Abstract
Water content in solid materials is usually measured by loss on drying and Karl Fischer titration (KFT) methods. The validation of KFT methods requires the certified reference materials (CRMs) for the water content in solids, especially those with lower water content. In the present study, two mixtures of sodium tartrate dihydrate and 4-methoxybenzoic acid were prepared using the gravimetric method and then used as candidates of CRMs with water content of 10.0 and 1.0 mg·g−1, respectively. Furthermore, the CRM of lactose monohydrate and that of 4-methoxybenzoic acid were also developed. The stability against the humidity of the raw materials was characterized using water sorption isotherms. The thermal stability of the raw materials was characterized by the thermogravimetric analyzer. These four CRMs for water content were certified using Karl Fischer coulometric and volumetric titration. To make the water content traceable, both KFT methods were calibrated using a home-made water content standard prepared by the gravimetric method. The average value of the two methods was used as the certified value. The certified water contents and their expanded uncertainties (U, k = 2) of four CRMs were (50.7 ± 0.6) mg·g−1, (9.90 ± 0.2) mg·g−1, (0.878 ± 0.044) mg·g−1 and (0.142 ± 0.013) mg·g−1, respectively. This series of CRMs were suitable for the validation of KFT methods for the water contents in solids.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Water is a common constituent of solid materials and the water content affects their physical and chemical properties. For example, as the water content of food increases, the storage life decreases [1]. The water content of pharmaceuticals is related to their chemical and physical stability, and a high water content might facilitate the microbial growth [2]. Because wood shrinks and warps as it dries, its water content should be reduced to an appropriate level before further processing and installation [3]. Calculating the content of elementals, volatile and ash, and the calorific value of coal and biomass from “as-received” values to dry basis need accurate water contents [4]. When the purity of pure organic substances is determined using the mass balance approach, the water content of substances should be determined accurately [5]. Thus, accurate measurement of the water content in solid materials is essential.
There are various methods for water content measurements in solids. The direct methods such as loss on drying (LOD) [6], Karl Fischer coulometric titration (KFCT) [7] and azeotropic distillation [8] are usually used to measure the water content of solids without calibration. Conversely, indirect methods, including capacitance [9], resistance [10], microwave resonance [11] and infrared absorption [11], should be calibrated using reference materials for water content.
LOD methods, including normal oven drying-weighing, portable oven drying-weighing and thermogravimetric analysis (TGA), weigh the mass fraction of evaporated constituents as the water content. LOD methods are simple, convenient, and low cost although they are not water specific. Karl Fischer titration (KFT) coupled with oven drying (oven-KFT), evolved vapor coulometric technique [12] and reaction with calcium hydride (CaH2) [13], are water specific and can measure the mass of evaporated water with a high sensitivity. The measured water content using these methods is probably less than the actual value due to part of the water remaining in the matrix after heating.
KFT methods, including the KFCT and Karl Fischer volumetric titration (KFVT), are widely used for determining the water content of solids and liquids. In the Karl Fischer reaction, water reacts with iodine (I2) according to the stoichiometry of 1:1; thus, the amount of water in the analyte is equal to that of I2 which is calculated through the amount of electrical charge according to Faraday’s law [7]. In KFT method with the direct sample addition, the sample is dissolved in the Karl Fischer reagent and thus all of the water in the sample is determined. For liquid samples, KFT methods with the sample injection have repeatable results. For solid samples, when the sample was added in the titration cell directly, the moisture from ambient atmosphere flowed in and resulted in a high drift, which makes the result unrepeatable and inaccurate.
Therefore, the methods for water content in solids require the validation using certified reference materials (CRMs). Compounds containing crystal water are traditionally used as reference materials because of their good thermal stability and stability against humidity [14,15,16]. Bell et al. [14] developed a series of CRMs of compounds containing crystal water with the contents of evaporated water ranging from 50 mg·g−1 to 470 mg·g−1. Authors developed CRMs of sodium tartrate dihydrate and potassium citrate monohydrate with water contents of 156.3 mg·g−1 and 55.8 mg·g−1, respectively [15]. Recently, Medvedevskikh et al. [16] developed a CRM of sodium molybdate dihydrate with a content of evaporated water of 148.8 mg·g−1.
Pure organic substances usually contain a water content ranging from 10 mg·kg−1 to 10 mg·g−1, therefore, CRMs with a lower water content are needed for the validation of methods [5]. Furthermore, special Karl Fischer reagents without methanol are used in the measurement of ketones and aldehydes; thus, CRMs like ketones or aldehydes are also needed for the validation of the KFT methods [7].
In the present study, two mixtures of one compound with a high water content and the other with an extremely low water content were prepared by the gravimetric method and used as the candidates of CRMs with low water contents (10.0 mg·g−1 and 1.0 mg·g−1). Furthermore, CRM of lactose monohydrate and CRM of 4-methoxybenzoic acid with water contents of 50.7 and 0.142 mg·g−1, respectively, were also developed. The KFCT and KFVT methods were used to certify these four CRMs.
2 Experimental
2.1 Reagents and Chemicals
1-Octanol, sodium tartrate dihydrate, lactose monohydrate, 4-methoxybenzoic acid, benzoic acid, methyl benzoic acid, oxy benzoic acid ethyl ester, amino benzoic acid, nitrobenzoic acid, oxy benzoic acid methyl ester and acetanilide were purchased from Sigma-Aldrich Inc. (St. Louis, MO, USA). Hydranal Coulomat AG, AK, and Hydranal Titrant 2 and Solvent were purchased from Honeywell Inc. (Morristown, NJ, USA).
2.2 Instruments
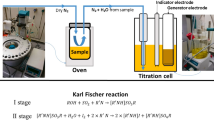
A Karl Fischer coulometric titrator (DL 39, Mettler-Toledo Inc., Switzerland) and a Karl Fischer volumetric titrator (V30, Mettler-Toledo Inc., Switzerland) were used. A glove box (Ultra-1, Alke gas purifying instrument Inc., Beijing, China) was used to contain the Karl Fischer titrator (Fig. 1). A 3D blender mixer was used to mix the raw materials of CRMs. The mass-vs-temperature curves were characterized using TGA (Pyris 1, Perkin-Elmer Inc., Waltham, MA, USA). The temperature of the TGA was calibrated using the CRMs of Alumel, nickel and iron for Curie point (GBW 13 239, 13 240 and 13 241, National Institute of Metrology (NIM) of China). The mass of the TGA was calibrated using the weight of F1 grade. The water sorption isotherms (WSIs) were characterized using a WSI meter (Aqua Lab VSA, Decagon Inc., Pullman, WA, USA). The water activity of the WSI meter was calibrated using the commercial reference materials of aqueous solution of lithium chloride, sodium chloride and potassium chloride for water activity (No. 1356206, 1364109, 1364006 and 1348381, Decagon Inc., Pullman, WA, USA).
2.3 Preparation of CRMs
Four CRMs were labeled as GBW 13 517, 13 518, 13 519 and 13 520, respectively. For GBW 13 517, 1 kg of lactose monohydrate was mixed using the 3D blender mixer for 15 min and then packaged into 200 bottles with screw-tops. For GBW 13 518 with a water content of approximately 10 mg·g−1, a mixture of sodium tartrate dihydrate (66.1 g) and 4-methoxybenzoic acid (980.1 g), was mixed using the 3D mixer for 15 min and then packaged into 200 bottles. Similarly, for GBW 13 519 with a water content of approximately 1.0 mg·g−1, 5.65 g of sodium tartrate dihydrate and 1021.1 g of 4-methoxybenzoic acid were mixed and then packaged. For GBW 13 520, 1 kg of pure 4-methoxybenzoic acid was mixed and then packaged into 200 bottles.
2.4 Preparation of Home-Made Water Content Standard
The home-made water content standard was prepared using anhydrous 1-octanol and pure water by means of the gravimetric method. 10-mL anhydrous 1-octanol was added in a 20-mL crimp neck headspace vial with a rubber septum and then the mass of 1-octanol was weighed. Then, 0.16 g of deionized water was added in the vial, and the vail was sealed immediately. Then the mass of added water was weighed. The water content of the anhydrous 1-octanol was determined using the KFCT method. So, the water content of this home-made standard was calculated based on the mass of 1-octanol, the mass of added water and the water content of anhydrous 1-octanol.
2.5 Analytical Methods
Both Karl Fischer coulometric and volumetric titrators were placed in the glove box in order to eliminate the interference from the moisture in the atmosphere (Fig. 1).
The KFCT measurement was performed using a DL 39 titrator equipped with a diaphragmless electrode. Hydranal Coulomat AG was used as the anolyte solution. The water content (x) was calculated according to Eq. 1.
where x is water content, g·g−1; Q is amount of electrical charge, C; M is molecular weight of water, 18.015 g·mol−1; f is water recovery; 2 is number of electrons needed for the production of one iodine molecule (I2); F is Faraday’s constant, 96 485 C·mol−1; m is sample mass, g.
The conditions were as follows: the sample masses of GBW 13 517, 13 518, 13 519, and 13 520 were 50 mg, 300 mg, 300 mg, and 400 mg, respectively; electrolysis rate, normal; polarization current, 2 μA; end voltage, 100 mV; extraction time, 180 s to 300 s; and titration time, 180 s to 300 s. The drift standby determined over 180 s before the sample addition was not more than 2 μg·min−1. Before the measurement, the water recovery (f) of the KFCT method was measured using the home-made water content standard. Solid sample was added in a copper tube closed at one end and weighed using an electric balance. The tube was then transferred into the glove box and then the sample was added into the titration cell. The tube was transferred outside the glove box and weighed again, and the mass of the added sample was calculated.
The KFVT measurement was performed using a V30 titrator. Hydranal titrant 2 and Hydranal solvent were used. The water content was calculated according to Eq. 2. The conditions were as follows: the sample mass and the end voltage were the same as those for the KFCT measurement. When the drift of titration decreased to 1 μg·min−1 higher than the drift standby, the titration was terminated. The titer of the titrant was measured using the home-made water content standard (Eq. 2):
where x is water content, g·g−1; V is volume of titrant for sample, mL; T is titer of titrant, mg·mL−1; m is sample mass, g; m0 is mass of home-made water content standard, g; x0 is prepared water content of home-made water content standard, g·g−1; V0 is volume of titrant for home-made water content standard, mL.
When the mass-vs-temperature curves were characterized, the temperature increased from room temperature up to 200 °C with a heating rate of 1 °C·min−1. The sample mass was approximately 10 mg.
When WSIs were measured, a chilled mirror hygrometer was used to measure the water activity. The maximum water activity for the WSI measurements was set as aw = 0.85. The flow rate of the gas was 20 mL·min−1. The temperature of the analyte was 25 °C.
3 Results and Discussion
3.1 Preparation of CRM Candidates for Water Content
The CRM should have a good homogeneity as well as a stability. Furthermore, the CRM should dissolve in Karl Fischer reagent that is mainly composed of alcohol and imidazole. So, lactose monohydrate with a water content of approximately 50 mg·g−1 was used as the raw material of CRM.
Compounds containing crystal water usually have water contents greater than 50 mg·g−1, and thus are unsuitable as the candidate of CRM with a low water content. To screen for the compound with a low water content, eight common organic compounds were measured using the KFCT method. As shown in Fig. 2, their water content results were 5.5 mg·kg−1, 137 mg·kg−1, 263 mg·kg−1, 270 mg·kg−1, 470 mg·kg−1, 674 mg·kg−1, 2020 mg·kg−1 and 3690 mg·kg−1, and the relative standard deviations (RSDs) repeatability of the results were 53 %, 18 %, 25 %, 5.2 %, 51 %, 4.0 %, 5.2 % and 6.4 %, respectively. The results above indicated that the water contents of these compounds were inhomogeneous. Although these eight compounds cannot represent all organic compounds, the results above indicated that organic compounds containing absorbed water might be unsuitable as CRM candidates with a low water content because of possible inhomogeneity. Furthermore, the absorbed water content of organic compounds might be different from batch to batch, which makes the repreparation of the CRMs difficult. Therefore, the mixture of one compound with a high water content and the other with an extremely low water content was prepared using the gravimetric method and then used as the candidate of CRM with a low water content. Sodium tartrate dihydrate was used as the compound with a high water content due to its stability and dissolubility in methanol [15]. As shown in Fig. 2, the water content of benzoic acid is extremely low (5.5 mg·kg−1), but it is difficult to mix benzoic acid and sodium tartrate dihydrate homogeneously because of the static electricity of benzoic acid. 4-Methoxybenzoic acid, with a low water content of 270 mg·kg−1 and a low RSD of 5.2 %, was employed as the compound with an extremely low water content (Fig. 2). Sodium tartrate dihydrate and 4-methoxybenzoic acid were mixed according to the mass proportions described above. The prepared water contents of the two CRM candidates were 10.0 mg·g−1 and 1.0 mg·g−1, respectively.
3.2 Characterization of the Stability of Raw Materials Against Humidity
The WSIs of lactose monohydrate, sodium tartrate dihydrate and 4-methoxybenzoic acid are shown in Figs. 3, 4, 5. The initial water activity of the three samples at 25 °C was 0.282, 0.193, and 0.235, respectively (Figs. 3, 4 and 5), which indicates that their water contents are stable at the humidity approximately 28.2 %rh, 19.3 %rh and 23.5 %rh, respectively. As the water activity increased from the initial value up to 0.85, the increase of mass of lactose monohydrate and sodium tartrate dihydrate were 0.16 % and 0.07 %, respectively, which indicates that two raw materials are stable against humidity and are suitable as the CRM candidate for water content (Figs. 3 and 4). On the contrary, as the water activity increased, the increase of mass of 4-methoxybenzoic acid was approximately 0.30 %, indicating that it is prone to absorb water at high humidity (Fig. 5). Therefore, the CRM of 4-methoxybenzoic acid, and the other two CRMs containing it, should be sealed and stored in a dry place.
3.3 Characterization of the Thermal Stability of Raw Materials
Figure 6c shows the mass-vs-temperature and the derivative mass-vs-temperature curves of lactose monohydrate, sodium tartrate dihydrate and 4-methoxybenzoic acid, respectively. As the temperature increased, the mass of lactose monohydrate decreased and the mass loss peak appeared at about 142 °C (Fig. 6a). This loss of mass indicates that lactose monohydrate decomposed under heating and then one of products, water, evaporated. When the temperature was higher than 155 °C, the mass decreased gradually (Fig. 6a), which is due to the slow decomposition of anhydrous lactose. Similarly, as the temperature increased, sodium tartrate dihydrate decomposed under heating (Fig. 6b). Two peaks of mass loss at about 64 °C and 136 °C correspond to the evaporation of water in different forms (Fig. 6b). The mass of 4-methoxybenzoic acid decreased from about 110 °C and the rate of mass loss increased gradually without an obvious peak (Fig. 6c). The melting point and boiling point of 4-methoxybenzoic acid is 182 °C and 275 °C, respectively. Thus, the mass loss from about 110 °C was likely because of the sublimation before melting.
The TGA curves indicated that the raw materials of CRMs including lactose monohydrate, sodium tartrate dihydrate and 4-methoxybenzoic acid were stable at room temperature. Because sodium tartrate dihydrate decomposes at about 64 °C, during the transportation of GBW 13 518 and 13 519, heat preservation should be performed to avoid any possible decomposition of sodium tartrate dihydrate.
3.4 Certification of CRMs
Prior to the certification, the homogeneity of water content of GBW 13 517 (lactose monohydrate) was tested using the TGA method. The homogeneity of water content of GBW 13 518, 13 519, and 13 520 were tested using the KFCT method. According to ISO Guide 35, one-way analysis of variance (ANOVA) was used to evaluate the between-bottle homogeneity [17]. All the statistics of F for four CRMs were less than the critical values (F0.05(m, n)), which indicate that the dispersity between the bottles is not obvious compared with that within the bottles, namely that the CRM candidates are homogeneous. Furthermore, the long-term stability of the water content of these four CRMs candidates was tested according to ISO Guide 35 using the KFCT method [17]. The results indicate that the water contents of the CRM candidates are stable within 12 months.
According to ISO Guide 35, the CRM should be certified using two methods with different principles [17]. In the present study, the KFCT and KFVT method were used. In determination using two KFT methods, the sample was added in the titration cell directly. The environmental conditions for the sample addition including the ambient atmosphere, the protection by carbon dioxide and the dry atmosphere in the glove box were compared using the KFCT method and sodium tartrate dihydrate. First, when the sample was added in the ambient atmosphere where the humidity was approximately 20 %rh, the RSD repeatability of the water content results was 0.5 %. Second, when the sample was added in the ambient atmosphere, dried carbon dioxide flowed into the titration cell to avoid the introduction of moisture; the RSD repeatability of the water content results was 1.5 %. Third, the titrator was placed in the glove box where the water content of nitrogen was not higher than 4 mg·kg−1. After the titration cell was opened and closed several times, the drift standby remained at 1 μg·min−1. By this way, the RSD repeatability of the water content of sodium tartrate dihydrate was 0.14 %. When the solid analyte was added directly into the titration cell in the ambient atmosphere, moisture in the air entered the cell and then the drift standby increased, which made the water content results unrepeatable and inaccurate. Titration in the extremely dry atmosphere in the glove box reduced the interference of moisture from the environment, which endowed the results a high repeatability.
In the determination of GBW 13 517 (lactose monohydrate) using the KFCT method, Hydranal Coulomat AG, a common Karl Fischer anolyte, was used. After the sample addition, the drift remained high and the titration could not terminate. When Hydranal Coulomat AK, a Karl Fischer anolyte without methanol, was used, the drift quickly decreases to the drift standby level and the titration could terminate in several minutes. Comparatively, in the determination of GBW 13 517 using the KFVT method, both the titrant and the solvent contained methanol. Aldehydes and ketones can react with methanol and produce acetals or ketals along with water [7]. During the KFCT, the hemiacetal group of lactose might also react with methanol in Hydranal Coulomat AG and produce water. When the anolyte without methanol was used, the interference of methanol was avoided and thus the titration could terminate. Lactose does not react with methanol in Hydranal Solvent during the KFVT, probably because the pH value of Hydranal Solvent is unsuitable for the reaction of lactose and methanol.
The results of the four CRMs using the KFCT and KFVT method are shown in Table 1. The average water content and percent RSD of GBW 13 517 using the KFCT and KFVT method was 50.57 mg·g−1 (0.28 %) and 50.79 mg·g−1 (0.22 %), respectively (Tables 1 and 2). The repeatability of both the KFCT and KFVT measurements was as good as those of liquid CRMs with similar water contents [18]. Furthermore, the results of two methods were consistent, indicating the accuracy of two methods. The average value of two methods (50.7 mg·g−1) was used as the certified value. Similarly, the certified values for GBW 13 518, 13 519 and 13 520 were 9.90 mg·g−1, 0.878 mg·g−1 and 0.142 mg·g−1, respectively (Table 1). As the water contents of the CRMs decreased, the repeatability of the results decreased (Tables 2, 3, 4 and 5). The repeatability of the results were worse than that of the liquid CRMs with similar water contents [18], which is because of the inhomogeneity within bottle and between bottle of water content.
The uncertainties of the certified values were evaluated according to ISO Guide 35 [17]. For each method used in the certification, the sources of uncertainty include type A and type B uncertainty (Eq. 3). Type A uncertainty comes from the dispersion of measured results and is equal to its standard deviation repeatability. Type B uncertainty comes from the factors other than statistical analysis of results. For the KFCT method, type B uncertainty was a combination of the uncertainties of the mass of sample (ur(m)), the amount of electrical charge (ur(Q)), Faraday’s constant (ur(F)), the molecular weight of water (ur(M)) and the water recovery (ur(f)), as shown in Eq. 4. ur(Q), ur(F) and ur(M) were very little and thus were omitted (Eq. 4). Because the water recovery of the KFCT method was determined using the home-made water content standard, the relative standard uncertainty of prepared water value of the home-made standard (ur(x0), 0.18 %) was used as ur(f):
For the KFVT method, type B uncertainty was a combination of the uncertainties of the volume of titrant for sample (ur(V)), the mass of sample (ur(m)), the mass of home-made water content standard (ur(m0)), the prepared water content of home-made standard (ur(x0)) and the volume of titrant for home-made standard (ur(V0)), as shown in Eq. 5:
The uncertainty from the homogeneity (uH), stability in long term (uls) and stability in short term (uss) were evaluated according to ISO Guide 35 [17]. The uncertainty from the KFCT, the KFVT, the homogeneity and the stability were combined (Eq. 6):
where ur,C is relative standard combined uncertainty; ur,KFCT is relative uncertainty of the KFCT method, and ur,KFVT is relative uncertainty of the KFVT method.
All the components of uncertainties of four CRMs are listed in Tables 2, 3, 4 and 5, respectively. Finally, the expanded uncertainties (U with k = 2) of GBW 13 517, 13 518, 13 519 and 13 520 were 0.6 mg·g−1, 0.2 mg·g−1, 0.044 mg·g−1, and 0.013 mg·g−1, respectively (Tables 2, 3, 4 and 5).
4 Discussion
CRMs for water contents in liquid were certified using the KFCT and KFVT method [16, 18,19,20,21]. In our previous work, CRMs for water content of sodium tartrate dihydrate and potassium citrate monohydrate were also certified using the KFCT and KFVT method [15]. A series of CRMs for water content of compounds containing crystal water reported by Bell et al. [14] were certified using the evolved vapor coulometric technique and LOD. The CRM for water content of sodium molybdate dihydrate reported by Medvedevskikh et al. [16] was certified using air thermal drying and thermogravimetric analysis with mass spectrometric detection (TGA–MS). The methods for the mass fraction of evaporated water including LOD, oven-KFT and evolved vapor coulometric technique do not determine the content of water remains in matrix after heating. If the sample decomposes under heating, the results of the LOD method would have a positive systematic error. If the decomposition products contain water, the results of oven-KFT and evolved vapor coulometric technique would have a positive systematic error. So, these methods are suitable for the sample with a good thermal stability, such as sodium molybdate [16]. Furthermore, when the water content of sample decreased, the repeatability of results based on the LOD method decreased [15]. Therefore, in order to determine the total water content of CRMs in solids accurately, authors used the KFCT and KFVT method with a direct sample addition.
The KFCT method determines the water mass through the amount of electrical charge according to Faraday’s law, so it is regarded as an absolute method for water content conventionally [19, 20]. However, in our previous work, it is found that the water recovery was affected by the type of electrode structure (diaphragm or diaphragmless) and the electrolyte. In the present study, the KFCT using a diaphragmless electrode and Hydranal Coulomat AK electrolyte had a water recovery of 104.5 %. Comparatively, the KFCT using the same electrode and Hydranal Coulomat AG electrolyte had a water recovery of 99.8 %. The systematic error of the water recovery was because of side reactions of Karl Fischer reaction. For example, Bunsen reaction that water, sulfur dioxide and iodine react to form sulfuric acid and hydrogen iodide, resulted a negative error of water content (Eq. 7) [7]. The iodine generated by the anode might be reduced at the cathode because of the lack of diaphragm, which resulted in a positive error of water content [21]. Furthermore, the electrolysis efficiency of iodine is less than 100 %, which resulted in a positive error of water content. Consequently, the KFCT method should be calibrated in order to acquire accurate results. In our study, the home-made water content standard was employed to determine the water recovery of the KFCT method. The prepared value of the home-made water content standard traces to the International System of Units (SI) of the mass of pure water and has a relative standard uncertainty (ur) of 0.18 % [21]. This home-made standard in a crimp neck headspace vial with a rubber septum is unstable in a long term, so it is unsuitable for the candidate of CRMs [21]. The KFCT and KFVT methods traced to SI of the mass of pure water through this home-made standard and thus achieved the accurate results.
The accuracies of these four CRMs in solids were compared with those of CRMs for the water content in liquids. The uncertainty of GBW 13 517 (Ur, 1.2 %) was similar to that of GBW 13 512 with a water content of 10 mg·g−1 (Ur, 1.3 %). The uncertainties of GBW 13 518, 13 519 and 13 520 (Ur, 2.0 %, 5.0 % and 9.4 %) were greater than that of GBW 13 512, 13 513, and 13 514 that have similar water contents (Ur, 1.3 %, 2.9 % and 8.7 %) [18]. These differences were mainly because of the poor between-bottle and within-bottle homogeneity of the mixture of solids. There is still room for improvement in the preparation of CRMs for water content in solids.
These four CRMs have been offered to customers from pharmaceuticals and organic chemical metrology. When they measured sample using KFT methods with the direct sample addition, they measured these CRMs and evaluated the accuracy of their method based on the repeatability and indicated error of results. When they measured sample using the oven-KFT method, they measured these CRMs and estimate the water recovery of their method based on the indicated errors of results. However, there remains a need of CRMs for the mass fraction of evaporated water of solids to evaluate the accuracy of the oven-KFT method and evolved vapor coulometric technique. Related investigation is ongoing.
5 Conclusion
In the present study, four CRMs for water content in solids, including pure lactose monohydrate, two mixtures and pure 4-methoxybenzoic acid, were developed. Two mixtures of sodium tartrate dihydrate and 4-methoxybenzoic prepared by the gravimetric method were used as the candidates of CRMs with a water content of 10 mg·g−1 and 1.0 mg·g−1. WSI and TGA measurements were used to characterize the stability against the humidity and the thermal stability of the raw materials, respectively. These four CRMs for water content were certified by the KFCT and KFVT method, and the average value of the two methods was used as the certified value. The home-made water content standard prepared by the gravimetric method was used to calibrate both KFT methods to make the water content traceable. The certified water contents and their expanded uncertainties (U with k = 2) of GBW 13 517, 13 518, 13 519, and 13 520 were (50.7 ± 0.6) mg·g−1, (9.90 ± 0.2) mg·g−1, (0.878 ± 0.044) mg·g−1 and (0.142 ± 0.013) mg·g−1, respectively.
References
M. Butt, M. Nasir, S. Akhtar, K. Sharif, Internet J. Food Saf. 4, 1 (2004)
L. Frink, C. Weatherly, D. Armstrong, J. Pharm. Biomed. Anal. 94, 111 (2014)
C. Skaar, Wood-water relations (Springer-Verlag, Berlin Heidelberg, 1988)
ISO 1928–2009, Solid mineral fuels—determination of gross calorific value by the bomb calorimetric method and calculation of net calorific value (ISO, Geneva, 2009)
K. Ishikawa, N. Hanari, Y. Shimizu, T. Ihara, A. Nomura, M. Numata, T. Yarita, K. Kato, K. Chiba, Accred Qual. Assur. 16, 311 (2011)
P. Miao, S.A. Bell, M. Rujan, M. Georgescu, C. McIlroy, Report on literature review of recent development in loss on drying method for moisture determination, NPL REPORT ENG 52, March 2014
E. Scholz, Karl Fischer Titration (Springer Verlag, Heidalberg, 1984)
W. Fetzer, Anal. Chem. 23, 1062 (1951)
W. Whalley, T. Dean, P. Izzard, J. Agr. Eng. Res. 52, 147 (1992)
T. Williams, J. Hydrol. 46, 385 (1980)
C. Corredor, D. Bu, D. Both, Anal. Chim. Acta 696, 84 (2011)
S. Bell, P. Miao, P. Carroll, Int. J. Thermophys. 39, 50 (2018)
W. Lück, Feuchtigkeit (R. Oldenbourg Verlag, Munich, 1964)
S. Bell, P. Miao, P. Carroll, New reference materials for calibration of moisture analysers, (2016). https://www.dropbox.com/sh/yixc7iusk8qad2k/AABkr2SwqZHWOUC4zVQaQlJja/Workshop%20Taastrup?dl=0&preview=02+-+S+Bell+New+reference+materials+20160510.pdf&subfolder_nav_tracking=1
W. Liu, H. Wang, X. Gu, C. Quan, X. Dai, Anal. Methods 8, 2845 (2016)
M. Medvedevskikh, A. Sergeeva, M. Krasheninina, O. Shokhina, Measur. Tech. 62, 475 (2019)
ISO Guide 35, Reference materials-general and statistical principles for certification (ISO, Geneva, 2006)
H. Wang, K. Ma, W. Zhang, T. Huang, X. Dai, J. Li, G. Sun, H. Li, Accred Qual. Assur. 17, 589 (2012)
S. Margolis, M. Levenson, Fresenius J. Anal. Chem. 367, 1 (2000)
S. Inagaki, M. Numata, Y. Kitamaki, N. Hanari, R. Iwasawa, Accred Qual. Assur. 21, 361 (2016)
H. Wang, K. Ma, W. Zhang, J. Li, G. Sun, H. Li, Food Chem. 134, 2362 (2012)
Acknowledgments
This study was supported by the National Quality Infrastructure Program of China (No. 2017YFF0205300) and the Ability Promotion Program of the National Institute of Metrology of China (No. 31-ANL1814).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Selected Papers of the 14th International Symposium on Temperature and Thermal Measurements in Industry and Science.
Rights and permissions
About this article
Cite this article
Zhi, X., Wang, H., Wu, Z. et al. Development of Four Certified Reference Materials for Water Content in Solids. Int J Thermophys 41, 50 (2020). https://doi.org/10.1007/s10765-020-02634-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10765-020-02634-7