Abstract
Epidermal growth factor receptor (EGFR) and its ligands are commonly expressed by synovial cells. The aim of the present study was to detect the potential effect of lapatinib an inhibitor of EGFR tyrosine kinases on collagen-induced arthritis. Thirty Wistar albino female rats were randomized into three groups. Arthritis was induced by intradermal injection of chicken type II collagen with incomplete Freund’s adjuvant. Serum TNF-α, IL-17, and malondialdehyde (MDA) levels were analyzed. Tissue superoxide dismutase (SOD), catalase and glutathione peroxidase (GPx) activities, and nuclear factor erythroid 2-related factor-2 (Nrf2) and heme oxgenase-1 (HO-1) expressions were determined. TNF-α, IL-17 and MDA levels, and Nrf2 and HO-1 expressions were lower in lapatinib-treated (30 mg/kg/day) group compared to sham group, while SOD, catalase, and GPx activities were higher (p < 0.05). Moreover, lapatinib ameliorated perisynovial inflammation and cartilage–bone destruction (p < 0.001). In conclusion, EGFR may have prominent pathogenic role and lapatinib may be an effective therapeutic option for arthritis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Rheumatoid arthritis (RA) is a disease of uncertain etiopathogenesis, characterized by chronic synovial inflammation and cartilage–bone erosions. Synovial hyperplasia and angiogenesis play critical roles on the pathogenesis of the deformities in RA [1–5]. Angiogenesis constitutes a very early event of synovial hyperplasia and thus causes the destruction in the joint cartilage and bone [2–4]. In addition to supplying hyperplastic synovium, the angiogenesis promotes the persistence of synovial inflammation through the influx of inflammatory cells to create the disease-specific microenvironment [2]. It also increases synovial inflammation by synthesizing pro-inflammatory cytokines activating the endothelial cells [2]. Furthermore, angiogenic factors synthesized in RA exacerbate the joint destruction through their direct activation of osteoclasts and osteoclast precursors [2]. The inhibition of angiogenesis has been shown to reduce the severity of arthritis and synovial hyperplasia as well as the destruction of joint bones and cartilage [3–5].
Histopathological studies revealed that epidermal growth factor (EGF), ErbB-2, and other tissue growth factor receptors are elevated in rheumatoid synovium [6, 7] and this elevation is associated with the synovial hyperplasia. The potential cause of increased EGF level in RA may be related to the angiogenesis [8]. The blockage of the EGF receptor (EGFR) directly produces critical anti-angiogenic effects [8] and indirectly inhibits the synthesis of other factors which have strong angiogenic effects [8, 9]. Besides, EGF has been shown to stimulate the synthesis of matrix metalloproteinase (MMP), which is known to be critically involved in joint destruction [10]. These data suggest that the inhibition of EGFR may prevent joint destruction in RA.
Lapatinib, a 4-anilinoquinoline derivate, inhibits the tyrosine kinase associated with EGFR [11]. It reversibly binds to the intracellular tyrosine kinase sites of EGFR-1 and EGFR-2 and inhibits substrate phosphorylation [12]. It thus prevents the flow in several important pathways including MAPK and P13K. Consequently, it performs anti-angiogenic and anti-proliferative actions by impacting the cell cycle progression, apoptosis, angiogenesis, and cell adhesion [12, 13]. It has been approved to use in the treatment of breast cancers in oncology due to its action on angiogenesis and cell proliferation.
The purpose of the present study is to explore the efficacy of lapatinib treatment in a collagen-induced arthritis (CIA) model.
MATERIALS AND METHODS
The experimental study was conducted on 30 Wistar albino female rats which were 8 to 10 weeks old and weighed between 200 and 250 g. The rats were randomized into three groups (with n = 10 in each group): control, arthritic sham, and lapatinib groups. The study was approved by the Animal Experiments Ethics Committee of Firat University.
Experimental procedures
Type 2 collagen (Sigma-Aldrich, St. Louis, USA) obtained from chicken sternum was diluted with 0.1 M acetic acid (1 mg/mL). The collagen solution was emulsified with an equal amount of incomplete Freund’s adjuvant (Difco Laboratories, Detroit, USA). The resulting solution was administered to the rats of the second and third groups with intradermal injections into the tail dorsal (100 μg/rat) and back paws (50 μg to each paw and total 200 μg to each rat). Seven days after the first administration, booster injection (100 μg/rat) was given through the tail dorsal.
Following the collagen injection, each rat was assessed on a daily basis for the development of arthritis and the clinical scoring of arthritis. The clinical scoring of arthritis was performed on both back paws on a scale between 0 and 4 points as described previously [14]. One day after the development of arthritis, the rats of the third group were given 30 mg/kg/day lapatinib (GlaxoSmithKline, Istanbul, Turkey) introduced through an orogastric probe.
All rats were sacrificed by decapitation on day 29 at the 15th day after the onset of arthritis. Blood samples were collected and the back paws were amputated from the knee down for further histopathological analysis. The blood samples collected were centrifuged at 3000 rpm for 10 min and the sera separated as such were kept at −20 °C until the day of analysis. Harvested tissue samples were cut into two parts; one part was fixed with 10 % formalin solution and embedded in paraffin for histopathological examination, and the other was stored immediately at −80 °C for Western blot analysis
Histopathological Evaluations
Tissue samples fixed in formalin solution were decalcified with 10 % nitric acid (30 days) to prepare paraffin blocks. Cross-sections taken from the blocks were stained with Hematoxylin-Eosin (H&E). Then, they were examined by a specialist pathologist under ×40, ×100, ×200, and ×400 magnifications in a light microscope to assess inflammatory cell infiltration, pannus formation, and bone destruction around the joint. The samples were scored for inflammatory status and the severity of arthritis on the scales between 0 and 4 points for histopathological scoring as described previously [15, 16].
Biochemical Analysis
Serum TNF-α (Invitrogen, Camarillo, CA, USA) and IL-17 (Uscn Life Science Inc., China) levels were studied using the relevant commercial kits according to the enzyme-linked immunosorbent assay (ELISA) method. Serum superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) activities were analyzed using appropriate commercial kits (Cayman Chemical Company, Ann Arbor, MI, USA) in accordance with the ELISA method. Serum malondialdehyde (MDA) levels were measured with a high-performance liquid chromatography device (Shimadzu, Japan).
Western Blot Analysis
Joint tissue samples were analyzed for the expression of nuclear factor erythroid 2-related factor-2 (Nrf2) and heme oxgenase-1 (HO-1) using the Western blot technique. In all groups, the hind paws were excised rapidly from sacrificed rats and then quickly frozen at −80 °C. Small pieces of the paw joints in each group of animals were pooled together for Western blot analysis. Homogenates were prepared in ice-cold lysis buffer containing 50 mM Tris–HCl (pH, 8.0), 150 mM NaCl, 5 mM EDTA, 1 % Triton X-100, 0.26 % sodium deoxycholate, 50 mM sodium fluoride, 10 mM b-glycerophosphate, 0.1 mM sodium orthovanadate, 10 μg/mL leupeptin, and 50 μg/mL phenylmethylsulfonyl fluoride (PMSF), and incubated on ice for 40 min [17]. Eighty microliters of 10 % Nonidet P–40 (NP–40) solution was added to the homogenates, and the mixture was then centrifuged for 2 min at 14,000g. at 4 °C for removing the cellular debris and isolating total protein. Concentration of the protein was determined according to the procedure described by Lowry et al. [18] using a protein assay kit supplied by Sigma (St. Louis, MO, USA). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis sample buffer containing 2 % b-mercaptoethanol was added to the supernatant. Equal amounts of protein (50 μg) were electrophoresed and subsequently transferred to nitrocellulose membranes (Schleicher and Schuell Inc., Keene, NH, USA). Nitrocellulose blots were washed twice for 5 min each in phosphate-buffered saline (PBS) and blocked with 1 % bovine serum albumin in PBS for 1 h prior to application of the primary antibody. The antibody against Nrf2 and HO-1 was purchased from Abcam Inc. (Abcam, Cambridge, UK). Primary antibody was diluted (1:1000) in the same buffer containing 0.05 % Tween-20. The nitrocellulose membrane was incubated overnight at 4 °C with protein antibody. The blots were washed and incubated with horseradish peroxidase-conjugated goat anti-mouse IgG (Abcam, Cambridge, UK). Specific binding was detected using diaminobenzidine and H2O2 as substrates. Protein loading was controlled using a monoclonal mouse antibody against β-actin antibody (A5316; Sigma). Blots were performed at least three times to confirm the reproducibility of the results. Bands were analyzed densitometrically using an image analysis system (Image J; National Institute of Health, Bethesda, USA).
Statistical Analysis
Data were presented as mean ± standard deviation. Statistical evaluations were performed using the SPSS package program, version 21. Kruskal–Wallis one-way analysis of variance was used for comparisons among the groups, and the Mann–Whitney U test was used for dual comparisons. Differences in continuous values (clinical scoring of arthritis on the 14th and 29th days) were assessed using the Wilcoxon rank-sum test. A p value of <0.05 was considered as significant.
RESULTS
Clinical Scoring of Arthritis
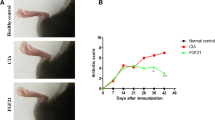
Arthritis was clinically developed in all rats of the second and third groups at 13 to 14 days after the injection of collagen. While the arthritis scores were similar in the sham- and lapatinib-treated groups on day 14 (p > 0.05), they were lower in the lapatinib group than in the sham group on day 29 (p < 0.001) (Table 1, Fig. 1). The arthritis score on day 29 was lower than the 14th day score in the lapatinib-treated group (p = 0.004) while it was decreased in the sham group (p = 0.008).
Assessments of daily arthritis score in the all study groups. Multiplication sign indicates the mean 14th day clinical arthritis scores were higher in the sham and lapatinib groups than in the control group (p < 0.001 for both). Plus sign indicates the mean 29th day score of sham group was higher than in lapatinib groups (p < 0.001). Asterisk indicates the mean 29th day scores was decreased in lapatinib group while it was increased in the sham group compared to the own 14th day score (Wilcoxon Rank test; p = 0.004 and p = 0.008, respectively).
Histopathological Evaluations
The histopathological scoring of joint tissue samples showed a significant decrease in inflammation and destruction scores of the rats in the lapatinib-treated group, in comparison to those of sham group (p < 0.001 for both) (Table 1 and Figs. 2 and 3).
Histopathological sections of joints in study groups (H&E ×40). Normal perisynovial tissue and cartilage–bone appearance in the control group (a). Obvious perisynovial inflammation and destruction of cartilage–bone in the sham group (b). Minimal perisynovial inflammation and synovial hyperplasia in the lapatinib-treated group (c).
Inflammation (a) and cartilage–bone destruction (b) scorings of the study groups. Histopathological scorings of inflammation and cartilage–bone destruction were increased in the Sham group compared to the control group (*p < 0 < 001 for both). On the other hand, they were decreased in the lapatinib group compared to the sham group (**p < 0.05 for both).
Serum Pro-inflammatory Cytokine Levels
Serum TNF-α and IL-17 levels were higher in the sham group than in the control group I (p < 0.01 for both). However, serum TNF-α and IL-17 levels were lower in the lapatinib-treated group than in the sham group (p < 0.01 for both). Serum TNF-α levels were similar in the control and lapatinib-treated groups, while serum IL-17 level was higher in the lapatinib group (p < 0.01) (Table 1).
Serum Malondialdehyde Levels
Serum MDA level was higher in the sham group than in the control group (p < 0.01). In the lapatinib-treated group, serum MDA level was higher than those in the control group and lower than those in the sham group (p < 0.01 for both) (Table 1).
Serum Anti-oxidants
SOD, CAT, and GPx activities were lower in the sham group than in the control group (p < 0.01 for all). However, they were higher in the lapatinib-treated group than in the control group (p < 0.05, p < 0.01, and p < 0.01, respectively). The lapatinib-treated group had lower SOD and CAT activities than the control group (p < 0.01 and p < 0.05, respectively). There was no significant difference between the control and lapatinib-treated groups in terms of the GPx activities (p > 0.05) (Table 1).
Western Blot Analysis
The Nrf2 and HO-1 expressions were lower in the sham group when compared to the control group (p < 0.05 for both). Lapatinib-treated group had the increased Nrf2 and HO-1 expressions compared to the sham group (p < 0.05 for both) (Fig. 4).
Western blot analysis and densitometric quantifications of Nrf2 (a) and HO-1 (b). The expressions of Nrf2 and HO-1 were lower in the sham group compared to the sham group (p < 0.05 for both). On the other hand, they were higher in the lapatinib-treated group compared to the sham group (p < 0.05 for both). Representative blots, repeated at least three times (n = 4), are shown. Actin was included to ensure equal protein loading. The densitometric quantifications were normalized to actin densities for each sample and expressed as mean ± SD. Different superscripts (a, b) indicate group mean differences (p < 0.05). Nrf2, nuclear factor erythroid 2-related factor-2, HO-1 heme oxgenase-1.
DISCUSSION
In the present study, the efficacy of lapatinib treatment, a tyrosine kinase inhibitor, on an experimental model of arthritis induced by collagen was observed. Administration of type II chicken collagen emulsified with incomplete Freund’s adjuvant to Wistar albino rats on day 1 and day 8 induced the development of arthritis on days 12–13. The rats in the arthritis group with no additional procedure developed diffuse inflammation in the perisynovial tissue, synovial hypertrophy and hyperplasia, pannus formation, and marked cartilage and bone erosion. Additionally, the serum levels of TNF-α, IL-17, and MDA increased, while SOD, CAT, and GPx enzyme activities decreased in the arthritis group. Lapatinib treatment, however, clinically reduced the arthritis score, histopathologically decreased synovial inflammation and cartilage–bone erosion, caused a decline in serum TNF-α and IL-17 levels, and increased anti-oxidant enzyme activities.
The type I family of tyrosine kinase receptors includes 4 homologous members: ErbB1 also EGFR, ErbB2 also known as human EGFR2 (HER2), ErbB3, and ErbB4 [11]. Multiple signal transduction pathways lie downstream of these activated receptors, including the mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-kinase (PI3K) pathways. Phospholipase Cγ and signal transducers and activators of transcription (STAT) also lie downstream of the ErbB receptors. The outcome of signaling regulates cell proliferation, angiogenesis, pro-inflammatory cytokine production, apoptosis, or cell adhesion [11–13]. Agents that target EGFR exert anti-proliferative and anti-angiogenic effects and they are used in cancer therapy.
Several studies [6, 7] have demonstrated that EGFR is expressed in synovial fibroblasts, in patients with RA. In addition, the increased concentrations of serum and joint EGF and EGFR have been demonstrated in RA patients [6, 7]. In addition, Yamane et al. [19] demonstrated that recombinant human amphiregulin which is a ligand for EGFR stimulated the proliferation of synovial fibroblast. These data suggest that EGF and EGFR have important roles in RA pathogenesis.
Synovial hyperplasia and angiogenesis are the hallmarks of RA pathogenesis. Owing to the synovial hyperplasia and the infiltration of inflammatory cells, the synovial lining increases greatly in mass. This hyperplastic synovial lining necessitates an increase in the vascular supply to the synovia, to cope with the increased requirement for oxygen and nutrients. When angiogenesis constitutes synovial hyperplasia, it transforms into a pannus tissue, ultimately leading to cartilage and bone destruction. Due to an ability to promote angiogenesis and cell proliferation, EGFR may contribute to cartilage and bone destruction in RA. EGFR tyrosine kinase inhibitors are used in oncology for their anti-angiogenic and anti-proliferative action. Swanson et al. [20] have showed in an experimental arthritis model that EGFR blockage with erlotinib reduces the severity of arthritis. In the present study, lapatinib an EGFR tyrosine kinase inhibitor leads to a decrease in synovial hyperplasia and pannus formation and prevents the development of cartilage and bone destruction.
The inhibition of EGFR may reduce production of pro-inflammatory cytokines. Yamane et al. [19] have demonstrated that EGFR ligand amphiregulin stimulates synthesis of the mRNA expression levels of VEGF, IL-8, GM-CSF, and IL-6 by cultured synovial fibroblast. Swanson et al. [20] have revealed that EGF induces RA synovial fibroblasts to produce VEGF, IL-8, MCP-1, and MMP-3 and concurrent EGFR tyrosine kinase inhibitor erlotinib treatment reduced the production of these cytokines. Currently, TNF-α is known to play a critical role in the pathogenesis of inflammatory joint disease, and the therapeutic efficacy of TNF-α inhibitors is recognized. Successful results obtained from inflammatory joint diseases with anti-TNF-α drugs demonstrate the significance of TNF-α in inflammation. In our study, the TNF-α level was elevated in the mice with CIA, while it was reduced in the lapatinib-treated mice. The decrease in TNF-α level may be one cause of the anti-arthritic action of lapatinib treatment.
Interleukin-17 is a cytokine synthesized primarily by CD + 4 T (Th17) cells. However, it is also known to be synthesized by other immune cells. It exhibits a synergistic effect with TNF-α and stimulates the synthesis of other pro-inflammatory cytokines [21, 22]. In RA, IL-17 initiates the release of destructive enzymes from the synovial fibroblasts [21]. Besides, it activates chondrocytes and macrophages which synthesize these destructive enzymes act to promote joint erosion [23]. It is known that MMPs are the enzymes playing the major part in cartilage–bone destruction. IL-17 has been shown to increase the synthesis of MMP from the synovial cells, fibroblasts, and macrophages [23]. Furthermore, it has been demonstrated that IL-17 activates osteoclasts which are known to have a critical function in bone resorption and stimulates the synthesis of prostaglandin E2 and nitric acid, which is thought to be involved in cartilage destruction [24, 25]. Chabaud et al. [26] have reported that recombinant mouse IL-17 injected into the normal rat joint causes joint destruction and showed the role of IL-17 in joint destruction in RA. On the other hand, Lubberts and colleagues [27] have proved in the CIA model that anti-IL-17 antibody treatment prevented cartilage and bone erosion. Similarly, it was established in our study that lapatinib treatment reduced IL-17 levels and impeded cartilage and bone destruction.
Reactive oxygen radicals increase dramatically in inflammatory conditions, particularly in inflammatory joint diseases. Of these radicals, superoxide anion (O2−) plays a critical role in inflammation. SOD enzyme and other anti-oxidant enzymes neutralize oxygen radicals and offset the damage inflicted by these highly aggressive products [28]. Reactive oxygen radicals are produced abundantly from the inflammatory foci together with cytokines and prostaglandins, and this production is associated with reduced anti-oxidant enzyme levels [28]. Reactive oxygen products are significantly involved in the development of cartilage damage [28–30]. Intra-articular SOD administration was shown to be related to clinical recovery and a decline in the level of inflammation [31, 32]. In our study, the serum levels of MDA increased, while SOD, CAT, and GPx enzyme activities decreased in the arthritis group. On the other hand, lapatinib treatment restored anti-oxidants and oxidant.
Nrf2, a redox-sensitive transcription factor, binds to anti-oxidant response elements (ARE) encoding many anti-oxidant enzymes and related stress-responsive proteins including glutathione S-transferase, GPx, and HO-1. Thus, Nrf2 regulates redox status and plays key roles in cellular defense by enhancing the removal of reactive oxygen species [33]. It has been documented that Nrf2-knockout mice have more severe cartilage injuries and more oxidative damage during experimentally induced arthritis [34, 35]. These results [34, 35] support a protective role of Nrf2 against arthritis. In the present study, Nrf2 and HO-1 expressions were decreased in the arthritis group, while lapatinib treatment increased their expressions. It may be concluded that anti-inflammatory effects of lapatinib lead to the restoration of oxidative stress. However, at the end of the treatment, anti-oxidant potentials of lapatinib may contribute its anti-arthritic effects.
The present study has several limitations. Firstly, our study did not examine angiogenesis. Lapatinib may have actions on angiogenesis. Secondly, intracellular signaling pathways may be potential targets of lapatinib and they could be studied in our study.
In conclusion, lapatinib, an EGFR tyrosine kinase inhibitor, decreases the levels of TNF-α and IL-17, increases anti-oxidant activity, and prevents cartilage–bone destruction in the CIA model. Therefore, these results suggest that EGFR may play a pathogenic role in RA and it may be concluded that lapatinib appears to be a promising agent in the treatment of RA.
References
Hirohata, S., and J. Sakakibara. 1999. Angioneogenesis as a possible elusive triggering factor in rheumatoid arthritis. Lancet 353: 1331.
Pap, T., and O. Distler. 2005. Linking angiogenesis to bone destruction in arthritis. Arthritis and Rheumatism 52: 1346–1348.
Clavel, G., N. Bessis, and M.C. Boissier. 2003. Recent data on the role for angiogenesis in rheumatoid arthritis. Joint, Bone, Spine 70: 321–326.
Nagashima, M., H. Tanaka, H. Takahashi, A. Tachihara, K. Tanaka, T. Ishiwata, et al. 2002. Study of the mechanism involved in angiogenesis and synovial cell proliferation in human synovial tissues of patients with rheumatoid arthritis using SCID mice. Laboratory Investigation 82: 981–988.
Sone, H., Y. Kawakami, M. Sakauchi, Y. Nakamura, A. Takahashi, H. Shimano, et al. 2001. Neutralization of vascular endothelial growth factor prevents collagen-induced arthritis and ameliorates established disease in mice. Biochemical and Biophysical Research Communications 281: 562–568.
Kusada, J., T. Otsuka, N. Matsui, T. Hirano, K. Asai, and T. Kato. 1993. Immuno-reactive human epidermal growth factor (h-EGF) in rheumatoid synovial fluids. Nippon Seikeigeka Gakkai Zasshi 67: 859–865.
Satoh, K., S. Kikuchi, M. Sekimata, Y. Kabuyama, M.K. Homma, and Y. Homma. 2001. Involvement of ErbB-2 in rheumatoid synovial cell growth. Arthritis and Rheumatism 44: 260–265.
Perrotte, P., T. Matsumoto, K. Inoue, H. Kuniyasu, B.Y. Eve, D.J. Hicklin, et al. 1999. Anti-epidermal growth factor receptor antibody C225 inhibits angiogenesis in human transitional cell carcinoma growing orthotopically in nude mice. Clinical Cancer Research 5: 257–265.
Goldman, C.K., J. Kim, W.L. Wong, V. King, T. Brock, and Gillespie. 1993. Epidermal growth factor stimulates vascular endothelial growth factor production by human malignant glioma cells: a model of glioblastoma multiforme pathophysiology. Molecular Biology of the Cell 4: 121–133.
Zhang, Z., T. Song, Y. Jin, J. Pan, L. Zhang, L. Wang, and P. Li. 2009. Epidermal growth factor receptor regulates MT1-MMP and MMP-2 synthesis in SiHa cells via both PI3-K/AKT and MAPK/ERK pathways. International Journal of Gynecological Cancer 19: 998–1003.
Kim, H.P., Y.K. Yoon, J.W. Kim, S.W. Han, H.S. Hur, J. Park, et al. 2009. Lapatinib, a dual EGFR and HER2 tyrosine kinase inhibitor, downregulates thymidylate synthase by inhibiting the nuclear translocation of EGFR and HER2. PloS One 16: 5933.
Rusnak, D.W., K. Lackey, K. Affleck, E.R. Wood, K.J. Alligood, N. Rhodes, et al. 2001. The effects of the novel, reversible epidermal growth factor receptor/ErbB-2 tyrosine kinase inhibitor, GW2016, on the growth of human normal and tumor-derived cell lines in vitro and in vivo. Molecular Cancer Therapeutics 1: 85–94.
Hirata, A., S. Ogawa, T. Kometani, T. Kuwano, S. Naito, M. Kuwano, et al. 2002. ZD1839 (Iressa) induces antiangiogenic effects through inhibition of epidermal growth factor receptor tyrosine kinase. Cancer Research 62: 2554–2260.
Trentham, D.E., A.S. Townes, and A.H. Kang. 1977. Autoimmunity to type II collagen an experimental model of arthritis. The Journal of Experimental Medicine 146: 857–868.
Hoffmann, M.S., S. Hayer, and G. Steiner. 2009. Immmunopathogenesis of rheumatoid arthritis; induction of arthritogenic autoimmune responses by proinflammatory stimuli. Annals of the New York Academy of Sciences 1173: 391–400.
Larsson, P., S. Kleinau, R. Holmdahl, and L. Klareskog. 1990. Homologous type II collagen-induced arthritis in rats. Characterization of the disease and demonstration of clinically distinct forms of arthritis in two strains of rats after immunization with the same collagen preparation. Arthritis and Rheumatism 33: 693–701.
Choi, J., B.J. Yoon, Y.N. Han, K.T. Lee, J. Ha, H.J. Jung, and H.J. Park. 2003. Antirheumatoid arthritis effect of rhus verniciflua and of the active component, sulfuretin. Planta Medica 69: 899–904.
Lowry, O.H., N.J. Rosebrough, A.L. Farr, and R.J. Randall. 1951. Protein measurement with the folin phenol reagent. Journal of Biological Chemistry 193: 165–175.
Yamane, S., S. Ishida, Y. Hanamoto, K. Kumagai, R. Masuda, K. Tanaka, et al. 2008. Proinflammatory role of amphiregulin, an epidermal growth factor family member whose expression is augmented in rheumatoid arthritis patients. Journal of Inflammation 5: 5.
Swanson, C.D., E.H. Akama-Garren, E.A. Stein, J.D. Petralia, P.J. Ruiz, A. Edalati, T.M. Lindstrom, and W.H. Robinson. 2012. Inhibition of epidermal growth factor receptor tyrosine kinase ameliorates collagen-induced arthritis. Journal of Immunology 188: 3513–3521.
Li, X., F.L. Yuan, W.G. Lu, Y.Q. Zhao, C.W. Li, J.P. Li, and R.S. Xu. 2010. The role of interleukin-17 in mediating joint destruction in rheumatoid arthritis. Biochemical and Biophysical Research Communications 397: 131–135.
Van Bezooijen, R.L., L. Van Der Wee-Pals, S.E. Papapoulos, and C.W. Löwik. 2002. Interleukin 17 synergises with tumour necrosis factor alpha to induce cartilage destruction in vitro. Annals of the Rheumatic Diseases 61: 870–876.
Koenders, M.I., J.K. Kolls, B. Oppers-Walgreen, L. van den Bersselaar, L.A. Joosten, J.R. Schurr, et al. 2005. Interleukin-17 receptor deficiency results in impaired synovial expression of interleukin-1 and matrix metalloproteinases 3, 9, and 13 and prevents cartilage destruction during chronic reactivated streptococcal cell wall-induced arthritis. Arthritis and Rheumatism 52: 3239–3247.
Kamiya, S., C. Nakamura, T. Fukawa, K. Ono, T. Ohwaki, T. Yoshimoto, et al. 2007. Effects of IL-23 and IL-27 on osteoblasts and osteoclasts: inhibitory effects on osteoclast differentiation. Journal of Bone and Mineral Metabolism 25: 277–285.
LeGrand, A., B. Fermor, C. Fink, D.S. Pisetsky, J.B. Weinberg, T.P. Vail, et al. 2001. Interleukin-1, tumor necrosis factor alpha, and interleukin-17 synergistically up-regulate nitric oxide and prostaglandin E2 production in explants of human osteoarthritic knee menisci. Arthritis and Rheumatism 44: 2078–2083.
Chabaud, M.G., G. Page, and P. Miossec. 2001. Enhancing effect of IL-1, IL-17, and TNF-alpha on macrophage inflammatory protein-3alpha production in rheumatoid arthritis: regulation by soluble receptors and Th2 cytokines. Journal of Immunology 167: 6015–6020.
Lubberts, E., L. van den Bersselaar, B. Oppers-Walgreen, P. Schwarzenberger, C.J. Coenen-de Roo, J.K. Kolls, et al. 2003. IL-17 promotes bone erosion in murine collagen-induced arthritis through loss of the receptor activator of NF-kappa B ligand/osteoprotegerin balance. Journal of Immunology 170: 2655–2662.
Afonso, V., R. Champy, D. Mitrovic, P. Collin, and A. Lomri. 2007. Reactive oxygen species and superoxide dismutases: role in joint diseases. Joint, Bone, Spine 74: 324–329.
Sandhu, J.K., S. Robertson, H.C. Birnboim, and R. Goldstein. 2003. Distribution of protein nitrotyrosine in synovial tissues of patients with rheumatoid arthritis and osteoarthritis. Journal of Rheumatology 30: 1173–1181.
Mathy-Hartert, M., G. Martin, P. Devel, G. Deby-Dupont, J.P. Pujol, J.Y. Reginster, et al. 2003. Reactive oxygen species downregulate the expression of pro-inflammatory genes by human chondrocytes. Inflammation Research 52: 111–118.
Goebel, K.M., U. Storck, and F. Neurath. 1981. Intrasynovial orgotein therapy in rheumatoid arthritis. Lancet 1: 1015–1017.
Salvemini, D., and S. Cuzzocrea. 2003. Therapeutic potential of superoxide dismutase mimetics as therapeutic agents in critical care medicine. Critical Care Medicine 31: 29–38.
Hayes, J.D., and A.T. Dinkova-Kostova. 2014. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends in Biochemical Sciences 39(4): 199–218.
Wruck, C.J., A. Fragoulis, A. Gurzynski, L.O. Brandenburg, Y.W. Kan, and K.K. Chan. 2011. Role of oxidative stress in rheumatoid arthritis: insights from the Nrf2-knockout mice. Annals of the Rheumatic Diseases 70: 844–850.
Maicas, N., M.L. Ferrándiz, R. Brines, L. Ibáñez, A. Cuadrado, M.I. Koenders, W.B. van den Berg, and M.J. Alcaraz. 2011. Deficiency of Nrf2 accelerates the effector phase of arthritis and aggravates joint disease. Antioxidants and Redox Signaling 15(4): 889–901.
Conflict of Interest
None.
Ethics Approval
Approval was obtained from the Ethics Committee of Firat University.
Provenance and Peer Review
Not commissioned; externally peer reviewed.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ozgen, M., Koca, S.S., Karatas, A. et al. Lapatinib Ameliorates Experimental Arthritis in Rats. Inflammation 38, 252–259 (2015). https://doi.org/10.1007/s10753-014-0028-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-014-0028-6