Abstract
Avastin is the monoclonal antibody for vascular endothelial growth factor (VEGF). This study aimed to investigate therapeutic effect of Avastin on type II collagen-induced arthritis. Type II chicken collagen was injected into the tails of Wistar rats, and 60 modeled female rats were randomly divided into three groups (n = 20): Avastin group, Etanercept group, and control group. Arthritis index and joint pad thickness were scored, and the pathology of back metapedes was analyzed. The results showed that compared to control group, the arthritis index, target-to-non-target ratio, synovial pathological injury index, serum levels of VEGF and tumor necrosis factor alpha, and VEGF staining were decreased significantly 14 days after Avastin or Etanercept treatment, but there were no significant differences between Avastin group and Etanercept group. These data provide evidence that Avastin exhibits similar effects to Etanercept to relieve rheumatoid arthritis in rat model and suggest that Avastin is a promising therapeutic agent for rheumatoid arthritis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Rheumatoid arthritis (RA) is a systemic autoimmune disease with unknown etiology and main features of chronic, symmetry, and erosive arthritis. RA is characterized by chronic inflammation of the synovium of joint, pannus formation, and the destruction of articular cartilage and remodeling, eventually leading to joint deformity and loss of function.
New vessels continue to transport inflammatory cells to the synovial fluid and supply the oxygen and nutrients for synovium abnormal hyperplasia to maintain the chronic inflammation of synovium and increase pannus invasiveness. Accumulating evidence suggests that angiogenesis plays an important role in the process of erosion and destruction of RA [1]. The most important cytokine to promote RA pannus angiogenesis is vascular endothelial growth factor (VEGF) [2]. VEGF interacts with its receptor VEGFR and other cytokines such as tumor necrosis factor alpha (TNF-α) and integrin αvβ3 to promote pannus formation, resulting in invasive synovitis and joint dysfunction [3].
Therefore, targeting pannus formation not only reduces the synovium blood supply but also suppresses the aggressive biological behavior of synovitis. In recent years, the application of blood vessel formation antagonist for RA treatment has been extensively investigated. The inhibitors of pannus angiogenesis include TNF-α antagonist, IL-1 antagonist, and IL-6 antagonist [4, 5]. Bevacizumab (Avastin) is a VEGF humanized monoclonal antibody and has been widely used for the inhibition of angiogenesis and tumorigenesis of colon cancer, lung cancer, and kidney cancer [6].
Collagen-induced arthritis (CIA) has been used as an experimental RA model [7]. In this study, we aimed to evaluate the therapeutic effects of Avastin on RA. We established type II collagen-induced arthritis (CIA) model in Wistar rats, which were treated with Avastin and Etanercept, a biological preparation that has been proven to be effective for clinical treatment of RA. We compared the morphology, iconography, serology, histopathology, and other indicators between Avastin and Etanercept treatment.
MATERIAL AND METHODS
Experimental Animals
All animals were maintained in accordance with the guidelines of the NIH (Guide for the Care and Use of Laboratory Animals, 1996), and the animal experiments were performed under approved protocols of the Animal Care and Use Committee of Inner Mongolia Medical College.
Female Wistar rats (weight 200 ± 20 g, 4−6 weeks old) were purchased from the Laboratory Animal Center at Inner Mongolia University and kept in the Animal Room of Department of Microbiology and Immunology at Inner Mongolia Medical College at room temperature of 20 °C, lighting/dark time of 12:12 h. For CIA model, chicken type II collagen (Sigma, USA) was injected into the tails of Wistar rats. Twenty-one days after initial immunity, type II collagen was intraperitoneally injected. The 60 successfully modeled female Wistar rats were randomly divided into three groups (n = 20): Avastin group, Etanercept group, and control group. For Avastin group, 30 mg/kg Avastin (Roche Pharmaceuticals, USA) was injected into the tail vein once a week for 2 weeks. For Etanercept group, 6 mg/kg Etanercept (Shanghai CP Guojian Pharmaceutical) was injected into the rats subcutaneously once a week for 2 weeks. For control group, 0.9 % sodium chloride solution (1 ml) was injected into the tail vein once a week for 2 weeks.
Determination of Arthritis Index
Arthritis index (AI) was determined as described previously [8]. According to the degree and range of joint swelling and joint swelling deformation, AI was counted as follows: 0 point for no arthritis; 1 point for local erythema or slight swelling of toe or ankle or single footpad; 2 points for erythema and mild swelling extending from the ankle to the end of the toe joints, feet, foot pads or inflammation at over two regions of ankle; 3 points for erythema and moderate swelling extending from the ankle to the end of the toe joints and mild dysfunction; and 4 points for severe redness and swelling of the entire foot including the ankle and toe, the disability to bear weight, dysfunction, and limited mobility.
Determination of Foot Pad Thickness
The vernier caliper was used to measure three positions at the foot pads of rats’ back foots at random, taking their mean as the final foot pad thickness.
Determination of Joint Pathological Injury Index and Immunohistochemistry Staining
The joint pathological injury index was determined as described previously [9]. The toe joints were taken without fur and tendon tissue, fixed in 10 % formaldehyde solution, and then embedded in paraffin. The joints were cut as 3-μm consecutive slices and stained by hematoxylin, eosin, and safranin O. The slices were observed under low power lens (×100) blindly and independently by two pathologists. The joint pathological injury index scores were counted as follows: 0 point for normal, 1 point for synovitis, 2 points for pannus formation, 3 points for articular cartilage destruction, and 4 points for the destruction of articular bone. For immunohistochemistry, the slices were incubated in 3 % H2O2 for 10 min at room temperature, to block endogenous peroxidase activity, then blocked with 2 % goat serum in 0.01 M PBS containing 0.3 % Triton X-100 (PBS-X) for 1 h at room temperature. Next, the slices were incubated at 4 °C overnight with rabbit antibody against VEGF (sc-152, Santa Cruz, USA). Afterwards, the slices were subjected to immunohistochemical staining using ABC method. For negative controls, the primary antibody was replaced with PBS.
Semi-quantitative scoring was based on the degree of positive staining: 0 point for no staining, 1 point for pale yellow, 2 points for brownish yellow, and 3 points for tan, and the percentage of positive cells: 0 point for negative, 1 point for 10 % or less, 2 points for 11−50 %, 3 points for 51–75 %, and 4 points for 75 % or more. The final scores were rated based on the product of staining intensity and the percentage of positive cells.
SPECT Imaging
Animals in the Avastin group and the Etanercept group underwent SPECT scan before treatment and 2 weeks after treatment, respectively. Rats were anesthetized via peritoneal injection of 0.7 ml 10 % chloral hydrate. Then, 99mTc-3P4-RGD2 (China Institute of Atomic Energy) was slowly injected into rat tail vein while avoiding piercing into skin. About 30 min later, the rats were placed under a SPECT scanning probe for SPECT scan. SPECT acquisition was the planar static acquisition, and the acquisition time was 6 min with peak of 140 kV, window width of 20, magnification of 1.0, and matrix of 256 × 256. The xeleris post-processing system workstation was used to perform image processing and measure the T/NT (target-to-non-target ratio).
Determination of Serum VEGF, TNF-α, and CTX-II Levels
The levels of VEGF, TNF-α, and C-terminal telopeptide of type II collagen (CTX-II) in rat serum were measured using VEGF-C, TNF-α, and CTX-II ELISA kits (eBioscienc, US) following the manufacturer’s instructions.
Statistical Analysis
Data were expressed as the mean ± SD and analyzed using the SPSS version 13.0 statistical analysis package (SPSS Inc., Chicago, IL, USA). The comparison between two groups was tested by paired sample t test. The comparison between several groups adopted the analysis of variance, and then, further pairwise comparisons were made by Newman–Student–Keul test. Linear regression analysis was used for the correlation analysis, and Levene was used to test the homogeneity of variance. P < 0.05 was considered as statistically significant.
RESULTS
The Establishment of RA Model
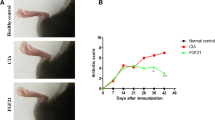
Twenty-four days after immunization, joint swelling was observed first at two back feet of the rats, and then joint swelling spread to the front feet and tail, increasingly deteriorating. The swelling reached the peak on the 34th day, shown as multiple and symmetrical joint swelling and redness. Some joints had ankylosis, deformity, and limited mobility. The animal had weight loss, even with ear and tail inflammation occasionally. Arthritis symptoms lasted for 50 days. Among the 100 rats used for modeling in this experiment, 61 had arthritis, and the success rate of modeling was 61 %.
Foot Pad Thickness and Arthritis Index in Three Groups
In Avastin group and Etanercept group 2 weeks after treatment, the foot pad thickness of back feet was reduced significantly compared with that before treatment, and the difference was statistically significant (P < 0.05). For the control group, the feet pad thickness before and after treatment had no significant difference (P > 0.05). Compared with the control group, foot pad thickness was reduced after treatment in Etanercept and Avastin groups, and the difference was statistically significant. However, there was no significant difference in foot pad thickness between the two treatment groups (Table 1).
In Avastin group and Etanercept group 2 weeks after treatment, the AI score was reduced significantly compared with that before treatment, and the difference was statistically significant (P < 0.05). For the control group, the AI score before and after treatment had no significant difference (P > 0.05). Compared with control group, AI score was lower after treatment in Etanercept and Avastin groups, and the difference was statistically significant. However, there was no significant difference in AI score between the two treatment groups (Table 2).
SPECT Imaging of Three Groups
The rat limbs had abnormal radioactivity concentrations. In Avastin group and Etanercept group 2 weeks after treatment, the T/NT was reduced significantly compared with that before treatment (P < 0.05). For the control group, the T/NT had no significant difference before and after treatment (P > 0.05). Compared with the control group, the T/NT was reduced after treatment in Etanercept and Avastin groups, and the difference was statistically significant (P < 0.05). However, the T/NT was not significantly different between the Avastin group and Etanercept group after treatment (Table 3, Fig. 1).
Serum VEGF, CTX-II, and TNF-α Levels in Three Groups
After 2 weeks of treatment, in both Avastin and Etanercept groups, serum VEGF, CTX-II, and TNF-α levels were significantly lower than those before treatment (P < 0.05). In control group, we observed no significant differences in serum VEGF, CTX-II, and TNF-α levels before and after treatment (P > 0.05). After 2 weeks of treatment, serum VEGF, CTX-II, and TNF-α levels were not significantly different between the Avastin group and Etanercept group (Tables 4, 5, and 6).
Joint Pathological Injury Index and VEGF Expression in Three Groups
Hematoxylin and eosin staining showed the infiltration of neutrophilic granulocyte and monocytes in synovial tissues. In addition, we observed synovial cells hyperplasia, increased number of layers, disorganized tissues, fibrin exudates, and feature of synovitis, and even articular cartilage and bone destruction of some tissues. Safranin O staining showed synovial cells hyperplasia, irregular forms, and invasion into articular cartilage surface. With severe synovitis, there was obvious articular cartilage and bone destruction. The joint pathological injury index was significantly higher in the control group than in the Avastin group and the Etanercept group (P < 0.05). In addition, immunohistochemical staining of VEGF in joint tissues was significantly higher in control group than in Avastin group and Etanercept group (P < 0.05). However, there were no significant differences in joint pathological injury index and VEGF expression between the Avastin group and Etanercept group (Table 7, Fig. 2).
Histological analysis of the toe joints of the rats in different groups after 2 weeks of treatment. Shown were representative images. A–C HE staining. (×100). D–F Safranin O staining (×200). G–I VEGF immunochemical staining (×400). A, D, G Avastin group. B, E, H Etanercept group. C, F, I control group.
Correlation of Pathological Injury Index, VEGF Staining, and Serum VEGF and TNF-α Levels in Different Groups
In the control group, after 2 weeks, the pathological injury index was increased, along with increased serum VEGF and TNF-α levels, and these changes were positively correlated (r = 0.734, P = 0.016; r = 0.813, P = 0.004, respectively). In addition, immunohistochemical staining of VEGF score was positively correlated with serum VEGF and TNF-α levels (r = 0.952, P = 0.000; r = 0.908, P = 0.000, respectively). Furthermore, the pathological injury index had a positive correlation with immunohistochemical staining of VEGF (r = 0.718, P = 0.019), while serum TNF-α level and serum VEGF level had a positive correlation (r = 0.943, P = 0.000) (Tables 8 and 9).
Similarly, we observed the positive correlation between pathological injury index, VEGF staining, and serum VEGF and TNF-α levels in Avastin and Etanercept groups (Tables 8 and 9).
DISCUSSION
RA is a chronic autoimmune disease. Although the pathogenesis of RA remains incompletely understood, pannus formation is generally recognized as the characteristic pathological change of RA. Up to now, a variety of cytokines have been shown to contribute to the formation of RA pannus, among which VEGF is the most important cytokine that promotes the formation of pannus by specially acting on the vascular endotheliocytes [10].
Currently, several mechanisms have been proposed to explain the promotion of RA pannus formation by VEGF [11]. First, as a mitogen of endothelium, VEGF promotes vascular endothelial cell differentiation and migration, eventually leading to the formation of pannus. Second, VEGF improves microvascular permeability, promoting the extravasation of plasma fibrin and its deposition in the extracellular matrix. Third, VEGF induces the expression and secretion of a variety of proteases by vascular endothelial cells, including urokinase-type and tissue-type plasminogen activator, which degrade extracellular matrix components to facilitate endotheliocyte migration [12]. Finally, VEGF plays a cooperative role with other factors such as TGF-β, FGF, IL-1, and TNF-α generated by vessels to promote pannus angiogenesis of RA.
By immunohistochemistry, VEGF has been shown to be expressed in the macrophage-like synovium stave cells and articular cartilage cells [13]. In addition, VEGF level was high in synovium, joint synovial fluid, and serum of patients with RA [14]. Clavel et al. demonstrated that serum VEGF level and joint swelling, the disease activity score, the ESR, and the CRP of patients with early RA were positively correlated, and proposed that VEGF level may serve as an evaluation index for RA [15]. Consistent with these data, in this study, we found that serum VEGF level, pathological injury index, and VEGF immunohistochemical staining score were positively correlated in CIA rat model.
Avastin is a custom-crafted humanized monoclonal antibody against VEGF and became the first drug approved by FDA to inhibit tumor angiogenesis. It is widely used in colon cancer, kidney cancer, and lung cancer. Given the crucial role of VEGF in the process of RA pannus formation and synovitis, antagonizing VEGF should be effective in the treatment of rheumatoid arthritis [16]. In this study, treatment of CIA rats by Avastin led to significant decreases in serum VEGF level, arthritis index, foot pad thickness, T/NT, and other evaluation indexes compared to the rats before treatment. In addition, serum VEGF level, pathological injury index, and VEGF immunohistochemical score were positively correlated in the treated rats.
The other drug used in this study to treat CIA rats was Etanercept. As a humanized recombinant human tumor necrosis factor-Fc, Etanercept could bind TNF-α to block TNF-α activity and has been used for RA treatment [17]. TNF-α is known to activate vascular endothelial cells, enhances the expression of endotheliocyte adhesion molecule, and promotes the secretion of VEGF and pannus formation [18]. Our data showed that CIA rats treated by Etanercept exhibited improved outcomes, such as reduced serum VEGF and TNF-α levels, reduced synovitis pathological injury index, and reduced VEGF staining in synovial tissue, compared to before treatment.
The imaging reagent 3P4-RGD2 used in this study for SPECT imaging contained amino acid residues Arg-Gly-Asp. Marked by radioactive nuclide 99mTc, this imaging reagent has affinity and selectivity for integrin αVβ3 receptor, which is expressed in vascular endothelial cell surface, especially during tumor angiogenesis [19]. In this study, we used 99mTc-3P4-RGD2 to observe the absorption of imaging agent at the limb joints of the rats. Before treatment there was abnormally high radioactive concentration at the limb joints of CIA rats, indicating pannus angiogenesis in these areas. However, in CIA rats treated with Avastin or Etanercept, which could inhibit VEGF bioactivity and expression, the radioactivity was significantly decreased, suggesting the inhibition of pannus angiogenesis.
The comparison between the Avastin group and Etanercept group showed that the gross morphology, serum VEGF and TNF-α levels, synovitis pathological injury index, imaging, and VEGF staining in synovial tissue were not significantly different in the rates treated by Avastin and those treated by Etanercept. These data provide evidence that Avastin exhibits similar effects to Etanercept to relieve RA in the rat model and suggest that Avastin is a promising new therapeutic agent for RA.
References
Konisti, S., S. Kiriakidis, and E.M. Paleolog. 2012. Hypoxia—a key regulator of angiogenesis and inflammation in rheumatoid arthritis. Nat Rev Rheumatol 8: 153–162.
Szekanecz, Z., T. Besenyei, G. Paragh, et al. 2009. Angiogenesis in rheumatoid arthritis. Autoimmunity 42: 563–573.
Szekanecz, Z., and A.E. Koch. 2008. Targeting angiogenesis in rheumatoid arthritis. Curr Rheumatol Rev 4: 298–303.
Aaltonen, K.J., L.M. Virkki, A. Malmivaara, et al. 2012. Systematic review and meta-analysis of the efficacy and safety of existing TNF blocking agents in treatment of rheumatoid arthritis. PLoS One 7: e30275.
Alonso-Ruiz, A., J.I. Pijoan, E. Ansuategui, A. Urkaregi, M. Calabozo, and A. Quintana. 2008. Tumor necrosis factor alpha drugs in rheumatoid arthritis: systematic review and metaanalysis of efficacy and safety. BMC Musculoskelet Disord 9: 52.
Heinzerling, J.H., and S. Huerta. 2006. Bowel perforation from bevacizumab for the treatment of metastatic colon cancer: incidence, etiology, and management. Curr Surg 63: 334–337.
Luo, X., X. Zuo, X. Mo, Y. Zhou, and X. Xiao. 2011. Treatment with recombinant Hsp72 suppresses collagen-induced arthritis in mice. Inflammation 34: 432–439.
Palmblad, K., H. Erlandsson-Harris, K.J. Tracey, et al. 2001. Dynamics of early synovial cytokine expression in rodent collagen-induced arthritis: a therapeutic study using a macrophage-deactivating compound. Am J Pathol 158: 491–500.
Romas, E., N.A. Sims, D.K. Hards, et al. 2002. Osteoprotegerin reduces osteoclast numbers and prevents bone erosion in collagen-induced arthritis. Am J Pathol 161: 1419–1427.
Yoo, S.A., S.K. Kwok, and W.U. Kim. 2008. Proinflammatory role of vascular endothelial growth factor in the pathogenesis of rheumatoid arthritis: prospects for therapeutic intervention. Mediat Inflamm 2008: 129873.
Paleolog, E.M. 2009. The vasculature in rheumatoid arthritis: cause or consequence? Int J Exp Pathol 90: 249–261.
Szekanecz, Z., T. Besenyei, A. Szentpétery, and A.E. Koch. 2010. Angiogenesis and vasculogenesis in rheumatoid arthritis. Curr Opin Rheumatol 22: 299–306.
Nagashima, M., H. Tanaka, H. Takahashi, et al. 2002. Study of the mechanism involved in angiogenesis and synovial cell proliferation in human synovial tissues of patients with rheumatoid arthritis using SCID mice. Lab Invest 82: 981–988.
Hashimoto, A., I.H. Tarner, R.M. Bohle, et al. 2007. Analysis of vascular gene expression in arthritic synovium by laser-mediated microdissection. Arthritis Rheum 56: 1094–105.
Clavel, G., N. Bessis, D. Lemeiter, et al. 2007. Angiogenesis markers (VEGF, soluble receptor of VEGF and angiopoietin-1) in very early arthritis and their association with inflammation and joint destruction. Clin Immunol 124: 158–164.
Thairu, N., S. Kiriakidis, P. Dawson, et al. 2011. Angiogenesis as a therapeutic target in arthritis in 2011: learning the lessons of the colorectal cancer experience. Angiogenesis 14: 223–234.
van Luijn, J.C., M. Danz, J.W. Bijlsma, F.W. Gribnau, and H.G. Leufkens. 2011. Post-approval trials of new medicines: widening use or deepening knowledge? Analysis of 10 years of Etanercept. Scand J Rheumatol 40: 183–191.
Cha, H.S., E.K. Bae, J.H. Koh, et al. 2007. Tumor necrosis factor-alpha induces vascular endothelial growth factor-C expression in rheumatoid synoviocytes. J Rheumatol 34: 16–19.
Su, Z.F., G. Liu, S. Gupta, Z. Zhu, M. Rusckowski, and D.J. Hnatowich. 2002. In vitro and in vivo evaluation of a Technetium-99m-labeled cyclic RGD peptide as a specific marker of alpha(V)beta(3) integrin for tumor imaging. Bioconjug Chem 13: 561–570.
Competing Interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Authors Yong Wang and Gula Da contributed equally.
Rights and permissions
About this article
Cite this article
Wang, Y., Da, G., Li, H. et al. Avastin Exhibits Therapeutic Effects on Collagen-Induced Arthritis in Rat Model. Inflammation 36, 1460–1467 (2013). https://doi.org/10.1007/s10753-013-9687-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-013-9687-y