Abstract
The fish communities in the Yangtze River (YR) basin is in a degraded state due to the influence of human factors. Here, we used environmental DNA (eDNA) metabarcoding technology to conduct a survey on the fish diversity in the upper reaches of the YR from Jiangjin to Fuling to search for better monitoring methods. We set up 12 sampling sites in this river section, collected 36 environmental samples, and obtained 5,067,423 valid sequences. After conducting an annotated comparative analysis utilizing the NCBI public database, a total of 104 freshwater fish species were identified, belonging to 8 orders, 24 families, and 72 genera. This included six nationally protected fish species, as well as endemic, introduced, and previously unrecorded species within this basin. The results of this survey indicate a trend toward miniaturization of species and a decline in endemic fish species as well as an increase in exotic fish species in the region, which implied the fish communities in the upper YR region remains in a degraded state. However, the species composition at the family level has remained relatively stable over the past decade. Furthermore, the diversity analysis revealed that fish composition and diversity exhibit variability across different locations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Yangtze River (YR) is the world’s third-longest river, having a total length of 6380 km (Shen et al., 2019). The upper reaches of YR refer to the mainstream section from Yibin to Yichang, which includes a variety of geomorphological types, such as mountains, hills, and plains, with a substantial elevation variation in the riverbed of more than 1500 m, alternating slow and fast currents and complex flow patterns (He et al., 2010; Wei, 2012; Liu et al., 2019; Meng et al., 2019). It is home to several rare and endemic fish species, as well as being a critical location for the conservation of the genetic germplasm and biodiversity of fish (Xie & Chen, 1999; Qin et al., 2008). The intricate topography and heterogeneous climate of the YR basin have engendered a wide variety of aquatic habitats, thereby fostering a diverse array of aquatic communities, comprising over 400 fish species (approximately 360 freshwater fish species), with endemic fish constituting up to 42% of the overall fish number (Cao, 2011; Liu et al., 2019). Meanwhile, there are 286 fish species in the upper YR, including 124 endemic fish, accounting for 79.49% of all endemic fish in the YR (He et al., 2010; Cao, 2011; Wei, 2012; Meng et al., 2019). With a total length of nearly 200 km, the section of the YR from Jiangjin to Fuling is located in the middle of the upper reaches. It is a crucial section in the upper reaches that includes the National Aquatic Germplasm Resources Conservation Area of the four major Chinese carps in the Chongqing Section of the Yangtze River (the reserve of the four major Chinese carps (Ctenopharyngodon idella (Valenciennes 1844), Hypophthalmichthys molitrix (Valenciennes 1844), Hypophthalmichthys nobilis (Richardson 1845), and Mylopharyngodon piceus (Richardson 1846)) and the connecting section of the national nature reserve of rare and endemic fish in the upper YR and the reserve of the four major Chinese carps.
As an essential part of the water ecosystem, fish impact the water environment and numerous aquatic organisms via upstream and downstream effects (Reis et al., 2020). However, due to anthropogenic factors, including dam construction, dredging and quarrying, and channel regulation, the endangerment of fish in the YR is increasing, and the number of threatened fish species is highest in the upper reaches of the YR basin (Jiang et al., 2016b). According to recent research, miniaturization of fish species is a serious problem in the upper YR, and endemic fish communities have been drastically reduced (Gao et al., 2015; Yang et al., 2017; Wang et al., 2019; Zhou et al., 2022). Meanwhile, the number of some fish species that are not native to the upper mainstream area of the YR, such as Neosalanx taihuensis (Chen 1956) and Pseudolaubuca engraulis (Nichols 1925), which prefer slow or still water habitats, have increased in the upper reservoir area (Gao et al., 2015; Yang et al., 2017; Wang et al., 2019; Zhou et al., 2022). According to previous research, the development of the upper YR cascade hydropower will fragment the habitats of 134 species of fish, restrict the migratory paths of 35 species, and impact the reproduction of 26 species which reproduce by drifting eggs fish (Cheng et al., 2015). Hence, the fish communities in the upper YR must be protected immediately.
Fish diversity is an essential aspect of biodiversity in aquatic ecosystems and a critical indicator that can be used to assess the health of aquatic ecosystems (Holmlund and Hammer 1999; Zou et al., 2020). Several researchers have explored and analyzed the upper reaches of the YR, which is a hotspot for fish diversity research. Traditional fishing methods (e.g., gill nets, ground cages, etc.) are still widely employed in the YR to monitor the fish diversity (Wu et al., 2011; Gao et al., 2013; Xing et al., 2021), which is not only time-consuming and the results are contingent, but is also hazardous to fish and to the environment. Further, it is challenging to discover rare or small-sized fish with this technique (Becker et al., 2015; Sales et al., 2018). Furthermore, acoustic detection has been utilized to monitor YR fish (Shi 2019), however, this approach focuses more on the density of species than on species-specific identification. Simultaneously, in a non-invasive manner, eDNA technology has been used extensively due to its ability to gather information on the presence of species and even produce quantitative estimations of species (Doi et al., 2017).
By extracting DNA from the environment, selecting appropriate primers, and integrating molecular biology techniques such as PCR and DNA sequencing, eDNA techniques are utilized for the qualitative or quantitative analysis of species. All DNA present in environmental samples, including mixed DNA from inside and outside the cells of the target organism, such as epidermal cells, secretions, germ cells, and so on, are referred to as eDNA. This allows us to determine the presence of the species in the approximate range without collecting organisms (Taberlet et al., 2012; Thomsen et al., 2015; Balasingham et al., 2018). The technology is also commonly used in the YR basin (Qu et al., 2020; Li et al., 2021; Wang et al., 2022). Even though eDNA is easily affected by many environmental factors, including the temperature, ultraviolet light, and pH (Bohmann et al., 2014; Strickler et al., 2015), studies have shown that the detection efficiency and sensitivity of this technology can be higher than those of traditional monitoring methods (Dejean et al., 2012; Jerde et al., 2013; Valentini et al., 2016), indicating that eDNA technology is an effective complementary tool for traditional monitoring methods (Hinlo et al., 2017).
On January 1, 2021, the “10-year ban on fishing in the YR basin” came into effect. Traditional resource survey approaches have been constrained in the context of YR preservation. In this study, the eDNA technique was used to analyze the fish species composition in the upper reaches of the YR from Jiangjin to Fuling to explore its applicability for fish monitoring and provide data for the “10-year ban on fishing the YR.”
Materials and methods
Study area
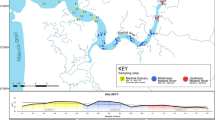
The river section from Jiangjin (106° 26′ 44.43″ E, 29° 21′ 9.72″ N) to Fuling (107° 30′ 30.35″ E, 29° 53′ 6.02″ N) is roughly 200 km long and is located in the upper reaches of the YR. A total of 12 sampling sites were established in this river section, with four points in the non-protected areas, Luo Huang (LH), Da Dukou (DDK), Chao Tianmen (CTM), and Xia Kou (XK), and eight points in the protected areas, Guang Yangdao (GYD), Mu Dong (MD), Ma Liu (ML), Chang Shou (CS), Shi Tuo (ST), Li Du (LD), Qing Xi (QX), and Nan Tuo (NT) (Fig. 1).
eDNA sampling and processing
In early August 2021, water collectors gathered 8 l of surface water within 50 m of each sampling point along the riverbank, which was further mixed, and then 6 l of the water samples were divided into three polyethylene bottles (3 replicate samples), a total of 36 samples. Before collecting water samples from several sampling sites, all equipment and sampling bottles were rinsed with a 10% bleach solution and replaced with disposable gloves to prevent exogenous DNA contamination (Dibattista et al., 2017). Within 24 h, the water samples were immediately stored under refrigeration and pumped through a vacuum peristaltic pump onto a 0.45 µm mixed cellulose membrane (Whatman, UK). Water samples with sediments were pre-filtered using sterile medical gauze before collection (Stewart et al., 2017). Similarly, all filtering apparatus were sterilized before sample filtration to avoid cross-contamination between samples. A negative control was also set up with distilled water at the time of filtration (Zhang et al., 2020). Finally, the membranes were frozen at − 80°C until use in the next DNA extraction step.
The total DNA from the water samples was extracted from the filter membrane using the PowerWater DNA Isolation Kit (Qiagen) in accordance with the instructions of the manufacturer. Next, 1% gel electrophoresis was used to detect the quality of the extracted eDNA. Each sample was extracted separately, and a blank filter membrane was established as a negative control simultaneously. Finally, the extracted DNA samples were stored at − 80°C until the next step of PCR amplification.
The samples were amplified using universal primers (tele02-F: 5′-AAA CTC GTG CCA GCC ACC-3′; tele02-R: 3′-GGG TAT CTA ATC CCA GTT TG-5′), which amplify a short fragments of the 12S rRNA mitochondrial gene (Taberlet et al., 2018). The 20 µl TransStart Fastpfu DNA polymerase amplification system contains 4 µl of 5 × FastPfu buffer, 2 µl of dNTPs (2.5 mM), 0.4 µl of FastPfu polymerase, 1–2 µl of template DNA (10 ng), 0.8 µl of each of the upstream and downstream primers (10 µM in total). It was made up to 20 µl with ddH2O. The following PCR reaction conditions were used: predenaturation at 95°C for 5 min, 35 cycles of 95°C for 30 s, 58°C for 30 s, and 72°C for 45 s, and a final extension at 72°C for 10 min. The ddH2O was used as a template for the PCR negative control to determine whether contamination occurred during the PCR amplification. The PCR amplification process was repeated three times for each sample, and their PCR products were mixed and detected by 2% agar gel electrophoresis. The target bands in this investigation were obtained by electrophoresis for 36 samples and were found to be around 167 bp in length, but none of the negative controls had a target band. Finally, the purified PCR products were recovered, then the Illumina PE250 library preparation was constructed and sequenced via paired-end sequencing by the Illumina sequencing facility.
Bioinformatic analyses and taxonomic assignment
Firstly, some poor-quality sequences, such as those less than 100 bp in length, were filtered out using Trimmomatic v.0.36 (Bolger et al., 2014), and pairs of reads were merged into a sequence using FLASH (v.1.2.11) (Magoč and Salzberg 2011). Then, the chimeras were removed using Usearch software combined with the reference sequences of the GOLD database and denovo sequences. Using Usearch (version 10 http://drive5.com/uparse/), high-quality sequences were combined as “parent–child” sets with 97% similarity (Zhang et al., 2022), and the unique sequences were obtained. Later, the comparison and taxonomic annotation were done on the public database, NCBI (https://www.ncbi.nlm.nih.gov/) with the Blastn tool using the uclust algorithm. A preliminary taxonomic (i.e., molecular operational taxonomic unit (MOTU)) annotation table was obtained by searching the unique sequences in the public database using criteria of ≥ 97% similarity (Sales et al., 2020), e ≤ 10–5, and coverage ≥ 0.9. The results obtained from the annotations were manually filtered to remove MOTUs sequences of non-fishes, and then combined with historical data from the Yangtze River basin to screen out MOTUs sequences of fish that are unlikely to belong to the region. Finally, MOTUs with sequence numbers of greater than ten were retained (Shu 2022; Zhang et al., 2022).
Reads from each sample were randomly selected using QIIME v.1.9.0 to normalize all eDNA sample data by the smallest number actually sequenced in all samples (Caporaso et al., 2010). After standardization, the relative sequence abundance (read ratio) of each species in each sample was kept constant. The averaged values of three parallel samples were taken for subsequent analyses such as species composition, alpha diversity, and beta diversity. The species composition mapping was based on the species sequence abundance ≥ 1%, where parts with an abundance of less than 1% can be merged with others (Wang et al., 2022). The alpha diversity, often expressed as the species richness, is the diversity of species within a relatively small area and reflects the results within a sample (Tuomisto 2010). The beta diversity analysis shows the variation in species characteristics across sites, reflecting the relationships among samples (Anderson et al., 2011). Based on the Bray–Curtis distance, the principal co-ordinates analysis (PCoA) and non-metric multidimensional scaling (NMDS) analysis were selected for the beta analysis.
Based on the relative sequence abundance of the species, the Shannon index (Shannon 1948), Simpson index (Simpson 1949), and Pielou index were calculated for each site, and the McNaughton index was used to determine the dominant species (Ling et al., 2022; Woodland et al., 2019). The Shannon index measures the community diversity by taking the richness and evenness of the community into consideration, with a higher index values indicating higher community diversity (Shannon 1948). The Simpson index commonly represents an area biodiversity, where higher values indicate a lower community diversity (Simpson 1949). The index is calculated as follows:
In the formulas, N is the total number of fish sequences detected; ni is the number of sequences of the i-th fish species; H is the Shannon index; Hmax is the maximum Shannon index that can be achieved with the same species richness (i.e., when the abundance of all species in the community is identical); Yi is the dominance index of the i-th fish species (species with Yi > 0.02 are dominant); and fi is the frequency of occurrence of species i. The statistical analysis was performed and figures were generated using the Biozeron Cloud Platform (http://www.cloud.biomicroclass.com/CloudPlatform).
Results
Sequence information and taxonomic assignment
A total of 5,067,423 valid sequences were collected from 12 sample locations. The sequenced data are published in the NCBI sequence Read Archive (SRA) database (accession numbers: SRR19217018-SRR19217053). A total of 62 MOTUs were shared by all sampling sites, which accounted for 59.62% of the total number of MOTUs in the fish with this river section (Fig. 2). A comparative annotation analysis of the database the fish in the Jiangjin to Fuling section of the upper YR included eight orders, 24 families, 72 genera, and 104 fish species based on recovered MOTUs (Table 1).
Fish species composition based on historical information and eDNA
Over the last 10 years, we have collected data from the YR section from Yibin to Wushan (Li et al., 2013; Xiong et al., 2015; Wei et al., 2021). In this section, there are 175 fish species belonging to 11 orders, 28 families, and 98 genera, included 11 national-level protected fish species, 12 municipal-level protected fish species, and 79 endemic fish species (Table S1). The Cyprinidae represent the most species-rich family in the region, accounting for 56.57% of the population, followed by the Cobitidae at 9.14% and Bagridae at 8.57%, while the specie number of all other families are low (Fig. 3b).
In the eDNA data, based on number of species, Cyprinidae (n = 54) was shown to have the highest share of 51.92%, followed by Cobitidae (n = 11) with 10.58% and Bagridae (n = 10) with 9.62%. The remaining families contained no more than three species each (Fig. 3a). The results of this study also included six national protected fish species Acipenser sinensis (Gray 1835), Coreius guichenoti (Sauvage et Dabry 1874), Rhinogobio ventralis (Sauvage et Dabry 1874), Schizothorax davidi (Sauvage 1880), Leptobotia rubrilabris (Dabry 1872), Myxocyprinus asiaticus (Bleeker 1864), three municipal-level protected fish species Ochetobibus elongatus (Kner 1867), L. rubrilabris, Leptobotia microphthalma (Fu et Ye 1983), and 19 species of fish that are endemic to the upper YR, along with 17 exotic species, accounting for 16.35% of the total number of species.
The species composition differed between sampling sites at the genus level, as shown in Fig. 4. The McNaughton index was used to determine the species dominance, and the eight dominant fish species in the YR section were shown to be C. idella, Hemiculter tchangi (Fang 1942), Rhodeus sinensis (Günther 1868), Pseudorasbora parva (Temminck et Schlegel 1846), Cyprinus carpio (Linnaeus 1758), Triplophysa cf. rosa (Chen et Yang 2005), Tachysurus fulvidraco (Richardson 1846), and Rhinogobius cliffordpopei (Nichols 1925), which all exhibited extremely high levels of relative sequence abundance at all sampling sites. In contrast, M. asiaticus, Elopichthys bambusa (Richardson 1845), O. elongatus, Acheilognathus cf. rhombeus (Temminck et Schlegel 1846), and 16 other fish species were found exclusively at a few sampling points (< 5 sampling points) with extremely low relative sequence numbers. Moreover, the overall relative sequences abundance of the “Four major Chinese carp” varied considerably in this river section. C. idella was dominant in terms of its relative sequence abundance among the “Four major Chinese carps,” followed by H. molitrix, then H. nobilis and finally M. piceus.
On one hand, the division of fish by habitat stratum at each sampling site revealed that demersal fish accounted for more than half of all fish species, whereas benthopelagic fish accounted for 40% to 54%, based on the relative sequence abundance. In contrast, the spawning types of results demonstrated, based on the species number and relative sequence abundance, that adhesive eggs accounted for more than 50% of all egg types at each sampling site. Between the sampling points, the percentages of different habitat layers or different spawning types were relatively similar (Fig. 5).
Alpha diversity analysis
Each sampling point had a coverage of between 0.999959 and 0.999908, suggesting that the sequencing depth included all sequences and reflects the true situation of the samples (Chen, 2020). The mean Shannon index value for the 12 sampling sites ranged from 2.346796 to 2.721591. The LH had the highest Shannon index value among all sampling sites, indicating the highest community diversity. QX had the lowest value, indicating that it had the lowest community diversity. Despite the fact that the Simpson index showed the opposite trend to the Shannon index, it had roughly the same meaning. Furthermore, the Pielou index of each sampling site was not significantly different, suggesting that the species distribution was consistent across the sampling sites.
Beta diversity analysis
The X-axis PC1 accounted for 21.56% of the variance in the sample composition, the Y-axis PC2 explained 18.537%, and the Z-axis PC3 accounted for 11.264%. These values were calculated by the PCoA of each sampling point (Fig. S1). Except for four sampling sites, LH, MD, ML, and XK, which were far apart from each other, all sampling points were closer to each other, indicating that the fish compositions of the LH, MD, ML, and XK locations varied more than at the other sampling points.
We divided the 12 sampling sites into 12 groups (three parallel samples from each sampling site were grouped) and used the Bray–Curtis distance matrix to conduct an NMDS analysis of the species composition at the species level. We obtained a stress score of 0.10969, indicating that the results have some explanatory significance. The findings revealed that the fish composition was dissimilar between the subgroup samples (nmds1, R2 = 0.21, P = 0.02; nmds2, R2 = 0.58, P = 0.30). In Fig. S2, MD, XK, and GYD are scattered and far apart from other sampling sites, implying that the fish compositions at these three sampling points is more differed from that of other sampling points. This result is similar to that obtained in the PCoA.
Discussion
Species composition
In the upper reaches of the YR from Jiangjin to Fuling, a total of 104 species of freshwater fish were found using eDNA technology, with a coverage rate of 70%. This is compared to the 149 species of fish contained in the most recent reference in historical data. The composition results at the family level based on eDNA revealed proportions of 51.92% Cyprinidae, 10.58% Cobitidae, and 9.62% Bagridae. These results are similar to those obtained with traditional methods in the previous decade, indicating that eDNA technology has some applicability in this watershed and could be a significant complementary tool for conventional investigation methods (Jiang et al., 2016a; Wang et al., 2022).
According to the survey findings, the “Four major Chinese carp” sequence abundance vary considerably in this river section. It may be related to their living habitat, in terms of the relative sequence abundance, the results showed a ranking of C. idella > H. molitrix > H. nobilis > M. piceus. Based on this, C. idella is the most active and lives closest to the surface, followed by H. molitrix and H. nobilis, M. piceus is the least active (Xian et al., 2010; Xu et al., 2017). Our survey was primarily conducted in surface water, leading to more mixed DNA of C. idella and H. molitrix, which have active habits. Hence, they have higher relative sequence abundances than the other two fish species. Furthermore, the amount and rate of eDNA released into the water was shown to vary among the species, and the activity of an organism was demonstrated to influence the amount of eDNA released into the water column (Geerts et al., 2017; Minamoto et al., 2017). This suggests that we should use a mixture of upper, middle, and lower water samples for eDNA sampling.
The current status of fish communities
Twenty-two species included in the present survey results, such as Misgurnus cf. mizolepis (Günther 1888), T. rosa, and Sinobdella cf. sinensis (Bleeker 1870), are not contained in historical data. It is possible that the scarce numbers or specific habits of these species resulted in them not being observed in this river section by conventional methods [e.g., O. elongatus, Hemiculterella sauvagei (Warpachowski 1888)]. In addition, it has been shown that traditional DNA macrobarcoding is unable to distinguish some sibling species (Shen et al., 2019). In the eDNA method, the barcodes are shorter, which would result in a low taxonomic resolution and therefore a failure to distinguish some sibling species [e.g., Siniperca cf. obscura (Nichols 1930) and Siniperca chuatsi (Basilewsky 1855)], which would have to be further validated by traditional methods (Sales et al., 2020, 2021). This is a limitation of the current eDNA method. Meanwhile, the Yangtze River has an extremely rich fish diversity, and there is also a possibility that some species could not be assigned to the correct species because their information was not included in the reference database (Li et al., 2019). However, these are the cases of a very small fraction of species and need to be handled with caution when making relevant descriptions.
Previous studies have shown that fish in the upper YR tend to undergo species miniaturization (Wei et al., 2021), and our study results support this conclusion. The study revealed that the dominant fish species in the Chongqing section of the YR mainstream in 2019 included H. nobilis, Saurogobio dabryi (Bleeker 1871), and H. molitrix (Wang et al., 2021). However, our results showed that small fish species, such as H. tchangi, R. sinensis, and P. parva had the highest relative sequence abundances, which may be related to the overfishing that occurred before the fishing ban (Chen, 2016). However, it is also possible that smaller fish are more active and shed more DNA (Minamoto et al., 2017), or the results may be related to the sampling time and frequency. Therefore, it is important to perform multi-season and multi-frequency sampling to obtain more accurate results from the eDNA method.
Meanwhile, the number of endemic fish species was shown to account for 18% of all fish species contained in the eDNA results and 20% of the endemic fish species in the historical data, which implies a possible declining trend in the number of endemic fish species in the upper YR, while other studies have also shown a decline in the number of endemic fish in the Three Gorges reservoir area in the upper YR (Wei et al., 2021), possibly owing to habitat destruction (Yang et al., 2017). The study also discovered a high proportion of demersal fishes and adhesive egg fish in the upper reaches of the YR from Jiangjin to Fuling, which may be related to the formation of the Three Gorges Reservoir area (the nearest sampling site is about 492 km away from the Three Gorges Dam), leading to slower water flow, an increased water depth, sediment deposition, and increased bait organisms for demersal fish. The formation of the reservoir area is more favorable for the survival of demersal fish and adhesive egg fish (Cao, 2019). In summary, the current state of degradation of fish communities in the upper YR should be seriously considered, and necessary measures must be taken to protect them.
Fish diversity
Table 2 depicts the alpha diversity index of the fish community abundance. The Shannon and Simpson indices synthetically reflect the diversity and evenness of the species (He, 2016). The Shannon index and Simpson index values were the highest for LH, revealing that this sampling site contains the highest fish community diversity, which may be related to the richness of the fish species and the evenness distribution of sequences at this sampling site. Based on the above two indicators, QX was found to have the least number of fish species, which could be attributed to the sampling site proximity to a freight terminal and the high influence of human factors at this site, resulting in a reduced fish abundance. Nevertheless, different indices for assessing the fish diversity have different emphasis, and the community richness index is not a perfect indicator of high community diversity (Ling et al., 2021).
The PCoA and NMDS analyses of each sampling site revealed that the fish species compositions at the LH, GYD, MD, ML, and XK sampling sites differed from those at other sampling sites, which could be related to the river topography and anthropogenic activities carried out near the sampling sites. It has become clear that the abundance and density of fish species are linked to the horizontal area and dimensions of river basins. The species composition and diversity of these fish communities are directly influenced by the longitudinal slope drop and bed substrate of rivers, among other factors (Platts, 1979; Walters et al., 2003; Liao 2021). Anthropogenic activities such as cultivation, laundering of clothes, and machine operations can alter the nitrogen and phosphorus elemental contents and sediment the content of the water, affecting the distribution and diversity of fish (Liu et al., 2004; Shi et al., 2018). Other sampling sites showed comparable results close, implying that they had more similar fish species composition, probably because they are continuous rivers with no obstacles in the center, allowing fish to swim back and forth between nearby sampling sites. Meanwhile, eDNA can be transported downstream at least 50 km in large rivers. Thus it is possible that the similarities in the fish species composition are also due to the transmission of eDNA by rivers (Pont et al., 2018).
Exotic species
This survey discovered Acipenser schrenckii (Brandt 1869), Coilia brachygnathus (Kreyenberg et Pappenheim 1908), Neosalanx cf. tangkahkeii (Wu 1931), Protosalanx chinensis (Basilewsky 1855), and 17 other exotic species, accounting for 16.35% of the total number of eDNA fish species. In a recent study that investigated the fish diversity in the Three Gorges Reservoir area using traditional fishing methods, 20 exotic fish species were identified, accounting for 13.42% of all fish in the study (Wei et al., 2021). We detected a higher proportion of exotic species, indicating that the invasion risk in the Three Gorges Reservoir area is continuously increasing. Exotic species, such as A. schrenckii, Micropterus salmoides (Lacepède 1802), and N. tangkahkeii, which are commonly farmed in the Three Gorges Reservoir area, originate primarily from fish escaping from surrounding farms, blind introduction, and human release (Wu et al., 2007; Qiao et al., 2010; Ba & Chen, 2012). The lack of necessary escape prevention measures on the farms led to the invasion of these species into the YR. The impoundment of water in the Three Gorges Reservoir area has resulted in drastic changes in the water environment, destruction of structure and function of the original ecosystem, the severe vacancy of ecological niches, and resource enrichment, which have facilitated the settlement and spread of exotic species (Ba & Chen, 2012).
The invasion of exotic species can result in the simplification of community composition of ecosystem and the harshness of the original ecological environment. Exotic species can also compete with native species for resources, reduce the genetic diversity of native species, and cause the decline or even extinction of indigenous fish species. For example, the introduction of species such as N. taihuensis into the Dianchi lake has resulted in the depletion of endemic fish stocks (Xiong et al., 2006). Since the 1950s, C. idella, H. molitrix, and other fish from the middle and lower reaches of the YR have been introduced into the water bodies of the Yunnan–Guizhou Plateau on a large scale, resulting in the endangerment and extinction of many indigenous and endemic fish on the Plateau (Xie & Chen, 2001). Therefore, we should mitigate against the invasive hazards of exotic species by establishing a scientific selection and breeding assessment system for exotic species, developing a comprehensive set of laws and regulations related to exotic species, monitoring and alerting existing exotic species, and improving the protection of indigenous species (Xu et al., 2004; Qiao et al., 2010; Ba & Chen, 2012).
Conclusion
The eDNA results imply that fish communities in this area have tended to undergo species miniaturization, and the endemic fish may also be gradually declining as well as the exotic fish species are increasing in the upper reaches of the YR from Jiangjin to Fuling. However, the species composition at the family level has remained stable over the last decade. Simultaneously, eDNA technology detected 104 species of freshwater fish from 72 genera and 8 orders, and 24 families were detected in this river section, indicating that this technology has particular relevance in this river section and can be used as an auxiliary tool to the traditional method.
Data availability
The reference sequences are available on NCBI under the following Accession Numbers SRR19217018-SRR19217053.
References
Anderson, M. J., T. O. Crist, J. M. Chase, M. Vellend, B. D. Inouye, A. L. Freestone, N. J. Sanders, H. V. Cornell, L. S. Comita & K. F. Davies, 2011. Navigating the multiple meanings of β diversity: a roadmap for the practicing ecologist. Ecology Letters 14(1): 19–28.
Ba, J. & D. Chen, 2012. Invasive fishes in Three Gorges Reservoir area and preliminary study on effects of fish invasion owing to impoundment. Journal of Lake Sciences 24(2): 185–189.
Balasingham, K. D., R. P. Walter, N. E. Mandrak & D. Heath, 2018. Environmental DNA detection of rare and invasive fish species in two Great Lakes tributaries. Molecular Ecology 27(1): 112–127.
Becker, R., N. G. Sales, G. Santos, G. Santos & D. Carvalho, 2015. DNA barcoding and morphological identification of neotropical ichthyoplankton from the Upper Paraná and São Francisco. Journal of Fish Biology 87(1): 159–168.
Bohmann, K., A. Evans, M. T. P. Gilbert, G. R. Carvalho, S. Creer, M. Knapp, W. Y. Douglas & M. De Bruyn, 2014. Environmental DNA for wildlife biology and biodiversity monitoring. Trends in Ecology & Evolution 29(6): 358–367.
Bolger, A. M., M. Lohse & B. Usadel, 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30(15): 2114–2120. https://doi.org/10.1093/bioinformatics/btu170.
Cao, W., 2011. Fish resources of the Yangtze River basin and the tactics for their conservation. Jiangxi Fishery Science and Technology 2: 1–4.
Cao, W., 2019. Water ecological restoration in the cascade development of hydropower in the upper reaches of the Yangtze River. Technology and Economy of Changjiang 3(2): 6.
Caporaso, J. G., J. Kuczynski, J. Stombaugh, K. Bittinger, F. D. Bushman, E. K. Costello, N. Fierer, A. G. Peña, J. K. Goodrich & J. I. Gordon, 2010. QIIME allows analysis of high-throughput community sequencing data. Nature Methods 7(5): 335–336.
Chen, S., 2016. Studies on Fishery Capture and Fishery Biology in the Three Gorges Reservoir, China, University of Chinese Academy of Sciences, Beijing:
Chen, S., 2020. Analysis of Fish and Plankton Diversity in Chongde Lake of Southwest University Based on eDNA Macro Barcode Technology, Southwest University, Chongqing:
Cheng, F., W. Li, L. Castello, B. R. Murphy & S. Xie, 2015. Potential effects of dam cascade on fish: lessons from the Yangtze River. Reviews in Fish Biology and Fisheries 25(3): 569–585.
Dejean, T., A. Valentini, C. Miquel, P. Taberlet, E. Bellemain & C. Miaud, 2012. Improved detection of an alien invasive species through environmental DNA barcoding: the example of the American bullfrog Lithobates catesbeianus. Journal of Applied Ecology 49(4): 953–959.
Dibattista, J. D., D. J. Coker, T. H. Sinclair-Taylor, M. Stat, M. L. Berumen & M. Bunce, 2017. Assessing the utility of eDNA as a tool to survey reef-fish communities in the Red Sea. Coral Reefs 36(4): 1245–1252.
Doi, H., R. Inui, Y. Akamatsu, K. Kanno, H. Yamanaka, T. Takahara & T. Minamoto, 2017. Environmental DNA analysis for estimating the abundance and biomass of stream fish. Freshwater Biology 62(1): 30–39.
Gao, T., H. Tian, C. Ye & X. Duan, 2013. Fish composition and diversity in the mainstream of the National Nature Reserve for Rare and Endemic Fish in the Upper Yangtze River. Freshwater Fisheries 43(2): 36–42.
Gao, T., H. Tian, H. Wang, X. Duan, S. Liu & D. Chen, 2015. Spatial and temporal distributions and influence factors of brass gudgeon (Coreius heterodon) eggs in Jiangjin section of the Upper Yangtze River. Journal of Fisheries of China 39(8): 1099–1106.
Geerts, A. N., P. Boets, S. Heede, P. Goethals & C. Heyden, 2017. A search for standardized protocols to detect alien invasive crayfish based on environmental DNA (eDNA): a lab and field evaluation. Ecological Indicators 84(84): 564–572.
He, M., 2016. Fish diversity and its influencing factors in the upper and middle reaches of Tingjiang River Fujian. Journal of Agriculture Sciences 31(6): 566–574. https://doi.org/10.19303/j.issn.1008-0384.2016.06.003.
He, Y., J. Wang, L. Ang & S. Lek, 2010. Predicting assemblages and species richness of endemic fish in the upper Yangtze River. Science of the Total Environment 408(19): 4211–4220.
Hinlo, R., E. Furlan & L. Suitor, 2017. Environmental DNA monitoring and management of invasive fish: comparison of eDNA and fyke netting. Management of Biological Invasions 8(1): 89–100.
Holmlund, C. M. & M. Hammer, 1999. Ecosystem services generated by fish populations. Ecological Economics 29: 253.
Jerde, C. L., W. L. Chadderton, A. R. Mahon, M. A. Renshaw, J. Corush, M. L. Budny, S. Mysorekar & D. M. Lodge, 2013. Detection of Asian carp DNA as part of a Great Lakes basin-wide surveillance program. Canadian Journal of Fisheries and Aquatic Sciences 70(4): 522–526.
Jiang, W., H. Zhao, J. Deng, Q. Wang, F. Kong & H. Zhang, 2016a. Detection of aquatic species using environmental DNA. Journal of Hydroecology 37(5): 1–7. https://doi.org/10.15928/j.1674-3075.2016.05.001.
Jiang, Z., J. Jiang, Y. Wang, E. Zhang & Y. Zhang, 2016b. Red list of China’s vertebrates. Biodiversity Science 24(5): 500.
Li, L., Q. Wei, J. Wu, H. Du & X. Xie, 2013. Current status of fish assemblages in Yibin reaches of the Yangtze River resources and environment in the Yangtze Basin. Environmental Science 22(11): 1449–1457.
Li, H., X. Huang, S. Li & A. Zhan, 2019. Environmental DNA (eDNA)-metabarcoding based early monitoring and warning for invasive species in aquatic ecosystems. Biodiversity 27(5): 491–504.
Li, S., X. Liu, W. Jiang, K. Xiao, A. Huang & Q. Zhang, 2021. Application of environmental DNA technology in natural reproduction of the four major Chinese carps in Yichang section of Yangtzi River, China. The Journal of Applied Ecology 32(6): 2241–2248.
Liao, Z., 2021. Study on the characteristics of water flow and topography of fish habitat from Chaotianmen to fengdu in the Upper Yangtze River. Master, Chongqing Jiaotong University.
Ling, J., Y. Jiang, P. Sun, X. Yuan, H. Zhang & B. Tang, 2021. Application and evaluation of environmental DNA technology in the investingation of fish diversity in Xiangshan Harbor. Journal of Fishery Sciences of China 28(2): 205–214.
Ling, L., G. Fan, Y. Hu, J. Huang & C. Li, 2022. Integrating environmental DNA technology and traditional fish survey to reveal the diversity of fishes in the rivers on the Chongming lsland. Journal of Shanghai Ocean University 31: 1–13.
Liu, J., W. Cao & J. Chang, 2004. The relationship between fish diversity and watershed characteristics in the main rivers of the upper Yangtze River. Journal of Jishou University (natural Sciences Edition) 25(1): 42–47.
Liu, F., P. Lin, M. Li, X. Gao, C. Wang & L. Huan Zhang, 2019. Status quo of fish resources in the Yangtze River basin and countermeasures for protection. Acta Hydrobiologica Sinica.
Magoč, T. & S. L. Salzberg, 2011. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27(21): 2957–2963.
Meng, B., J. Zhang, H. Ye, F. Chang & Y. Jiang, 2019. Current situation and protection enlightenment of the function of fishing spawning grounds in the national nature reserve for the rare and endemic fishes, Upper Reaches of the Yangze River. Resources and Environment in the Yangtze Basin 28(11): 2772–2785.
Minamoto, T., M. Fukuda, K. R. Katsuhara, A. Fujiwara & R. Masuda, 2017. Environmental DNA reflects spatial and temporal jellyfish distribution. PLoS ONE 12(2): e0173073.
Platts, W. S., 1979. Relationships among stream order, fish populations, and aquatic geomorphology in an Idaho river drainage. Fisheries 4(2): 5–9.
Pont, D., M. Rocle, A. Valentini, R. Civade, P. Jean, A. Maire, N. Roset, M. Schabuss, H. Zornig & T. Dejean, 2018. Environmental DNA reveals quantitative patterns of fish biodiversity in large rivers despite its downstream transportation. Scientific Reports 8(1): 1–13.
Qiao, Y., W. Song & W. Li, 2010. Discussion on biological invasion effect on aquatic organism biodiversity and management countermeasure. Journal of Agro-Environment Science 29(S1): 321–323.
Qin, W., L. Liu, W. Xu, Z. Wang & M. Jiang, 2008. Prediction of ecological impacts of the planned xiaonanhai power dam on therare and endemic fishes nature reserve in the upper reaches of the Yangtze River. Journal of Ecology and Rural Environment 4: 23–26.
Qu, C., K. A. Stewart, R. Clemente-Carvalho, J. Zheng, Y. Wang, C. Gong, L. Ma, J. Zhao & S. C. Lougheed, 2020. Comparing fish prey diversity for a critically endangered aquatic mammal in a reserve and the wild using eDNA metabarcoding. Scientific Reports 10(1): 1–16.
Reis, Ad. S., M. P. Albrecht & S. E. Bunn, 2020. Food web pathways for fish communities in small tropical streams. Freshwater Biology 65(5): 893–907.
Sales, N. G., T. C. Pessali, F. R. Andrade Neto & D. C. Carvalho, 2018. Introgression from non-native species unveils a hidden threat to the migratory Neotropical fish Prochilodus hartii. Biological Invasions 20(3): 555–566.
Sales, N. G., Md. C. Kaizer, I. Coscia, J. C. Perkins, A. Highlands, J. P. Boubli, W. E. Magnusson, M. N. F. Da Silva, C. Benvenuto & A. D. Mcdevitt, 2020. Assessing the potential of environmental DNA metabarcoding for monitoring Neotropical mammals: a case study in the Amazon and Atlantic Forest. Brazil. Mammal Review 50(3): 221–225.
Sales, N. G., O. S. Wangensteen, D. C. Carvalho, K. Deiner, K. Præbel, I. Coscia, A. D. McDevitt & S. Mariani, 2021. Space-time dynamics in monitoring neotropical fish communities using eDNA metabarcoding. Science of the Total Environment 754: 142096.
Shannon, C. E., 1948. A mathematical theory of communication. The Bell System Technical Journal 27(3): 379–423.
Shen, Y., N. Hubert, Y. Huang, X. Wang, X. Gan, Z. Peng & S. He, 2019. DNA barcoding the ichthyofauna of the Yangtze River: insights from the molecular inventory of a mega-diverse temperate fauna. Molecular Ecology Resources 19(5): 1278–1291.
Shi, N., 2019. Temporal and spatial distribution characteristics of fish in the nationalaquatic germplasm resources conservation a rea of the four major fishesin the Chongqing section of the Yangtze River. Master, Chongqing Normal University.
Shi, R., L. Tang, G. High, D. Yang & X. Xu, 2018. Analysis of the relationship between fish diversity and watershed characteristics in the Yangtze River Basin. Journal Publishing Center of Tsinghua University Press 58(7): 650–657. https://doi.org/10.16511/j.cnki.qhdxxb.2018.22.033.
Shu, L., 2022. Assessment of fish diversity based on environmental DNA technology: Embryonic development dynamics, primers evaluation, and fish diversity monitoring in the Erhai Lake. Doctor, Southwest University.
Simpson, E. H., 1949. Measurement of diversity. Nature 163(4148): 688–688.
Stewart, K., H. Ma, J. Zheng & J. Zhao, 2017. Using environmental DNA to assess population-wide spatiotemporal reserve use. Conservation Biology 31(5): 1173–1182.
Strickler, K. M., A. K. Fremier & C. S. Goldberg, 2015. Quantifying effects of UV-B, temperature, and pH on eDNA degradation in aquatic microcosms. Biological Conservation 183: 85–92.
Taberlet, P., E. Coissac, M. Hajibabaei & L. H. Rieseberg, 2012. Environmental DNA. Molecular Ecology 21(8): 1789–1793.
Taberlet, P., A. Bonin, L. Zinger & R. Coissac, 2018. Environmental DNA: for biodiversity research and monitoring. Environmental DNA: For Biodiversity Research and Monitoring.
Thomsen, P. & E. Willerslev, 2015. Environmental DNA—an emerging tool in conservation for monitoring past and present biodiversity. Biological Conservation. 13: 4–18.
Tuomisto, H., 2010. A diversity of beta diversities: straightening up a concept gone awry. Part 1. Defining beta diversity as a function of alpha and gamma diversity. Ecography 33(1): 2–22.
Valentini, A., P. Taberlet, C. Miaud, R. Civade, J. Herder, P. F. Thomsen, E. Bellemain, A. Besnard, E. Coissac & F. Boyer, 2016. Next-generation monitoring of aquatic biodiversity using environmental DNA metabarcoding. Molecular Ecology 25(4): 929–942.
Walters, D., D. Leigh, M. C. Freeman, B. Freeman & C. Pringle, 2003. Geomorphology and fish assemblages in a Piedmont river basin, USA. Freshwater Biology 48(11): 1950–1970.
Wang, Z., A. T. Sathrajith, S. Xie & F. Cheng, 2019. Effect of the impoundment of dam cascade in the Jinsha River and increased water level of the three Gorges Reservoir on the distribution and abundance of Pseudolaubuca engraulis (Nichols) larvae in the Upper Mainstem of the Yangtze River. Acta Hydrobiologica Sinica 43(3): 606–611.
Wang, S., Y. Dan, Y. Li, F. Yu & H. Chen, 2021. Analysis on the composition and change of commercial fishes in Chongqing section of the main stream of Three Gorges Reservoir. South China Agriculture 15(25): 1–4. https://doi.org/10.19415/j.cnki.1673-890x.2021.25.001.
Wang, M., X. Yang, W. Wang, C. Duan, Z. Liu, Q. Chen, Y. Li & Y. Shen, 2022. Fish diversity in Chongqing section of the national nature reserve for rare and endemic fish in the upper Yangtze River based on eDNA technology. Acta Hydrobiologica Sinica 46(1): 15.
Wei, Q., 2012. Scientific Investigation Report on the National Nature Reserve of Rare and Endemic Fish in the Upper Yangtze River, Science Press, Beijing:
Wei, N., Y. Zhang, F. Wu, Z. Shen, H. Ru & Z. Ni, 2021. Current status and changes in fish assemblages in the Three Gorges Reservoir. Resources and Environment in the Yangtze Basin 30(8): 1858–1869.
Woodland, R. J., F. Y. Warry, Y. Zhu, R. Mac Nally, P. Reich, G. P. Jenkins, D. Brehm & P. L. Cook, 2019. Role of benthic habitat structure and riverine connectivity in controlling the spatial distribution and ecology of estuarine fish. Marine Ecology Progress Series 630: 197–214.
Wu, Q., X. Duan, S. Xu, C. Xiong & D. Chen, 2007. Studies on fishery resources in the Three Gorges Reservoir of the Yangtze River. Freshwater Fisheries 37(2): 70–75.
Wu, J., J. Wang, Y. He & W. Cao, 2011. Fish assemblage structure in the Chishui River, a protected tributary of the Yangtze River. Knowledge and Management of Aquatic Ecosystems 400: 11.
Xian, X., Z. Cao & S. Fu, 2010. The comparison of critical swimming speed and endurance at high speed of four species of juvenile fish. Journal of Chongqing Normal University (natural Science) 27(4): 16–20.
Xie, P. & Y. Chen, 1999. Threats to biodiversity in Chinese inland waters. Ambio 28: 674–681.
Xie, P. & Y. Chen, 2001. Invasive carp in China’s plateau lakes. Science 294(5544): 999–1000.
Xing, Y., J. Bai, H. Li, B. Liu & Y. Zhao, 2021. Species diversity of fishes in the Dingqu River Basin, tributary of the upper Yangtze River. China. Biodiversity Data Journal 9: e76669.
Xiong, F., W. Li, J. Pan, A. Li & T. Xia, 2006. Status and changes of fishresources in Lake Fuxian, Yunnan Province. Journal of Lake Sciences 18(3): 305–311.
Xiong, F., H. Liu, X. Duan, S. Liu & D. Chen, 2015. Present status of fishe ry resources in Yibin section of the upper Yangtze River. Journal of Southwest University (natural Science Edition) 37(11): 43–50.
Xu, H., S. Qiang, Z. Han, J. Guo, Z. Huang, H. Sun, S. He, H. Ding, H. Wu & F. Wan, 2004. The distribution and introduction pathway of alien invasive species in China. Biodiversity Science 12(6): 626–638.
Xu, C., Z. Ai & M. Xiao, 2017. A review of influencing factors on natural reproduction of four major Chinese carps in Yangtze River. Journal of China Three Gorges University (natural Sciences) 39(4): 27–30. https://doi.org/10.13393/j.cnki.issn.1672-948X.2017.04.006.
Yang, Z., Y. Tang, Y. Gong, C. Dong, X. Chen, C. Wan & J. Chang, 2017. The spatial-temporal distribution characteristics of the endemic fish in the upper reaches of the Yangtze River under the normal operation of the Three Gorges Reservoir. Ecology and Environmental Monitoring of Three Gorges 2(1): 1–10. https://doi.org/10.19478/j.cnki.2096-2347.2017.01.01.
Zhang, S., J. Zhao & M. Yao, 2020. A comprehensive and comparative evaluation of primers for metabarcoding eDNA from fish. Methods in Ecology and Evolution 11(12): 1609–1625.
Zhang, S., Y. Zheng, A. Zhan, C. Dong, J. Zhao & M. Yao, 2022. Environmental DNA captures native and non-native fish community variations across the lentic and lotic systems of a megacity. Science Advances 8(6): eabk0097.
Zhou, Q., F. Cheng, Z. Wang, J. Cao, H. Tang, Q. Zhu & S. Xie, 2022. Study on fish resources of the early life history stages and the effects of ecological regulation of the Xiangjiaba reservoir at the Hejiang section in the upper reaches of the Yangtze River. Environmental Monitoring in China 38(1): 95–103. https://doi.org/10.19316/j.issn.1002-6002.2022.01.09.
Zou, K., J. Chen, H. Ruan, Z. Li, W. Guo, M. Li & L. Liu, 2020. eDNA metabarcoding as a promising conservation tool for monitoring fish diversity in a coastal wetland of the Pearl River Estuary compared to bottom trawling. Science of the Total Environment 702: 134704.
Acknowledgements
The authors sincerely thank all the crews for their help with the manuscript writing and data analysis. This work was funded by the National Natural Science Foundation of China (grant number 32202939) and the Natural Science Foundation of Chongqing (CSTB2022NSCQ-MSX0793).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling editor: Christian Sturmbauer
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cheng, R., Luo, Y., Li, Q. et al. Application of eDNA metabarcoding for monitoring the fish diversity of the Jiangjin to Fuling section of the upper reaches of the Yangtze River. Hydrobiologia 850, 4067–4088 (2023). https://doi.org/10.1007/s10750-023-05297-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-023-05297-1