Abstract
Nickel determination is important because of its use in many industrial areas and its negative effects on human health. In this study, an ultraviolet-based photochemical vapor generation (UV-PVG) setup was combined with a T-shaped zirconium-coated slotted quartz tube-atom trapping (T-SQT-AT) apparatus to boost the sensitivity of a flame atomic absorption spectrophotometer for nickel determination. Nickel was separated from the sample matrix by converting it into its volatile species prior to online preconcentration by trapping on the zirconium-coated T-SQT inner surface. Analytical performance was maximized by optimizing all variable conditions. The limit of detection (LOD) and limit of quantification (LOQ) were found as 10 and 33 µg/L, respectively. Daphne tea and lake water samples were analyzed under optimum conditions, and there was no detectable nickel in the samples. For this purpose, spiking experiments were carried out for the samples in order to evaluate the applicability and accuracy of the method. The percent recovery values calculated for the two samples spiked at three different concentrations ranged between 90 and 112%. To our best knowledge, this is the first study in literature where UV-PVG was combined with T-SQT-AT for the determination of nickel in daphne tea and lake water samples prior to FAAS determination.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nickel is a transition metal on periodic table, and it is found in the ecosystem as the 24nd most abundant element (Prueitt & Goodman, 2015). This element is used in various industrial applications with a rising demand. It is widely utilized in nickel–cadmium batteries, jewelry, ceramics, and dyes and used as a catalyst for certain reactions (Gad, 2014). Nickel has several compounds having different health effects (Wallace, 1973). Thus, accurate determination of nickel in environmental samples is crucial for the protection of public health. Nickel is vital for plants in the concentration range of 0.01–5 µg/g dry wt. Meanwhile, overexposure of nickel may become toxic to the plants as with other heavy metals (Seregin & Kozhevnikova, 2006). For instance, tea drinks contaminated with nickel can be dangerous for human bodies. One study conducted by (Zhong et al., 2016) investigated different types of tea samples and nickel concentration were ranged between 2.70 and 13.41 mg/kg (Zhong et al., 2016). For this reason, daphne tea was selected in this study to investigate for its nickel content.
Generally, metals are determined using instruments such as inductively coupled plasma-mass spectrometry (ICP-MS) (Kulomäki et al., 2019), inductively coupled plasma-optical emission spectrometry (ICP-OES) (Gondal et al., 2020), electrothermal atomic absorption spectrometry (ETAAS) (López-García et al., 2017), flame atomic absorption spectrometry (FAAS) (Reclo et al., 2017), and atomic fluorescence spectrometry (AFS) (de Santana et al., 2016). FAAS has been widely employed in the determination of metals, using acetylene/air flame as atomizer (Soylak & Koksal, 2019). Despite the fact that it is a simple and rapid system, it is insufficient for trace level determinations relative to the more advanced instruments such as ICP-MS and ICP-OES (López-García & Hernández-Córdoba, 2015). FAAS systems have very low sample introduction efficiencies of about 5% (Welz & Sperling, 1998), but their low hardware and operation costs make them suitable for routine laboratory analyses (Fernández et al., 2019). Thus, FAAS systems have been combined with several systems such as online slotted quartz tube (SQT), slotted quartz tube-atom trapping (SQT-AT) (Ataman, 2008), hydride generation (HG) (Asiabi et al., 2016), photochemical vapor generation (PVG) (Mollo & Knochen, 2020), and offline preconcentration methods (various microextraction methods) (Iraji et al., 2012; Reclo et al., 2017; Seidi & Alavi, 2019; Sixto et al., 2019) to enhance their detection power. Moreover, the PVG when combined with the AAS system can increase the analyte’s signal-to-noise ratio (He et al., 2007). PVG systems are composed of a photoreactor, gas-liquid separator, and peristaltic pump (De Jesus et al., 2016). This system is based on the formation of radicals by irradiating organic acids having low molecular weight such as formic, acetic, and propionic acids with UV radiation. The radicals formed, in turn, attack analytes to form their volatile species (Giersz et al., 2017). PVG is a trending new approach among the chemical vapor generation methods such as hydride generation. Unlike HG, no toxic and expensive chemicals such as sodium borohydride are used in PVG, and it can be applied to several of the transition elements (Sturgeon, 2017).
In the literature, there are several studies for the determination of nickel by PVG. For example, PVG was combined with GF-AAS. Volatile species of nickel were trapped on the inner surface of the graphite tube after the formation of volatile species with formic acid under the UV irradiation (Zheng et al. 2009). In another study, PVG was integrated with ICP-MS to determine nickel. Nickel determination was acquired in alcoholic beverage, and the LOD value was presented as 0.1 µg/L (de Quadros & Borges, 2014).
The SQT was introduced by Watling as a means to enhance detection power of FAAS (Watling, 1977). Tubes produced from silica, graphite, stainless steel, and quartz are widely used in the literature (Matusiewicz, 1997). SQT is utilized based on two principles to enhance the sensitivity of FAAS systems. It can prolong the retention period of atoms in the light path of a hollow cathode lamp to increase atomic absorbance (Watling, 1978). It can also be used as an atom trapping surface for online preconcentration purposes. After the adsorption/trapping of metals atoms for an optimum period, they are re-volatilized by using a reducing agent such as methyl isobutyl ketone and methyl ethyl ketone to obtain narrow and transient signals (Ataman, 2008). In order to increase adsorption capacity of an SQT, the inner surface can be coated with high melting point materials such as molybdenum, tantalum, gold, zirconium, and osmium (Demirtaş et al., 2015).
The aim of this study was to determine nickel in lake water and daphne tea samples utilizing an FAAS system combined with the PVG-T-shaped SQT-AT. All variable parameters of the system were optimized by the univariate approach, and system analytical performance was evaluated under the optimum conditions.
Materials and methods
Reagents
The chemicals used throughout the study were as follows: formic acid, acetic acid, propionic acid, nitric acid, hydrochloric acid, potassium nitrate, sodium chloride, molybdenum(VI) oxide, zirconium(IV) chloride, and niobium(V) chloride. The chemicals were purchased from Merck (Germany), and all were of analytical grade. Nickel standard solution (1.00 g/L) was bought from High Purity Standards (USA) and used to prepare all intermediate and working solutions by diluting acidified nickel standard solution with distilled water.
In order to ensure that all glass apparatus were free from organic and inorganic contaminants, they were washed with detergent, rinsed, and soaked in a nitric acid bath (2.0% v/v) overnight.
Instrumentation
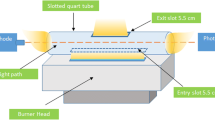
An atomic absorption spectrophotometer (ATI UNICAM 929 AA, England) was used for the detection of nickel at the 232.0 nm analytical line and 0.5 nm spectral bandpass. As radiation source, a nickel hollow cathode lamp (Varian) was used with 15.0 mA as operation current. Background correction was achieved by using a deuterium lamp (D2). Acetylene and air gas were used for generation of flame. Argon gas was used for transfer of the analytes into the atomizer unit, and hydrogen gas was also utilized as a reducing agent. The UV-PVG-T-SQT-AT system (Fig. 1) is comprised of a peristaltic pump (MiniPuls3, Gilson, USA), custom-made UV photoreactor, gas-liquid separator, ultrasonication bath, and T-shaped slotted quartz tube. The UV photoreactor and gas-liquid separator were built with unique designs in our laboratory, and further information can be found in the project completed (Bakırdere, 2020). The UV photoreactor that consists of quartz tube and UV radiation source (Sylvania 16W, UK) was covered with polypropylene tube. The quartz tube having eight equal sides was combined with zigzag pattern, and UV lamp was placed middle of these tubes. The GLS was designed in a cylindrical tube. Small spherical-shaped glass spheres were added inside the GLS for increasing the surface area where the sample/standard solutions pass. In addition, the GLS was placed into an ultrasonic bath to increase the separation efficiency of volatile analyte species from liquid part. The T-shaped quartz tube with 14.0-cm length, 1.8-cm outer diameter, and 1.0-mm wall thickness was used as an atom trapping surface. A 6.0-cm long and 2.0-mm wide entrance slot was cut at the bottom of the tube to allow complete entry of the acetylene-air flame into the tube when placed on the burner. In addition, eight circular holes with 2.0 mm diameter were drilled at the top part of the tube to lower the flame velocity in order to prolong the retention time of analyte species inside the tube. Furthermore, the inner surface of the quartz tube was coated with high melting point zirconium to increase trapping capacity. In this study, three different coating metals, niobium, molybdenum, and zirconium were tested for their trapping efficiency for nickel. Four identical T-SQTs were employed, where one was left bare, and the others were covered with the three different metals. In the coating process, solutions of the three metals having approximately 100 mg/L were prepared in separate tubes. The T-SQTs were placed on a fuel-lean flame, and the coating solutions were sent to the system via a nebulizer for 10 min. During this period, the quartz windows at both sides of the burner were covered with aluminum foil to prevent them from being coated with the metals.
Schematic representation of UV-PVG-T-SQT-AT-FAAS system (Bakırdere, 2020)
Procedure
Samples were acidified with 10 M propionic acid prior to analysis. The sample solutions were sent to the UV photoreactor using a peristaltic pump at a constant flow rate, which determined the time period that the samples entered and exited the reactor, equivalent to irradiation period. Liquid samples containing volatile nickel compounds were pumped into the gas-liquid separator in a dropwise manner. With the help of a sonicator, volatile compounds were separated from the liquid matrix and transferred into the quartz tube by argon gas at optimum flow rate and trapped on the inner surface of the tube under a fuel-lean flame medium. After 1.0-min trapping period, hydrogen gas set to optimum flow rate was sent to the system by opening the valve for simultaneous re-volatilization of trapped atoms to obtain narrow and transient absorbance signals. The total period of one analysis was approximately 4.5 min. Schematic representation of UV-PVG-T-SQT-AT-FAAS system is given in Fig. 1.
Safety considerations
International Agency for Research on Cancer (IARC) classifies nickel/nickel compounds as group 1 carcinogens for humans and animals. It was reported that adsorption of this element into the human body is higher than the other metals. Therefore, this metal can cause an allergic reaction to the skin. Nickel carbonyl has potential cancerogenic effects with serious damage to living cell. Moreover, this compound of nickel leads to acute poisoning (Barceloux, 1999). Hence, throughout the study, a fume hood was placed on the UV-PVG system and the burner head of FAAS system to remove harmful volatile species in order to protect the analyst.
Furthermore, output pressure of hydrogen gas was fixed to 5.0 bar, and hydrogen gas was sent to system using an adjustable valve for 1 s one valve in order to create small bursts. All experiments were performed under fume hood, and all precautions were taken to eliminate the possible risks.
Sample preparation
Daphne tea and lake water samples were used to validate accuracy and applicability of the developed method. Dried daphne tea samples were purchased from a local market (İstanbul, Turkey). Lake water samples were collected from Dam Lake (Kayseri, Turkey). The daphne tea sample (2.50 g) was weighed into a volumetric flask and completed to 250 mL with boiled deionized water. The volumetric flask was covered with watch glass. Then, this mixture was brewed for 10 min, just as it is prepared for tea consumption. The brewed sample solution (250 mL) was allowed to cool down to room temperature and then filtered before the ten times dilution for analysis. Likewise, the lake water sample was first filtered to remove solid particles and then diluted ten times with deionized water.
Results and discussion
Optimization of atom trapping parameters
Experimental conditions that affect the atom trapping process were optimized by the univariate method. Optimum parameters of the photochemical vapor generation and separation efficiency for nickel were taken from our previous study (Büyükpınar et al., 2020). Triplicate measurements were performed throughout the study, and analytical performance of the developed method was evaluated under optimum conditions.
Flame conditions
Volatile derivatives of nickel produced inside the UV photoreactor were separated from the liquid solution in a GLS and transferred to the T-SQT attached atomizer unit for adsorption onto the inner quartz surface. Due to the fact that atomization occurs at high temperatures, the attached quartz tubes are heated electrically or with the help of acetylene-air flame. The latter is the most suitable option for atom trapping studies because different flame conditions can be performed for both adsorption and re-volatilization processes. By adjusting the proportions of oxidant and fuel gases, the chemical character and temperature of the flame can be altered. The flame used in trap studies must provide the following conditions: (i) the flame temperature must be kept high enough to break down molecules containing the analyte atom, (ii) it must provide a reducing environment so that the released analyte atoms do not form their oxides, and (iii) it must be kept at a temperature low enough to increase the amount of atoms adsorbing onto the quartz surface (Ataman, 2008).
In order to modify the flame environment, the flow rate of acetylene was adjusted to various values, while keeping the airflow rate constant. Acetylene:air ratios of 0.20, 0.26, 0.42, and 0.53 were tested. The lowest absorbance value was observed for the 0.20 ratio, while the 0.26 ratio recorded the highest absorbance and was selected as optimum acetylene:air ratio.
The characteristic of the flame inside the quartz tube is also affected by the distance between the tube and the point where the flame forms on the burner. It was observed that the velocity of the flame in the tube decreased as the tube was moved away from the burner. Prolonged retention of the analyte derivatives is known to increase the amount of trapped species, and thus, the distance of SQT from the burner head was optimized. After performing experiments in the range of 0.0–3.0 mm with 1.0 mm intervals, 1.0 mm recorded the highest absorbance values was chosen as the optimum distance.
Argon, which is used in PVG studies to separate volatile derivatives from the liquid matrix and transfer them to the atomizer has an impact on the flame composition, rate of analyte transfer, and temperature in the quartz tube. Optimization of the carrier gas flow rate is therefore a very important parameter that affects the efficiency of trapping. In this optimization stage, flow rates lower than 1700 mL/min could not be obtained, and at flow rates higher than 2850 mL/min, liquid was forcefully carried from the GLS into the quartz tube. The highest absorbance values were recorded for the flow rate of 1700 mL/min, and a 60% reduction in absorbance occurred when the rate was increased to 2100 mL/min. This can be attributed to three reasons: increasing the amount of argon per unit time causes dilution of the transported volatile nickel derivatives, causes rapid exit of atoms from the flame, and decreases atomization efficiency as a result of reduced flame temperature. In the batch-type PVG-AAS system, a linear relationship was observed between the argon gas flow rate and the absorbance signals because the volatile derivatives must be transported to the atomizer collectively (Büyükpınar et al., 2020). On the contrary, in trapping studies, keeping the flow rate of the carrier gas as slow as possible ensures that volatile species are transferred at a moderate rate to adsorb properly onto the inner surface of the quartz tube.
Trapping conditions
The main purpose of a trapping system is to collect analytes on the SQT surface for a specified period to increase their concentrations and release them instantaneously to obtain higher absorbance signals relative to the conventional aspiration method. In this way, even samples with concentrations below the instrument’s detection limit can be easily determined. In the T-SQT-AT system (Bodur et al., 2019), trapping of analytes takes place on the inner surface of the quartz tube. During sampling, analyte derivatives accumulate on this surface, and their quantity increase with time. Therefore, a linear correlation is seen between the sampling period and the absorbance signal obtained. Since the tube has a limited surface area, the surface is completely covered after a while, and this linear relation reaches a plateau. Further sampling after this point only prolongs analysis time and increases consumption of the sample.
To determine the optimum trapping period for Ni in this study, periods in the range of 60–240 s with 60 s intervals were tested, and 120 s was selected as the optimum period for recording the highest absorbance value for an uncoated SQT.
Coating the quartz surface is a simple process that is performed to increase its trapping capacity for analytes. This procedure was first applied by Brown and Milner to prevent the vitrification of SQTs, increase their lifetime, and enhance trapping efficiency for several elements (Brown et al., 1985). Metals to be used for coating must have higher melting points than the boiling point of the analyte under study. This prevents the coating material from separating from the surface of the tube together with the analyte during the release step. Zr, Mo, and Ti meet this requirement and are commonly used as coating materials (Demirtaş et al., 2015).
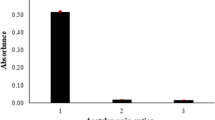
All three coating elements (Zr, Mo, and Nb) increased the trapping capacity of the T-SQT. Compared to bare T-SQT, Mo- and Nb-coated T-SQT provided approximately 146% and 115% enhancement in peak intensity, respectively, while about 175% increase in absorbance value was observed for the Zr-coated T-SQT. In addition, a single analyte signal was achieved with Zr-coated T-SQT while multiple signals were obtained with bare T-SQT when reducing agent was sequentially sent to the system. The signals belonging to Zr-coated T-SQT and bare T-SQT are given in Fig. 2.
In order to investigate the effect of enhanced trapping surface over the spontaneous release problem encountered with the bare T-SQT, the trapping period optimization process was repeated for the period range of 30–240 s. For the coated SQT, 60 s emerged as the optimum trapping period compared to 120 s for the bare SQT. This suggests that there was greater interaction and adsorption onto the quartz surface of the coated SQT within a short time period.
Re-volatilization step
Analyte derivatives preconcentrated on the inner surface of the quartz tube are released and atomized as the last step of the method. To achieve this goal, two different strategies have been proposed in the literature: fuel alteration and organic solvent aspiration (Korkmaz et al., 2002). In the fuel alteration process, a reducing flame is created by increasing the fuel ratio in the flame composition after sampling. The other strategy involves sending an organic solvent to the flame via the nebulizer unit to form a pulse of reducing flame. In the system proposed by Uslu et al., hydrogen gas was sent directly to the flame from the T-junction on the SQT in short bursts (Uslu et al., 2018). The use of hydrogen gas is more responsive and instant in a manner of changing the flame medium compared to the other two strategies, thus making the signal width narrower. The shortened time for a complete release of the trapped analytes leads to increased peak height. In this study, experiments were conducted with different hydrogen gas flow rates in the range of 440–2300 mL/min, and the highest absorbance values were recorded at 440 mL/min. A decrease in peak height as well as an increase in background (BG) signal was observed for the higher flow rates of hydrogen gas due to unstable flame environment for atomization and absorbance measurement.
Analytical figures of merit
Analytical performance of the PVG-T-SQT-AT-FAAS for the determination of nickel was investigated under the optimum experimental conditions given in Table 1. A series of standard nickel solutions acidified with 10.0 M of propionic acid were prepared and analyzed with triplicate measurements. A linear correlation between nickel concentration and signal height was observed with good linearity (R2 = 0.9992).
Detection and quantification limits (LOD and LOQ) of the developed system were calculated using the expressions 3 × S/m and 10 × S/m, respectively, where S is the standard deviation for 5 consecutive measurements of acidified nickel standard solution, and m is the slope of the calibration plot. All analytical figures of merit are summarized in Table 2. A 91-fold enhancement was achieved by the developed method by comparing its LOD value to the LOD value of the conventional FAAS system. Figures of merit reported for other methods in the literature are also presented in Table 2, and it can be clearly seen that they are comparable with the figures obtained in this study. In our previous study, a closed quartz atomization cell was used to provide atomization of volatile nickel species (Büyükpınar et al., 2020) with temperature controlling system in Analytic Jena NovAA 300 system having the detection limit of 147 µg/L. In this study, due to the problem in that instrument, different FAAS having LOD of 911 µg/L was used with an opened T-SQT allowing us to release the trapped analyte after adding the hydrogen gas. It is clear that enhancement in detection power in this study is about 91 (based on LOD comparison) while this value was 42 in our previous study. It is clear that trap has advantages in the enhancement of the detection power. On the other hand, the trapping strategy used in this study allowed us to separate the analyte species from other volatile interfering compounds by trapping the analyte species on the inner surface of the quartz tube.
In literature, cloud point extraction-flame atomic absorption spectrometry (CPE-FAAS) (Galbeiro et al., 2014) and room temperature ionic liquid-liquid extraction-flow injection-flame atomic absorption spectrometry (RTIL-LLE-FI-FAAS) (Dadfarnia et al., 2010) methods have been presented for the determination of nickel at trace level, but these methods possess tedious experimental steps and also highly toxic chemicals usage. On the other hand, the developed analytical method provided the opportunity to qualify/quantify µg/L levels of nickel with the green procedure. In addition, the PVG-atom trap proposed with several modifications in this study can be coupled with higher sensitive instruments such as ICP-OES, GF-AAS, and ICP-MS to lower the detection limits.
Interference experiments
The PVG involves a series of reactions between several ions and radicals; therefore, it is assumed that foreign ions present in the medium may interfere with the process. According to some studies in the literature, nickel volatile species are repressed by anionic interferents (Zheng et al., 2009). Since solutions that contain only anions do not reflect real samples, the interference effects on the method were investigated using in a medium that contained both cations and anions. Hence, sodium, potassium, magnesium, calcium, iron, selenium, and lead were added as cations, while chloride and nitrate were added as anions to represent the most abundant ions in environmental samples. The experiments were carried out with 1:1 and 1:10 molar ratio of acidified nickel solution to interferent anions/cations. The concentration of nickel in the samples was set at 3.4 × 10-3 mM and spiked with interferent mixed solution. Another nickel solution without interference was used for comparison. It was observed that the presence of ions at the same molar concentration as nickel caused about 13.7% decrease in absorbance signals. Furthermore, no absorbance signal was observed in the case with excess interferent ions. According to these data, the applicability of the method becomes difficult, especially in a complex matrix with low nickel content. This observation can be explained as a result of four different effects: (i) inhibition of photochemical vapor generation reactions, as studies in the literature have established; (ii) competitive reactions from other elements that produce more efficient volatile derivatives than nickel; (iii) coating of the quartz surface with possible volatile derivatives of other elements; and (iv) synergies of these effects.
Recovery studies
Accuracy of the developed method was tested by carrying out a series of analyses with real sample matrices. The developed method was applied to daphne tea and lake water samples, which were prepared according to the procedures given in the section Real Samples. Before the preparation of matrix matching calibration standards, blank analyses for the samples were carried out to confirm that nickel levels were below the detection limit, and no analytical signals were observed. Matrix matching calibration strategy was applied to eliminate possible matrix effects as indicated by the interference studies. Recovery results calculated via external calibration (Table 3) showed that there were negative and positive matrix effects on the analyte arising from lake water and daphne tea matrices, respectively. Therefore, standards were prepared in analyte-free real samples at five different concentration (75–250 µg/L for lake water and 150–350 µg/L for daphne tea), and the samples were introduced to the developed system. Then, matrix matching calibration was drawn via data obtained from the matrix matching calibration standards, and recovery results were calculated via matrix matching calibration. Different lake water and daphne tea samples after blank measurement were used to obtain matrix matching calibration. Daphne tea and lake water samples were spiked to final nickel concentrations of 200, 250, and 300 µg/L and 100, 150, and 200 µg/L, respectively. In addition, two sets of calibration standard solutions were prepared using each sample’s matrix, and the linear equations from the calibration plots were used to calculate the concentration and percent recovery of nickel. All results obtained from the recovery experiments are given in Table 3.
Conclusions
In the present study, UV-PVG-T-SQT-AT-FAAS was developed by combining photochemical vapor generation and atom trapping systems to increase the analytical performance of the conventional FAAS system for nickel detection in two sample matrices. LOD and LOQ values were found to be 10 and 33 µg/L, respectively. The enhancement factor was calculated as 91-folds by comparing the LOD of the developed method to that of the FAAS system. A very significant interference effect on nickel determination by the developed method was observed in the presence of various cations and anions. However, these effects can be overcome by using the matrix matching calibration method. Thus, spike recovery experiments for nickel in lake water and daphne tea samples produced percent recovery results in the range of 90–112%.
Data availability
The data sets generated during the current study are available from the corresponding author on reasonable request.
References
Asiabi, H., Yamini, Y., Seidi, S., Shamsayei, M., Safari, M., & Rezaei, F. (2016). On-line electrochemically controlled in-tube solid phase microextraction of inorganic selenium followed by hydride generation atomic absorption spectrometry. Analytica Chimica Acta, 922, 37–47. https://doi.org/10.1016/j.aca.2016.04.001
Ataman, O. Y. (2008). Vapor generation and atom traps: atomic absorption spectrometry at the ng/L level. Spectrochimica Acta - Part B Atomic Spectroscopy, 63(8), 825–834. https://doi.org/10.1016/j.sab.2008.03.013
Bakırdere, S. (2020). Project Report of "Determination of Pb, Cd, Ni, Co at trace levels by ultraviolet photochemical volatile compounds generation-atom trap-flame atomic absorption spectrometry system", The Scientific and Technological Research Council of Turkey (TÜBİTAK), Project Number: 117Z380
Barceloux, D. G. (1999). Nickel. Journal of Toxicology - Clinical Toxicology, 37(2), 239–258. https://doi.org/10.1081/CLT-100102423
Bodur, S., Erarpat, S., Selali Chormey, D., Büyükpınar, Ç., & Bakırdere, S. (2019). Determination of bismuth in bottled and mineral water samples at trace levels by T-shaped slotted quartz tube-atom trap-flame atomic absorption spectrometry. Analytical Letters. https://doi.org/10.1080/00032719.2018.1477790
Brown, A. A., Milner, B. A., & Taylor, A. (1985). Use of a slotted quartz tube to enhance the sensitivity of conventional flame atomic-absorption spectrometry. The Analyst, 110(5), 501–505. https://doi.org/10.1039/AN9851000501
Büyükpınar, Ç., San, N., Komesli, O. T., & Bakırdere, S. (2020). Combination of an efficient photochemical vapor generation system and flame atomic absorption spectrophotometry for trace nickel determination in wastewater samples. Analytical Letters. https://doi.org/10.1080/00032719.2020.1805749
Dadfarnia, S., Haji Shabani, A. M., Shirani Bidabadi, M., & Jafari, A. A. (2010). A novel ionic liquid/micro-volume back extraction procedure combined with flame atomic absorption spectrometry for determination of trace nickel in samples of nutritional interest. Journal of Hazardous Materials, 173(1–3), 534–538. https://doi.org/10.1016/j.jhazmat.2009.08.118
De Jesus, H. C., Grinberg, P., & Sturgeon, R. E. (2016). System optimization for determination of cobalt in biological samples by ICP-OES using photochemical vapor generation. Journal of Analytical Atomic Spectrometry, 31(8), 1590–1604. https://doi.org/10.1039/c6ja00069j
de Quadros, D. P. C., & Borges, D. L. G. (2014). Direct analysis of alcoholic beverages for the determination of cobalt, nickel and tellurium by inductively coupled plasma mass spectrometry following photochemical vapor generation. Microchemical Journal, 116, 244–248. https://doi.org/10.1016/j.microc.2014.04.015
de Santana, F. A., Portugal, L. A., Serra, A. M., Ferrer, L., Cerdà, V., & Ferreira, S. L. C. (2016). Development of a MSFIA system for sequential determination of antimony, arsenic and selenium using hydride generation atomic fluorescence spectrometry. Talanta, 156–157, 29–33. https://doi.org/10.1016/j.talanta.2016.04.063
Demirtaş, I., Bakirdere, S., & Ataman, O. Y. (2015). Lead determination at ng/mL level by flame atomic absorption spectrometry using a tantalum coated slotted quartz tube atom trap. Talanta, 138, 218–224. https://doi.org/10.1016/j.talanta.2015.02.044
Fernández, B., Lobo, L., & Pereiro, R. (2019). Atomic absorpton spectrometry | fundamentals, instrumentation and capabilities. In Encyclopedia of analytical science (pp. 137–143). Elsevier. https://doi.org/10.1016/B978-0-12-409547-2.14116-2
Gad, S. C. (2014). Nickel and nickel compounds. In Encyclopedia of toxicology: Third edition (pp. 506–510). Elsevier. https://doi.org/10.1016/B978-0-12-386454-3.00889-7
Galbeiro, R., Garcia, S., & Gaubeur, I. (2014). A green and efficient procedure for the preconcentration and determination of cadmium, nickel and zinc from freshwater, hemodialysis solutions and tuna fish samples by cloud point extraction and flame atomic absorption spectrometry. Journal of Trace Elements in Medicine and Biology, 28(2), 160–165. https://doi.org/10.1016/j.jtemb.2013.12.004
Giersz, J., Bartosiak, M., & Jankowski, K. (2017). Sensitive determination of Hg together with Mn, Fe, Cu by combined photochemical vapor generation and pneumatic nebulization in the programmable temperature spray chamber and inductively coupled plasma optical emission spectrometry. Talanta, 167, 279–285. https://doi.org/10.1016/j.talanta.2017.02.018
Gondal, M. A., Aldakheel, R. K., Almessiere, M. A., Nasr, M. M., Almusairii, J. A., & Gondal, B. (2020). Determination of heavy metals in cancerous and healthy colon tissues using laser induced breakdown spectroscopy and its cross-validation with ICP-AES method. Journal of Pharmaceutical and Biomedical Analysis, 183, 113153. https://doi.org/10.1016/j.jpba.2020.113153
He, Y., Hou, X., Zheng, C., & Sturgeon, R. E. (2007). Critical evaluation of the application of photochemical vapor generation in analytical atomic spectrometry. Analytical and Bioanalytical Chemistry. https://doi.org/10.1007/s00216-006-1044-7
Iraji, A., Afzali, D., Mostafavi, A., & Fayazi, M. (2012). Ultrasound-assisted emulsification microextraction for separation of trace amounts of antimony prior to FAAS determination. Microchimica Acta, 176(1–2), 185–192. https://doi.org/10.1007/s00604-011-0706-0
Korkmaz, D., Kumser, S., Ertaş, N., Mahmut, M., & Ataman, O. Y. (2002). Investigations on nature of re-volatilization from atom trap surfaces in flame AAS. Journal of Analytical Atomic Spectrometry, 17(12), 1610–1614. https://doi.org/10.1039/b207788d
Kulomäki, S., Lahtinen, E., Perämäki, S., & Väisänen, A. (2019). Determination of mercury at picogram level in natural waters with inductively coupled plasma mass spectrometry by using 3D printed metal scavengers. Analytica Chimica Acta, 1092, 24–31. https://doi.org/10.1016/j.aca.2019.09.075
López-García, I., & Hernández-Córdoba, M. (2015). Atomic absorption spectrometry. In M. de la Guardia, & S. Garrigues (Eds.), Handbook of mineral elements in food (pp. 189–217). Wiley. https://doi.org/10.1002/9781118654316.ch10
López-García, I., Rengevicova, S., Muñoz-Sandoval, M. J., & Hernández-Córdoba, M. (2017). Speciation of very low amounts of antimony in waters using magnetic core-modified silver nanoparticles and electrothermal atomic absorption spectrometry. Talanta, 162, 309–315. https://doi.org/10.1016/j.talanta.2016.10.044
Matusiewicz, H. (1997). Atom trapping and in situ preconcentration techniques for flame atomic absorption spectrometry. Spectrochimica Acta - Part B Atomic Spectroscopy, 52(12), 1711–1736. https://doi.org/10.1016/S0584-8547(97)00089-X
Mollo, A., & Knochen, M. (2020). Towards the abatement of nitrate interference on selenium determination by photochemical vapor generation. Spectrochimica Acta - Part B Atomic Spectroscopy, 169, 105875. https://doi.org/10.1016/j.sab.2020.105875
Prueitt, R. L., & Goodman, J. E. (2015). Nickel. In Hamilton and Hardy’s industrial toxicology: Sixth edition (pp. 173–182). Wiley. https://doi.org/10.1002/9781118834015.ch25
Reclo, M., Yilmaz, E., Soylak, M., Andruch, V., & Bazel, Y. (2017). Ligandless switchable solvent based liquid phase microextraction of nickel from food and cigarette samples prior to its micro-sampling flame atomic absorption spectrometric determination. Journal of Molecular Liquids, 237, 236–241. https://doi.org/10.1016/j.molliq.2017.04.066
Seidi, S., & Alavi, L. (2019). Novel and rapid deep eutectic solvent (DES) homogeneous liquid–liquid microextraction (HLLME) with flame atomic absorption spectrometry (FAAS) detection for the determination of copper in vegetables. Analytical Letters, 52(13), 2092–2106. https://doi.org/10.1080/00032719.2019.1598425
Seregin, I. V., & Kozhevnikova, A. D. (2006). Physiological role of nickel and its toxic effects on higher plants. Russian Journal of Plant Physiology, 53(2), 257–277. https://doi.org/10.1134/S1021443706020178
Sixto, A., Mollo, A., & Knochen, M. (2019). Fast and simple method using DLLME and FAAS for the determination of trace cadmium in honey. Journal of Food Composition and Analysis, 82, 103229. https://doi.org/10.1016/j.jfca.2019.06.001
Soylak, M., & Koksal, M. (2019). Deep eutectic solvent microextraction of lead(II), cobalt(II), nickel(II) and manganese(II) ions for the separation and preconcentration in some oil samples from Turkey prior to their microsampling flame atomic absorption spectrometric determination. Microchemical Journal, 147, 832–837. https://doi.org/10.1016/J.MICROC.2019.04.006
Sturgeon, R. E. (2017). Photochemical vapor generation: A radical approach to analyte introduction for atomic spectrometry. Journal of Analytical Atomic Spectrometry. https://doi.org/10.1039/c7ja00285h
Uslu, H., Büyükpınar, Ç., Unutkan, T., Serbest, H., SAN, N., Turak, F., & Bakırdere, S. (2018). A novel analytical method for sensitive determination of lead: Hydrogen assisted T-shape slotted quartz tube-atom trap-flame atomic absorption spectrometry. Microchemical Journal, 137, 155–159. https://doi.org/10.1016/j.microc.2017.10.015
Wallace, W. E. (1973). Nickel compounds. In Rare earth intermetallics (pp. 111–144). Elsevier. https://doi.org/10.1016/b978-0-12-732850-8.50014-3
Watling, R. J. (1977). The use of a slotted quartz tube for the determination of arsenic, antimony, selenium and mercury. Analytica Chimica Acta, 94(1), 181–186. https://doi.org/10.1016/S0003-2670(01)83645-X
Watling, R. J. (1978). The use of a slotted tube for the determination of lead, zinc, cadmium, bismuth, cobalt, manganese and silver by atomic absorption spectrometry. Analytica Chimica Acta, 97(2), 395–398. https://doi.org/10.1016/S0003-2670(01)93448-8
Welz, B., & Sperling, M. (1998). Atomizers and atomizer units. In M. Welz, & B. Sperling (Eds.), Atomic absorption spectrometry (pp. 149–219). https://doi.org/10.1002/9783527611690.ch4
Zheng, C., Sturgeon, R. E., & Hou, X. (2009). UV photochemical vapor generation and in situ preconcentration for determination of ultra-trace nickel by flow injection graphite furnace atomic absorption spectrometry. Journal of Analytical Atomic Spectrometry, 24(10), 1452–1458. https://doi.org/10.1039/b909962j
Zhong, W. S., Ren, T., & Zhao, L. J. (2016). Determination of Pb (lead), Cd (cadmium), Cr (chromium), Cu (copper), and Ni (nickel) in Chinese tea with high-resolution continuum source graphite furnace atomic absorption spectrometry. Journal of Food and Drug Analysis, 24(1), 46–55. https://doi.org/10.1016/j.jfda.2015.04.010
Funding
This work was financially supported by The Scientific and Technological Research Council of Turkey (TÜBİTAK) with a Grant Number of 117Z380.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Büyükpınar, Ç., Yazıcı, E., San, N. et al. Determination of nickel in daphne tea extract and lake water samples by flame atomic absorption spectrophotometry with a zirconium-coated T-shaped slotted quartz tube-atom trap and photochemical vapor generation sample introduction. Environ Monit Assess 193, 627 (2021). https://doi.org/10.1007/s10661-021-09430-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-021-09430-2