrane surface when a high voltage is applied. This could occur due to the small effective area of the membrane in the laboratory-scale ED cell, which limits the initial concentration of metal ions. The highest amount of metallic ions in the feed solution reported in previous researches was around 450 ppm (Chan et al. 2022). However, the metal concentration in the current study was remarkably lower than the maximum capacity of the ED systems; therefore, this parameter has an insignificant effect on the process.
Flow rate
The effect of feed flow rate changes on gold recovery (at a feed concentration of 3.5 ppm and a voltage of 30 V) is shown in Fig. 8. As can be seen, by increasing the feed flow rate up to 0.32 L/min, the Au recovery increased. This occurred due to turbulence in the flow and reduced mass transfer boundary layer at the membrane surface. It reduced the thickness of the layer formed around the membrane, which increased the penetration of ions and, as a result, the efficiency of the process. On the other hand, the gold recovery decreased at flow rates higher than the optimum condition due to the drop in the solution residence time on the membrane's effective surface. Furthermore, unspecific flow geometry and consequently increment in the cell’s electrical resistance as a consequence of high flow rate explain recovery decreases (Długołęcki et al. 2010). Although increasing the flow rate increased the setup capacity, deterioration or rupture of the membrane surface could occur at high flow rates (Aytaç and Altın 2017).
Experiment with gold plating bath wastewater
The gold plating tanks and cables are covered by a layer of gold. This layer should be removed regularly to increase the plating process efficiency and avoid gold overuse. Cleaning gold plating baths with hypochlorite and chloride-based solutions is a standard method in the industry. An experiment was carried out with actual wastewater containing gold ions obtained from gold plating bath washing to evaluate the application of ED method on an industrial scale. The gold plating bath was washed with a reagent containing 2% sodium hypochlorite, 1% hydrochloric acid and 1 g/L sodium chloride. The wastewater was used to run an experiment at the optimum condition obtained from optimization tests (voltage = 32.51 V and a flow rate of 0.32 L/min). The metal concentration of the feed, cathodic, and anodic solution was measured after 3 h (Table 4).
As it is concluded from Table 4, the recovery of gold in the cathode compartment is approximately 91% which is 4% less than the gold recovery when using a pure gold solution. This decrease in recovery can be caused due to membrane fouling. Membrane fouling in almost all membrane processes is commonly caused by the precipitation of ions on the membrane surface or membrane pores. As can be seen in SEM images (Fig. 9), some particles were deposited on the surface of the anodic membrane. The energy-dispersive X-ray spectroscopy (EDS) indicated that deposited particles were made from iron. Despite its low concentration, iron is considered to be one of the most common inorganic pollutants that generate oxides and hydroxides, causing membrane fouling (Melliti et al. 2022, 2019). The X-ray diffraction analysis confirms the presence of iron oxide on the membrane surface in the form of goethite (Fig. 10).
Conclusion
In this research, a three-compartment electrodialysis (ED) cell was used for gold recovery from hypochlorite liquors. The effect of three different parameters, including the applied voltage, gold concentration in solution and flow rate, were investigated in gold recovery. The gold was effectively recovered during the potentiostatic mode in an optimum condition, including voltage 32.51 V, the gold concentration of 4.1 ppm and a flow rate of 0.32 L/min. The results indicated that voltage had the highest impact on gold recovery, while the Au concentration in feed solution had the most negligible impact. The gold extraction percent of 95% was achieved during 3 h, representing the electromembrane process used in this research as an appropriate technique for recovering and purifying the remaining gold in waste plating bath reagent. A test also was carried out with wastewater obtained from washing gold plating bath with hypochlorite and chloride-based solutions, at optimum conditions. The results indicated that this method could also be used to efficiently separate ions from industrial wastewater.
Availability of data and materials
Not applicable.
Code availability
Not applicable.
References
Afifah DN, Ariyanto T, Supranto S, Prasetyo I (2018) Separation of lithium ion from lithium-cobalt mixture using electrodialysis monovalent selective ion exchange membrane. Eng J 22:165–179. https://doi.org/10.4186/ej.2018.22.3.165
Asiya SI, Pal K, Kralj S, El-Sayyad GS, de Souza FG, Narayanan T (2020) Sustainable preparation of gold nanoparticles via green chemistry approach for biogenic applications. Mater Today Chem 17:100327. https://doi.org/10.1016/j.mtchem.2020.100327
Aytaç E, Altın S (2017) Influence of flow rate on the removal of copper, lead and nickel from solution in electrodialysis process. Int J Sci Technol 3:24–35. https://doi.org/10.20319/mijst.2017.33.2435
Babilas D, Dydo P (2018) Selective zinc recovery from electroplating wastewaters by electrodialysis enhanced with complex formation. Sep Purif Technol 192:419–428. https://doi.org/10.1016/j.seppur.2017.10.013
Bazrgar Bajestani M, Moheb A, Dinari M (2020) Preparation of lithium ion-selective cation exchange membrane for lithium recovery from sodium contaminated lithium bromide solution by electrodialysis process. Desalination 486:114476. https://doi.org/10.1016/j.desal.2020.114476
Benneker AM, Rijnaarts T, Lammertink RGH, Wood JA (2018) Effect of temperature gradients in (reverse) electrodialysis in the Ohmic regime. J Membr Sci 548:421–428. https://doi.org/10.1016/j.memsci.2017.11.029
Chan KH, Malik M, Azimi G (2022) Separation of lithium, nickel, manganese, and cobalt from waste lithium-ion batteries using electrodialysis. Resour Conserv Recycl 178:106076. https://doi.org/10.1016/j.resconrec.2021.106076
Chaudhary AJ, Donaldson JD, Grimes SM, Yasri NG (2000) Separation of nickel from cobalt using electrodialysis in the presence of EDTA. J Appl Electrochem 30:439–445. https://doi.org/10.1023/A:1003966132167
Długołęcki P, Ogonowski P, Metz SJ, Saakes M, Nijmeijer K, Wessling M (2010) On the resistances of membrane, diffusion boundary layer and double layer in ion exchange membrane transport. J Membr Sci 349:369–379. https://doi.org/10.1016/j.memsci.2009.11.069
Generous MM, Qasem NAA, Akbar UA, Zubair SM (2021) Techno-economic assessment of electrodialysis and reverse osmosis desalination plants. Sep Purif Technol 272:118875. https://doi.org/10.1016/j.seppur.2021.118875
Ghassa S, Farzanegan A, Gharabaghi M, Abdollahi H (2021) Iron scrap, a sustainable reducing agent for waste lithium ions batteries leaching: an environmentally friendly method to treating waste with waste. Resour Conserv Recycl 166:105348. https://doi.org/10.1016/j.resconrec.2020.105348
Gmar S, Chagnes A (2019) Hydrometallurgy recent advances on electrodialysis for the recovery of lithium from primary and secondary resources. Hydrometallurgy 189:105124. https://doi.org/10.1016/j.hydromet.2019.105124
Golubenko D, Yaroslavtsev A (2020) Development of surface-sulfonated graft anion-exchange membranes with monovalent ion selectivity and antifouling properties for electromembrane processes. J Membr Sci 612:118408. https://doi.org/10.1016/j.memsci.2020.118408
Habashi F (2005) Gold—an historical introduction. In: Adams MD, Wills BA (eds) Advances in gold ore processing. Elsevier, Amsterdam, pp xxv–xlvii. https://doi.org/10.1016/S0167-4528(05)15045-5
Han J-H, Hwang K, Jeong H, Byeon S-Y, Nam J-Y, Kim C-S, Kim H, Yang S, Choi JY, Jeong N (2019) Electrode system for large-scale reverse electrodialysis: water electrolysis, bubble resistance, and inorganic scaling. J Appl Electrochem 49:517–528. https://doi.org/10.1007/s10800-019-01303-4
Hannapel J, Richter C (2004) Report from Washington: surface finishing challenges and opportunities—a look back and a preview for 2004. Met Finish 102:16–20. https://doi.org/10.1016/S0026-0576(04)90003-7
Heights B (1983) United States patent (19) 11
Johnson CA (2015) The fate of cyanide in leach wastes at gold mines: an environmental perspective. Appl Geochemistry 57:194–205. https://doi.org/10.1016/j.apgeochem.2014.05.023
Kang H, Kim E, Jung SP (2017) Influence of flowrates to a reverse electro-dialysis (RED) stack on performance and electrochemistry of a microbial reverse electrodialysis cell (MRC). Int J Hydrog Energy 42:27685–27692. https://doi.org/10.1016/j.ijhydene.2017.06.187
Kavitha E, Poonguzhali E, Nanditha D, Kapoor A, Arthanareeswaran G, Prabhakar S (2022) Current status and future prospects of membrane separation processes for value recovery from wastewater. Chemosphere 291:132690. https://doi.org/10.1016/j.chemosphere.2021.132690
Korolev I, Altınkaya P, Halli P, Hannula P, Yliniemi K, Lundström M (2018) Electrochemical recovery of minor concentrations of gold from cyanide-free cupric chloride leaching solutions. J Clean Prod. https://doi.org/10.1016/j.jclepro.2018.03.177
Lambrechts IA, Lall N (2021) Traditional usage and biological activity of Plectranthus madagascariensis and its varieties: a review. J Ethnopharmacol 269:113663. https://doi.org/10.1016/j.jep.2020.113663
Melliti E, Touati K, Abidi H, Elfil H (2019) Iron fouling prevention and membrane cleaning during reverse osmosis process. Int J Environ Sci Technol 16:3809–3818. https://doi.org/10.1007/s13762-018-1899-0
Melliti E, Van der Bruggen B, Elfil H (2022) Combined iron oxides and gypsum fouling of reverse osmosis membranes during desalination process. J Membr Sci 653:120472. https://doi.org/10.1016/j.memsci.2022.120472
Meng Q, Li G, Kang H, Yan X, Wang H, Xu D (2020) A study of the electrodeposition of gold process in iodine leaching solution. Metals (basel). https://doi.org/10.3390/met10010050
Mubita T, Porada S, Aerts P, van der Wal A (2020) Heterogeneous anion exchange membranes with nitrate selectivity and low electrical resistance. J Membr Sci 607:118000. https://doi.org/10.1016/j.memsci.2020.118000
Rafiee P, Ghassa S, Moosakazemi F, Khosravi R, Siavoshi H (2021) Recovery of a critical metal from electronic wastes: germanium extraction with organic acid. J Clean Prod 315:128223. https://doi.org/10.1016/j.jclepro.2021.128223
Reid FH (1973) Gold plating in the electronics industry—a review of the fourth symposium of the American Electroplaters’ Society. Gold Bull 6:77–81. https://doi.org/10.1007/BF03215011
Rezaei H, Ziaedin Shafaei S, Abdollahi H, Shahidi A, Ghassa S (2022) A sustainable method for germanium, vanadium and lithium extraction from coal fly ash: sodium salts roasting and organic acids leaching. Fuel 312:122844. https://doi.org/10.1016/j.fuel.2021.122844
Sadyrbaeva TZ (2012) Gold(III) recovery from non-toxic electrolytes using hybrid electrodialysis-electrolysis process. Sep Purif Technol 86:262–265. https://doi.org/10.1016/j.seppur.2011.10.007
Seyedhakimi A, Bastami SA, Ghassa S, RazaviChehreh Chelgani HS (2018) Exploring relationships between various activations of granular activated carbon on silver and gold adsorption: a kinetic and equilibrium study. Sep Sci Technol 56:1–12. https://doi.org/10.1080/01496395.2018.1540635
Shacham-Diamand Y, Osaka T, Okinaka Y, Sugiyama A, Dubin V (2015) 30years of electroless plating for semiconductor and polymer micro-systems. Microelectron Eng 132:35–45. https://doi.org/10.1016/j.mee.2014.09.003
Sina G, Mohammad N, Ziaedin SS, Hadi A, Fariborz G, Sara M (2022) Optimization of pyrite bio-oxidation to produce ferric reagent for sphalerite leaching. J Hazard Toxic Radioact Waste 26:4021035. https://doi.org/10.1061/(ASCE)HZ.2153-5515.0000644
Song Y, Zhao Z (2018) Separation and purification technology recovery of lithium from spent lithium-ion batteries using precipitation and electrodialysis techniques. Sep Purif Technol 206:335–342. https://doi.org/10.1016/j.seppur.2018.06.022
Sousa R, Regufe MJ, Fiúza A, Leite MM, Futuro A (2021) A systematic review of sustainable gold extraction from raw ores using alternative leaching reagents. Extr Ind Soc. https://doi.org/10.1016/j.exis.2021.101018
Strathmann H (2010) Electrodialysis, a mature technology with a multitude of new applications. DES 264:268–288. https://doi.org/10.1016/j.desal.2010.04.069
Tanaka Y (2015) Ion exchange membranes: fundamentals and applications. Elsevier, Amsterdam
Tonner JB, Tonner J (2004) Desalination and energy use. In: Cleveland CJBT-E of E (ed). Elsevier, New York, pp 791–799. https://doi.org/10.1016/B0-12-176480-X/00403-4
Van Grieken R, Aguado J, López-Muñoz MJ, Marugán J (2005) Photocatalytic gold recovery from spent cyanide plating bath solutions. Gold Bull 38:180–187. https://doi.org/10.1007/BF03215258
Vineyard D, Hicks A, Karthikeyan KG, Barak P (2020) Economic analysis of electrodialysis, denitrification, and anammox for nitrogen removal in municipal wastewater treatment. J Clean Prod 262:121145. https://doi.org/10.1016/j.jclepro.2020.121145
Vizserálek G, Balogh T, Takács-Novák K, Sinkó B (2014) PAMPA study of the temperature effect on permeability. Eur J Pharm Sci 53:45–49. https://doi.org/10.1016/j.ejps.2013.12.008
Wang W, Liu R, Tan M, Sun H, Niu QJ, Xu T, Nikonenko V, Zhang Y (2019) Evaluation of the ideal selectivity and the performance of selectrodialysis by using TFC ion exchange membranes. J Membr Sci 582:236–245. https://doi.org/10.1016/j.memsci.2019.04.007
Wilkinson P (1986) Understanding gold plating. Gold Bull 19:75–81. https://doi.org/10.1007/BF03214646
Yen WT, Pindred RA (1989) Gold and silver extraction with non-cyanide reagents from a refractory complex sulphide ore. In: Processing of complex ores. The Canadian Institute of Mining and Metallurgy. https://doi.org/10.1016/b978-0-08-037283-9.50042-1
Zafari M, Kikhavani T, Ashrafizadeh SN (2022) Hybrid surface modification of an anion exchange membrane for selective separation of monovalent anions in the electrodialysis process. J Environ Chem Eng 10:107014. https://doi.org/10.1016/j.jece.2021.107014
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
HR was involved in methodology, investigation, resources, writing—original draft; HA contributed to supervision, conceptualization, writing-review and editing; and SG was involved in formal analysis, writing—original draft.
Corresponding author
Ethics declarations
Conflict of interest
There are not any conflicts of interest.
Additional information
Editorial responsibility: Samareh Mirkia.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rezaei, H., Abdollahi, H. & Ghassa, S. Recovery of gold ions from wastewater using a three-compartment electrodialysis separation system. Int. J. Environ. Sci. Technol. 20, 4827–4838 (2023). https://doi.org/10.1007/s13762-023-04870-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-023-04870-4