Abstract
Poyang Lake is the largest freshwater lake in China, and its unique ecosystem plays an important role in maintaining biodiversity. However, the biodiversity of Poyang Lake is facing a serious threat as a result of human activities. Species investigation is the basis of biodiversity protection. In order to improve the water ecological monitoring system and achieve efficient and non-invasive species monitoring, environmental DNA metabarcoding was used in this study to assess biodiversity in different habitats in Poyang Lake. A total of 45 species including 31 fish species and six bivalve species were detected in water samples collected from 29 sampling sites in six habitats in Poyang Lake (Jiangxi section of the mainstream of the Yangtze River, channel connecting Poyang Lake and the Yangtze River, main lake area of Poyang Lake, Nanjishan Nature Reserve, Junshan Lake and Qinglan Lake). The species were detected through a standardized process involving water sample collection, filtration, environmental DNA extraction, genetic marker amplification, high-throughput sequencing and bioinformatics analysis. The 31 fish species, which included 11 alien species, were mainly cyprinids and lake dwellers. Alpha diversity analysis indicated a decline in biodiversity in Poyang Lake habitats and a serious need for water ecology conservation. Beta diversity analysis revealed significant differences in the biotic community structures of the six habitats in Poyang Lake. The results align with those obtained with more traditional methods, and hence, environmental DNA metabarcoding can be used as an alternative biodiversity monitoring tool for rapid detection of the diversity and spatial distribution of organisms (especially fish). This technique provides a new toolkit for biodiversity monitoring and aquatic ecological conservation in Poyang Lake area.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The loss of biodiversity is one of the most serious environmental crises facing the world, and the biodiversity decline in freshwater populations is even greater than that in marine and terrestrial ecosystems (Valentini et al. 2016). The development of rapid and effective tools to monitor biodiversity fluctuations is the focus of scientific research on conservation and management strategies. Traditional survey methods have been invaluable for monitoring aquatic biodiversity and developing management and conservation strategies, but they also have many shortcomings, such as inefficiency, selectivity, destructiveness or strict reliance on declining taxonomic expertise (Wheeler et al. 2004).
The recently developed environmental DNA metabarcoding technology can directly extract DNA from environmental samples (such as water, sediment, soil, etc.), apply universal primers for target groups, and identify multiple target species in environmental samples through polymerase chain reaction (PCR) amplification combined with high-throughput sequencing (Taberlet et al. 2012). There is no need to collect target organisms. The advantages of non-destructive sampling and high detection sensitivity compensate for the deficiencies of traditional morphological monitoring. The environmental DNA metabarcoding technology has great application potential for biodiversity assessment (Thomsen and Willerslev 2015). In recent years, environmental DNA metabarcoding has been widely used for fisheries management and diversity monitoring in freshwater ecosystems (Ruppert et al. 2019). A variety of amphibians, fish, mammals, insects and crustaceans have been found in environmental DNA biodiversity surveys in hundreds of ponds, streams and rivers in the USA (Thomsen et al. 2012). Evans et al. (2017) used three pairs of common fish primers to detect all fish species previously identified in a reservoir using traditional methods as well as 11 previously uncaptured fish species. Zhang et al. (2020) systematically evaluated the influence of spatial sampling design on the fish community structure in three lakes of different sizes based on environmental DNA technology, and the results confirmed that shoreline sampling was equally effective.
Located on the south bank of the middle and lower reaches of the Yangtze River, Poyang Lake is the largest freshwater lake in China and one of only three lakes connected to the Yangtze River (Zhang and Li 2007). Water flows into Poyang Lake from five major rivers; the Ganjiang, Fuhe, Xinjiang, Raohe and Xiushui Rivers. The water then passes into the Yangtze River in the Hukou region after being regulated and stored in the lake (Zhang 1993). In recent years the increase in human activities, especially the overexploitation and utilization of freshwater resources (e.g. reclaiming land from lakes, sand mining, damming rivers, dike breeding, overfishing, etc.,) has caused serious damage to the freshwater ecosystem in Poyang Lake. These activities place the lake at risk of degradation and have seriously affected the biodiversity (especially fish species), resulting in significant changes in the community structure (Hu et al. 2011; Chen et al. 2012). The biodiversity and ecological balance of Poyang Lake have attracted close attention from the state and Federal government. In recent years, relevant measures have been taken to ban fishing in Poyang Lake (Xu et al. 2020).
There is an urgent need to establish a rapid, effective, and environmentally friendly monitoring system for biodiversity conservation and ecological restoration in Poyang Lake. Baseline data can provide a scientific foundation for the formulation and implementation of fishery management and ecological protection policies.
In this study, environmental DNA metabarcoding technology was used for the first time to investigate the structure and constitutive mechanisms of biological communities in different habitats in Poyang Lake. This study can provide the required baseline data to enable better understanding of the degradation of the lake fishery habitat and the evolution of biodiversity patterns. It can also provide important data for the restoration of the lake habitat and biodiversity, and provide a theoretical framework for the conservation and adaptive management of important fishery lakes.
Materials and methods
Study area
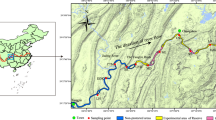
Poyang Lake is a seasonal lake formed by water from the Yangtze River and five other rivers, with obvious flood and drought rhythms. It covered an area of about 2000 km2 at the time of sampling. We divided Poyang Lake into six habitats (Jiangxi section of the mainstream of the Yangtze River, channel connecting Poyang Lake and the Yangtze River, main lake area of Poyang Lake, Nanjishan Nature Reserve, Junshan Lake and Qinglan Lake) (Fig. 1).
Environmental DNA sampling
In this study, samples were collected from Poyang Lake in April 2019. According to the geographical environment, hydrology and habitat characteristics of Poyang Lake, a total of 29 sampling sites were set up in six habitats (Fig. 1). A 1 L surface water sample was collected from each site in a sterile plastic bottle and stored at – 4 °C before filtration. We conducted a study on the vertical distribution differences for the environmental DNA diversity of fish in Poyang Lake, and the results showed that there were no significant differences (unpublished). Three duplicate samples were collected at each site. To reduce contamination between the sites, the surveyor followed a sterile protocol, e.g. wearing sterile gloves at each site, and the equipment was sterilized between each site. We filled the sample bottles with sterilized water to ensure there was no contamination on site or in the bottles.
Water sample filtration and environmental DNA extraction
The water was filtered within 24 h through a 0.45-μm mixed cellulose filter membrane (Jinteng, China) with an oil-free vacuum pump (Rocker 300, Taiwan). In order to assess the possible presence of exogenous DNA contamination, a field blank control was used during filtration. The membranes were folded and preserved in sterile 1.5 mL centrifuge tubes at − 20 °C. Environmental DNA was extracted from the samples and blanks using the DNeasy Blood & Tissue Kit (Qiagen, Germany) according to the method described by Zhang et al. (Zhang et al. 2020). Three extractions were performed for each site. A blank membrane was used as the negative control.
Amplification and high-throughput sequencing
Mitochondrial cytochrome b degenerate primers L14912-CYB: 5'-TTCCTAGCCATACAYTAYAC-3' (Y = C or T) and H15149-CYB: 5'-GGTGGCKCCTCAGAAGGACATTTGKCCYCA-3' (K = G or T, Y = C or T) were amplified by PCR with barcodes for an eight-base sequence unique to each sample (Miya and Nishida 2000). The product size was approximately 285 bp and the target site of the primer pair was a conserved region widely found in vertebrates (Minamoto et al. 2012). PCR reactions were performed in triplicate in a 25 µL mixture comprising 5 μL of 5 × reaction buffer, 5 μL of 5 × GC buffer, 2 μL of a deoxynucleotide (dNTP, 2.5 mM) mixture, 1 μL of forward and reverse primers (10 uM), 50 ng of DNA template, 0.25 μL of Q5 High-Fidelity DNA (2 U/μL) Polymerase (New England Biolabs, USA), and molecular biology-grade water added up to 25 μL. For all samples, PCR was performed as follows: 98 °C for 2 min, followed by 30 cycles of 98 °C for 15 s, 55 °C for 30 s, and 72 °C for 30 s, with a final extension at 72 °C for 5 min. Molecular biology-grade water was used as a template for the negative control in each PCR reaction. Amplicons were extracted from 2% agarose gels and purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, US) according to the manufacturer’s instructions. All negative controls had no target bands, indicating that there was no exogenous DNA contamination during sampling, filtration, DNA extraction and PCR amplification. Paired-end sequencing was performed on an Illumina MiSeq. The Mova-Seq-PE250 sequencing strategy was used. Low-quality sequences were removed using QIIME software according to the following conditions: (1) sequences less than 150 bp in length, (2) sequences with an average Phred quality score < 20, (3) sequences containing ambiguous bases other than N, (4) sequences with base mismatches for the 5' end primers > 1, and (5) sequences containing single nucleotide repeats > 8 bp (Caporaso et al. 2010). Sequence alignment was performed using the UCLUST tool in QIIME software (Edgar 2010). Clusters with high-quality sequence consistency over 97% were identified as operational taxonomic units (OTUs). The sequence with the highest abundance was selected from each OTU, screened against GenBank using the Basic Local Alignment Search Tool (BLAST), and assigned to the taxon with the highest total score (Chariton et al. 2010). Finally, OTUs with relative abundance values below 0.001% were removed (Bokulich et al. 2013). The relative abundance of all species at each site was estimated with pi = Ni/N, where Ni is the number of reads for species i and N is the total read for all species in the sample.
Data analysis
The parameters used for species annotation were identity value ≥ 97% and e-value < 10–10. OTUs that aligned to the same species were merged. If there was an OTU that could not be annotated at the species level, the analysis was carried out one level higher. The number of valid sequences of species in each sample was counted in an Excel table. A species was considered to be present at the sampling point if it was detected in at least two duplicate samples. Sequencing produced a total of 2.98 million paired end reads, with 2.51 million reads remaining after quality filtering. The average number of reads per site after filtering was 60,185.
The alpha diversity for each sample was calculated using four diversity indices: Shannon–Wiener index (\({H}^{\mathrm{^{\prime}}}=-\sum_{i=1}^{s}\left({p}_{i}\mathrm{In}{P}_{i}\right)\), Pielou index ((\({J}^{^{\prime}}={H}^{^{\prime}}/\mathrm{ln}S\))), Simpson index (C = \(\sum\nolimits_{i = 1}^{s} {\left[ {Ni\left( {Ni - 1} \right)/N\left( {N - 1} \right)} \right]}\)), and Margalef index (D = (S − 1)/lnN), where S is the total number of species in each sample, pi is the relative abundance, Ni is the number of reads for species i and N is the total read for all species in the sample. The differences in biodiversity between habitats were compared via non-parametric tests using IBM® SPSS® 25.0. The Kruskal–Wallis test was used to evaluate whether the distributions of multiple biodiversity indices were significantly different. Beta diversity refers to the differences or similarities in community composition among different groups of samples, determined through inter-group comparative analysis. Beta diversity was assessed using Principal Component Analysis (PCA), Nonmetric Multidimensional Scaling (NMDS) and cluster analysis. For NMDS, multivariate analysis of variance (Adonis) was used to evaluate the differences in community structure in different habitats.
Results
Major taxa at the class level based on environmental DNA metabarcoding
In the spring of 2019, Pisces species accounted for 68.9% of all species identified in Poyang Lake, followed by Bivalves at 13.3%. The Pisces species in the Yangtze River habitat (A1–A3) accounted for about 70% of the total. Other classes included Mammalia, Bivalvia and Insecta (Fig. 2). The number of classes found at each sampling site in the channel connecting Poyang Lake and the Yangtze River (B1–B8) varied considerably. The samples collected at the B1 site contained Pisces and Mammals, with the former accounting for about 65% of all species. The samples collected at the B2, B3, B7 and B8 sites contained Pisces, Mammalia and Mastigophora. Four or more classes were identified at B4, B5 and B6. Among the sampling sites in the main lake area of Poyang Lake, five classes were identified at C1 and C6, and three classes were identified at C2, C3, C4 and C5. Oligotricla was only found at C7. In Nanjishan Nature Reserve (D1–D3), Pisces species were absolutely dominant and the sample from D2 only contained fishes. In Junshan Lake and Qinglan Lake habitats, Pisces accounted for more than 50% of the species identified (Fig. 2).
Class-level diversity of major taxa across the surveyed sites in Poyang Lake. Jiangxi section of the Yangtze River (A1–A3), channel connecting Poyang Lake and the Yangtze River (B1–B8), main lake area of Poyang Lake (C1–C8), Nanjishan Nature Reserve (D1–D3), Junshan Lake (E1–E5), Qinglan Lake (F1–F2)
Species composition and abundance
A total of 45 species were identified in the six habitats investigated, including 31 species of Pisces and six species of Bivalves (Table 1). Among the habitats, the largest number of species (28 species) was identified in the channel connecting Poyang Lake and the Yangtze River, while the lowest number was identified in Qinglan Lake (10 species). Among the Pisces species identified, Cyprinus carpio were found in all habitats. Three fish species, Tachysurus fulvidraco, Hypophthalmichthys nobilis and Cyprinus rubrofuscus, were identified in at least four habitats. Some fishes, such as Myxocyprinus asiaticus, Gobiobotia filifer, Rutilus rutilus, Mugilogobius myxodermus, Oreonectes platycephalus, and Microphysogobio tafangensis, were identified in only one habitat. Among the Bivalves identified, Solenaia oleivora and Nodularia douglasiae were found in four habitats, Arconaia lanceolata and Acuticosta chinensis were found in three habitats, and Lamprotula leai and Limnoperna fortunei were found in only two habitats. Human sequences were present in all habitats. The amplified sequence contained a significant proportion of human sequences, which was normal because there were traces of human activity in the water. The primers used in this study need human sequence-inhibiting primers to be added during amplification. These primers were not added in our study, which was why the human sequences were present in all habitats.
In this study, 31 fish species from eight orders, 11 families and 24 genera were identified, including 20 indigenous species belonging to eight families and 15 genera as well as 11 exotic species from five families and 10 genera (Table 1). Among them, 70.97% were limnicolous fishes, 16.13% were river–lake migratory fishes, 9.68% were river-sea migratory fishes and 3.23% were potamophilous fishes. Among the fish sequences detected, 94.62% were for limnicolous, 5.61% for river–lake migratory fishes, 0.15% for river-sea migratory fishes, and 0.06% for potamophilous fishes. The Pisces species included the orders Cypriniformes, Siluriformes, Clupeiformes, Beloniformes, Osmeriformes, Gymnotiformes, Cyprinodontiformes and Perciformes, with Cypriniformes comprising the largest number of species at 19.
The highest abundance of C. carpio and Fundulus notatus was found in the Jiangxi section of the mainstream of the Yangtze River (Fig. 3). C. carpio and T. fulvidraco were most abundant in the channel connecting Poyang Lake and the Yangtze River habitat. Only C. carpio showed high abundance in both the main lake area of Poyang Lake and Nanjishan Nature Reserve. The species with the highest abundance in the Junshan Lake habitat were C. carpio and Xenocypris davidi. Hypophthalmichthys nobilis was the most abundant in Qinglan Lake.
Alpha diversity analysis of different habitats
The Shannon-Weiner diversity index for the different habitats ranged from 0.45 to 1.70. The highest diversity index was found at the Jiangxi section of the mainstream of the Yangtze River, followed by the channel connecting Poyang Lake and the Yangtze River. The lowest diversity value was found in Qinglan Lake (Fig. 4). The Pielou Index ranged from 0.20 to 0.61, with the highest value in the Jiangxi section of the mainstream of the Yangtze River and the lowest in Qinglan Lake. The Simpson index ranged from 0.22 to 0.84, with the highest value in Qinglan Lake and the lowest in the Jiangxi section of the mainstream of the Yangtze River. The Margalef index ranged from 1.04 to 2.62, with the highest value in the channel connecting Poyang Lake and the Yangtze River and the lowest in Nanjishan Nature Reserve. There was no significant difference (P = 0.416) in the diversity indices among different habitats (Fig. 4).
Beta diversity analysis in different habitats based on environmental DNA metabarcoding
PCA showed that the habitats in the channel connecting Poyang Lake and the Yangtze River, the main lake area of Poyang Lake, Nanjishan Nature Reserve and Junshan Lake had relatively similar species compositions. The Jiangxi section of the mainstream of the Yangtze River and Qinglan Lake were very different from the other habitats (Fig. 5a). After standardized conversion of the species abundance in the 29 sampling sites, clustering was performed based on the Euclidean distance and ward minimum connections. The results showed that the 29 sampling sites were clustered into three branches with a Euclidean distance D = 17.2. Branch I included all sampling sites in the Jiangxi section of the mainstream of the Yangtze River. Branch II included 24 sampling sites in four habitats, specifically the channel connecting Poyang Lake and the Yangtze River, the main lake area of Poyang Lake, Nanjishan Nature Reserve and Junshan Lake. The sampling sites at Qinglan Lake were part of branch III (Fig. 5b).
Principal component analysis and clustering analysis in six habitats, a Sample of principal component analysis. Treat 1: Jiangxi section of the mainstream of the Yangtze River, Treat 2: channel connecting Poyang Lake and the Yangtze River, Treat 3: main lake area of Poyang Lake, Treat 4: Nanjishan Nature Reserve, Treat 5: Junshan Lake, Treat 6: Qinglan Lake, b Sample hierarchical clustering tree based on OTU levels. Jiangxi section of the mainstream of the Yangtze River (A1–A3), channel connecting Poyang Lake and the Yangtze River (B1–B8), main lake area of Poyang Lake (C1–C8), Nanjishan Nature Reserve (D1–D3), Junshan Lake (E1–E5), and Qinglan Lake (F1–F2)
In this study, NMDS analysis was conducted on 5 groups of samples (Qinglan Lake was deleted due to the small number of sample points) at the species level. The NMDS stress value was 0.12, indicating a good result. The overall community structure was significantly different among habitats (R2 = 0.486, P < 0.001) (Fig. 6). Adonis analysis showed that there were significant differences between the Jiangxi section of the mainstream of the Yangtze River and the main lake area of Poyang Lake (R2 = 0.017, P = 0.025) and between the main lake area of Poyang Lake and Junshan Lake (R2 = 0.020, P = 0).
Nonmetric multidimensional scaling (NMDS) ordination of the community compositions of all environmental DNA samples colored according to the habitat. A—Jiangxi section of the mainstream of the Yangtze River, B—channel connecting Poyang Lake and the Yangtze River, C—main lake area of Poyang Lake, D—Nanjishan Nature Reserve, E—Junshan Lake, F—Qinglan Lake. (0.01 < *P ≤ 0.05, 0.001 < **P ≤ 0.01, ***P ≤ 0.001)
Discussion
Fish community structures in Poyang Lake based on environmental DNA
A total of 31 fish species were detected in this study using environmental DNA metabarcoding technology. The majority of fish were Cyprinidae (16 species), accounting for 51.61% of all fish species identified, which was consistent with the results obtained using traditional methods (Fang et al. 2016; Zhang and Li 2007). This confirms a previous report indicating that the majority of fish in Poyang Lake were Cyprinidae, and that limnicolous fish were the most abundant (Yang et al. 2015). In addition, 11 exotic species were detected in this study distributed in four orders. Cypriniformes were the most abundant, possibly due to their strong adaptability. The detection of the rare fish Coilia nasus in the channel connecting Poyang Lake and the Yangtze River as well as in the main lake area of Poyang Lake indicated that environmental DNA metabarcoding is effective for the detection of endangered species. The second-class state protected animal M. asiaticus was detected in the Junshan Lake habitat due to artificial cultivation of the species over a large surface area in this habitat. Cyprinus carpio was the dominant fish species in the Jiangxi section of the mainstream of the Yangtze River, the channel connecting Poyang Lake and the Yangtze River, the main lake area of Poyang Lake and Nanjishan Nature Reserve, as determined with environmental DNA metabarcoding. The dominant fish species caught using traditional methods are C. carpio and Carassius auratus (Wang et al. 2016; He et al. 2016; Xiong 2018; Hu et al. 2005). The dominant species observed in Qinglan Lake and Junshan Lake were closely related to the cultivated species at the time because dike breeding is performed in these two lakes.
Even with environmental DNA metabarcoding approaches, valuable input from taxonomic experts is often required (Evans and Lamberti 2018). Firstly, environmental DNA metabarcoding can only determine the presence of species based on the genetic information available in environmental samples. The technique cannot capture information about the population size, age structure, physiological status and growth developmental stage of the target species (Shu et al. 2020). Secondly, environmental DNA metabarcoding relies on the integrity of molecular databases, which can lead to false negatives when the target species sequence is missing from the database (Cristescu and Hebert 2018). In addition, the bias of PCR may lead to the failure of environmental DNA detection for some low abundance species (Carew et al. 2013) and biomass estimation errors (Elbrecht and Leese 2015, 2017). Therefore, in actual practice, traditional survey methods should be combined with environmental DNA detection technology to monitor aquatic biodiversity more comprehensively and efficiently. These methods will play a more active role in the construction of aquatic ecological civilizations (Ge et al. 2020).
Alpha diversity of biological communities in Poyang Lake
Higher Shannon-Weiner index values indicate higher diversity in a community. A Shannon-Weiner diversity index between 1.5 and 3.5 indicates the community has a high level of biodiversity (Magurran 1988). In this study, the Shannon-Weiner diversity indices for the habitats in the Jiangxi section of the mainstream of the Yangtze River, the channel connecting Poyang Lake and the Yangtze River and the Junshan Lake in Poyang Lake were between 1.5 and 3.5 (Fig. 4). This means the biodiversity in these habitats was high. The other habitats had low diversity, especially the Qinglan Lake habitat. The high biodiversity in the Jiangxi section of the mainstream of the Yangtze River and the channel connecting Poyang Lake and the Yangtze River was due to their special geographical location. The spatial and temporal continuity of rivers and lakes is an important reason for their high biodiversity. Some scholars believe that the convergence of rivers can help increase fish diversity (Fernandas et al. 2004; Röpke et al. 2015). For the channel connecting Poyang Lake and the Yangtze River, seasonal inundation makes the floodplain a rich habitat for aquatic animals. Vegetation along the lakeside and in the water can provide shelter for aquatic animals as well as spawning grounds for C. carpio, C. auratus and other fishes during the flood season (Qian et al. 2002). Due to the temperature gradient and vortex formed at the confluence, nutrients, wood debris and organic matter accumulate in these areas, which is conducive to the growth of phytoplankton and zooplankton and provide a rich source of food for aquatic animals (Gayoso and Podesta 1996). Due to the frequent dike aquaculture activities in Qinglan Lake, fishery farming has damaged the biological community structure of the water body, severely affecting the biological diversity. Aquatic plants were seriously damaged in Junshan Lake due to a large number of crab farms in the early twentieth century. Subsequently, the lake underwent a decade-long ecological restoration. Junshan Lake has a high Shannon-Weiner diversity index and Pielou index, which is attributed to the 10 years of ecological restoration. The biodiversity level of habitats in which dike farming was prohibited showed a decreasing trend due to overfishing. For example, the Shannon-Weiner diversity index recorded using traditional methods in the channel connecting Poyang Lake and the Yangtze River was greater than 2 in 2012 (He et al. 2016). Data based on net fishing in 2014 in Hukou indicated a Shannon-Weiner diversity index of 2.59 (Wang et al. 2016). Survey data from 2009 showed that there were 42 freshwater mussel species in Poyang Lake (Xiong et al. 2011). Survey data from 2016 to 2017 showed that there were 24 mussel species in Poyang Lake (Li et al. 2019). The species richness of mussels in Poyang Lake decreased significantly. The situation for water ecological protection is grim with the continuous decline of biodiversity in Poyang Lake. In an effort to improve ecological protection, a 10-year fishing ban will be imposed on the waters of the mainstream of the Yangtze River and the Hukou region in Poyang Lake from January 1, 2021.
Beta diversity analysis in six habitats
PCA and clustering tree analysis showed that the habitats in the channel connecting Poyang Lake and the Yangtze River, the main lake area of Poyang Lake, Nanjishan Nature Reserve and Junshan Lake had relatively similar community structures (Fig. 5). However, the community structures in these four habitats were obviously different from those in the Jiangxi section of the mainstream of the Yangtze River and Qinglan Lake (Fig. 5). This may be related to the fact that the Jiangxi section of the mainstream of the Yangtze River is located at the confluence of Poyang Lake and the river, resulting in a higher Shannon-Weiner diversity index and Pielou index compared to the other habitats. Due to frequent aquaculture and lack of timely ecological restoration, the community structure in the Qinglan Lake habitat is quite different from those of the other habitats. The species composition and abundance heat maps for all habitats were different (Table 1, Fig. 3) and there were significant differences in community structure among the habitats (Fig. 6). These results are of great significance for the protection, restoration and scientific management of fishery lakes in China. They can provide a foundation for decision-making by government management departments.
Conservation strategies for biodiversity in Poyang Lake
The current fishing ban policy is conducive to the restoration of biodiversity in Poyang Lake. Based on the results of this study, the following suggestions are proposed. Firstly, carry out artificial proliferation and release to curb the decline of fishery resources. Secondly, strengthen pollution control and strictly control the pollution of lakes. Thirdly, formulate a scientific and reasonable sand mining plan. Fourthly, regular monitoring and early warning systems should be established, such as by increasing seasonal sampling and developing monitoring plans.
Conclusions
Environmental DNA metabarcoding technology has great application potential for the monitoring and conservation of biodiversity. This study represents the first application of environmental DNA metabarcoding for the assessment of biodiversity in Poyang Lake and demonstrates the feasibility of using environmental DNA metabarcoding to detect species composition and the distribution of organisms (especially fish) in different habitats. The results of high-throughput sequencing showed a decreasing trend in the biodiversity and characteristics of the habitat community structure in Poyang Lake. Although environmental DNA metabarcoding cannot completely replace traditional fish survey methods, it can serve as an important complementary tool for rapid determination of the diversity and spatial distribution of organisms in water bodies. It can reduce potential damage to the ecosystem compared to traditional monitoring techniques, shorten the survey period, improve the detection efficiency, and provide reliable data to enable a rapid response for the aquatic ecological protection.
Data availability
The datasets analyzed during the current study are available from the corresponding author on a reasonable request.
References
Bokulich NA, Subramanian S, Faith JJ, Gevers D, Gordon JI, Knight R, Mills DA, Caporaso JG (2013) Quality filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat Methods 10:57–59. https://doi.org/10.1038/nmeth.2276
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336. https://doi.org/10.1038/nmeth.f.303
Carew ME, Pettigrove VJ, Metzeling L, Hoffmann AA (2013) Environmental monitoring using next generation sequencing: rapid identification of macroinvertebrate bioindicator species. Front Zool 10:45. https://doi.org/10.1186/1742-9994-10-45
Chariton AA, Court LN, Hartley DM, Colloff MJ, Hardy CM (2010) Ecological assessment of estuarine sediments by pyrosequencing eukaryotic ribosomal DNA. Front Ecol Environ 8:233–238. https://doi.org/10.1890/090115
Chen W, Zhang Y, Zhao C, Wang C (2012) Species composition and biodiversity of fish community in Hukou section of the Yangtze River. Resour Environ Yangtze Basin. 21:684–691
Cristescu ME, Hebert PDN (2018) Uses and misuses of environmental DNA in biodiversity science and conservation. Annu Rev Ecol Evol Syst 49:209–230. https://doi.org/10.1146/annurev-ecolsys-110617-062306
Edgar RC (2010) Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26:2460–2461. https://doi.org/10.1093/bioinformatics/btq461
Elbrecht V, Leese F (2015) Can DNA-based ecosystem assessments quantify species abundance? Testing primer bias and biomass-sequence relationships with an innovative metabarcoding protocol. PLoS ONE 10:e0130324. https://doi.org/10.1371/journal.pone.0130324
Elbrecht V, Leese F (2017) Validation and development of COI metabarcoding primers for freshwater macroinvertebrate bioassessment. Front Env Sci Switz 5:11. https://doi.org/10.3389/fenvs.2017.00011
Evans NT, Lamberti GA (2018) Freshwater fisheries assessment using environmental DNA: a primer on the method, its potential, and shortcomings as a conservation tool. Fish Res 197:60–66. https://doi.org/10.1016/j.fishres.2017.09.013
Evans NT, Li YY, Renshaw MA, Olds BP, Deiner K, Turner CR, Jerde CL, Lodge DM, Lamberti GA, Pfrender ME (2017) Fish community assessment with eDNA metabarcoding: effects of sampling design and bioinformatic filtering. Can J Fish Aquat Sci 74:362–1374. https://doi.org/10.1139/cjfas-2016-0306
Fang C, Chen W, Zhou H, Zhang Y, Fu P, He G, Wu B, Wang S (2016) Fish resources of Poyang Lake and its utilization proposal. Jiangsu J Agr Sci 44:233–243. https://doi.org/10.15889/j.issn.1002-1302.2016.09.067
Fernandas CC, Podos J, Lundberg JG (2004) Amazonian ecology: tributaries enhance the diversity of electric fishes. Science 305:1960–1962. https://doi.org/10.1126/science.1101240
Gayoso AM, Podesta GP (1996) Surface hydrography and phytoplankton of the Brazil-Malvinas currents confluence. J Plankton Res 18:941–951. https://doi.org/10.1093/plankt/18.6.941
Ge Y, Yan Y, Cheng Q (2020) Environmental DNA and its application in aquatic biodiversity. Fishery Inform Strat 35:55–62. https://doi.org/10.13233/j.cnki.fishis.2020.01.008
He G, Fang C, Chen W, Fu P, Zhou H, Zhang Y, Wu B, Wang S (2016) Fish community structure and diversity in Pingfeng section of the channel connecting the Poyang Lake and the Yangtze River. Jiangxi Fishery Sci 4:3–6. https://doi.org/10.3969/j.issn.1006-3188.2016.04.002
Hu M, Wu Z, Liu Y (2011) Fish diversity and community structure in Hukou area of Lake Poyang. J Lake Sci 23:246–250
Hu M, Wu Z, Zhou H, Zhang A, Song W, Zhang C (2005) The fisheries characters and resource status of Nanjishan Natural Reserve in Poyang Lake. Resour Environ Yangtze Basin 14:561–565
Li K, Liu X, Zhou Y, Xu Y, Lv Q, Ouyang S, Wu X (2019) Temporal and spatial changes in macrozoobenthos diversity in Poyang Lake Basin, China. Ecol and Evol 9:6353–6365. https://doi.org/10.1002/ece3.5207
Magurran AE (1988) Ecological diversity and its measurement. Princeton University Press, NewJersey
Minamoto T, Yamanaka H, Takahara T, Honjo MN, Kawabata ZI (2012) Surveillance of fish species composition using environmental DNA. Limnology 13:193–197. https://doi.org/10.1007/s10201-011-0362-4
Miya M, Nishida M (2000) Use of mitogenomic information in teleostean molecular phylogenetics: a tree-based exploration under the maximum-parsimony optimality criterion. Mol Phylogenet Evol 17:437–455. https://doi.org/10.1006/mpev.2000.0839
Qian X, Huang C, Wang Y, Xiong F (2002) The status quo of fishery resources of Lake Poyang and its environmental monitoring. Acta Hydrobiol Sin 26:612–617. https://doi.org/10.3321/j.issn:1000-3207.2002.06.006
Röpke CP, Amadio SA, Winemiller KO, Zuanon J (2015) Seasonal dynamics of the fish assemblage in a floodplain lake at the confluence of the Negro and Amazon Rivers. J Fish Biol 89:194–212. https://doi.org/10.1111/jfb.12791
Ruppert KM, Kline RJ, Rahman MS (2019) Past, present, and future perspectives of environmental DNA (eDNA) metabarcoding: a systematic review in methods, monitoring, and applications of global eDNA. Glob Ecol Conserv 17:e00547. https://doi.org/10.1016/j.gecco.2019.e00547
Shu L, Lin J, Xu Y, Cao T, Feng J, Peng Z (2020) Investigating the fish diversity in Erhai Lake based on environmental DNA metabarcoding. Acta Hydrobiol Sin 44:1080–1086. https://doi.org/10.7541/2020.125
Taberlet P, Coissac E, Hajibabaei M, Rieseberg LH (2012) Environmental DNA. Mol Ecol 21:1789–1793. https://doi.org/10.1111/j.1365-294X.2012.05542.x
Thomsen PF, Willerslev E (2015) Environmental DNA-an emerging tool in conservation for monitoring past and present biodiversity. Biol Conserv 183:4–18. https://doi.org/10.1016/j.biocon.2014.11.019
Thomsen PF, Kielgast J, Iversen LL, Wiuf C, Rasmussen M, Gilbert MTP, Orlando L, Willerslev E (2012) Monitoring endangered freshwater biodiversity using environmental DNA. Mol Ecol 21:2565–2573. https://doi.org/10.1111/j.1365-294X.2011.05418.x
Valentini A, Taberlet P, Miaud C, Civade R, Herder J, Thomsen PF, Bellemain E, Besnard A, Coissac E, Boyer F, Gaboriaud C, Jean P, Poulet N, Roset N, Copp GH, Geniez P, Pont D, Argillier C, Baudoin JM, Peroux T, Crivelli AJ, Olivier A, Acqueberge M, Brun ML, Møller PR, Willerslev E, Dejean T (2016) Next-generation monitoring of aquatic biodiversity using environmental DNA metabarcoding. Mol Ecol 25:929–942. https://doi.org/10.1111/mec.13428
Wang S, Duan X, Chen W, Zhang H, Fu P, He G, Wu B (2016) Status and changes of fish resources in the Hukou area of Poyang Lake. Freshwater Fish 46:50–55. https://doi.org/10.3969/j.issn.1000-6907.2016.06.009
Wheeler QD, Raven PH, Wilson EO (2004) Taxonomy: impediment or expedient? Science 303:285. https://doi.org/10.1126/science.303.5656.285
Xiong G (2018) Preliminary survey on fish resources in Wetland Inland Lakes of Poyang Lake. Jiangsu J Agr Sci 46:186–190. https://doi.org/10.15889/j.issn.1002-1302.2018.21.048
Xiong L, Ouyang S, Chen T, Qi T, Wu X (2011) Diversity patterns of freshwater mussels in poyang lake area. J Nanchang Univ (nat Sci) 35:288–295
Xu N, Xiong M, Shao K, Que Y, Li J (2020) Preliminary study on environmental dna metabarcoding for detecting biodiversity in the middle and lower reaches of the Yangtze River. Res Environ Sci 33:1187–1196. https://doi.org/10.13198/j.issn.1001-6929.2020.03.06
Yang S, Li M, Zhu Q, Wang M, Liu H (2015) Spatial and temporal variations of fish assemblages in Poyang Lake. Resour Environ Yangtze Basin 24:54–64
Zhang B (1993) The hydrological features and the renovative strategy of the Poyang Lake. Resour Environ Yangtze Basin 2:36–42
Zhang T, Li Z (2007) Fish resources and fishery utilization of Lake Poyang. J Lake Sci 19:434–444. https://doi.org/10.18307/2007.0412
Zhang S, Lu Q, Wang Y, Wang X, Zhao J, Yao M (2020) Assessment of fish communities using environmental DNA: effect of spatial sampling design in lentic systems of different sizes. Mol Ecol Resour 20:242–255. https://doi.org/10.1111/1755-0998.13105
Funding
This work was funded by the National Key R & D Program of China (Grant No. 2018YFD0900801). Thanks to Weiwei Sun, Weikai Wang and Dr. Xiongjun Liu at Nanchang University, China, for their help with sample collection. Thanks also to Quanfeng Lu at Nanchang University, China, for the kind data analysis.
Author information
Authors and Affiliations
Contributions
CZ and XW: conceived and designed the research; JC and TG: contributed to field sampling, filtration and environmental DNA extraction in the laboratory. CZ and JC: wrote the manuscript, CZ and SO: contributed to the Discussion section and, subsequently, to various iterations of the manuscript, all authors reviewed the manuscript before submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, C., Chen, J., Guo, T. et al. Environmental DNA metabarcoding reveals the biological community structure in Poyang Lake, China. Conservation Genet Resour 14, 437–448 (2022). https://doi.org/10.1007/s12686-022-01295-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12686-022-01295-y