Abstract
Gastrotricha and Platyhelminthes form a clade called Rouphozoa. Representatives of both taxa are main components of meiofaunal communities, but their role in the trophic ecology of marine and freshwater communities is not sufficiently studied. Traditional collection methods for meiofauna are optimized for Ecdysozoa, and include the use of fixatives or flotation techniques that are unsuitable for the preservation and identification of soft-bodied meiofauna. As a result, rouphozoans are usually underestimated in conventional biodiversity surveys and ecological studies. Here, we give an updated outline of their diversity and taxonomy, with some phylogenetic considerations. We describe successfully tested techniques for their recovery and study, and emphasize current knowledge on the ecology, distribution, and dispersal of freshwater gastrotrichs and microturbellarians. We also discuss the opportunities and pitfalls of (meta)barcoding studies as a means of overcoming the taxonomic impediment. Finally, we discuss the importance of rouphozoans in aquatic ecosystems and provide future research directions to fill in crucial gaps in the biology of these organisms needed for understanding their basic role in the ecology of benthos and their place in the trophic networks linking micro-, meio-, and macrofauna of freshwater ecosystems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Meiofauna constitutes a significant reservoir of biodiversity in aquatic ecosystems that is often overlooked. Sediments and vegetation in freshwater habitats, including freshwater ponds, lakes, and rivers, but also mosses, wet soils and semi-aquatic agricultural ecosystems (e.g., paddy fields), are teeming with hundreds of thousands, if not millions of poorly known or completely unrecorded species of these microscopic animals (Giere, 2009).
Traditional morphology-based sampling techniques to study biodiversity and ecology of meiofauna are usually addressed towards ecdysozoan taxa such as nematodes and copepods, and have so far failed to account for the sometimes equally abundant and diverse soft-bodied gastrotrichs and meiofaunal flatworms or so-called “microturbellarians” (Martens & Schockaert, 1986; Nesteruk, 2006; Schockaert et al., 2008; Giere, 2009). Perhaps more so than other meiofaunal groups, gastrotrichs and microturbellarians also exemplify the taxonomic impediment, including a lack of knowledge on their biodiversity and organismal biology, a lack of experts and a lack of taxonomic infrastructure. As such, they have lagged behind in species discovery and identification, uncovering (cryptic) speciation, biodiversity surveys, population genetics, and phylogeography. Traditional morphology-based identification methods are often time-, effort-, and resource-intensive, depend on a handful of taxonomic experts, and cannot uncover cryptic diversity. As shown by a few comprehensive studies of single taxa, the current number of described species of these two groups in fresh waters is significantly lower than the estimated species diversity (e.g., Catenulida: Larsson et al., 2008; Microstomum: Atherton & Jondelius, 2018, 2019; Gastrotricha: Balsamo et al., 2008).
In this contribution, we will focus on the micro- and meiofaunal freshwater representatives of these taxa and largely omit the numerous members of the macrofaunal flatworm group Tricladida, which, because of their large size, have been rather well studied and are far better known worldwide. Freshwater gastrotrichs and microturbellarians not only share a number of morphological and biological traits, but their parent phyla, Gastrotricha and Platyhelminthes, also recently emerged in a monophyletic clade within the Spiralia called Rouphozoa (Gr. Rouphao, ingesting by sucking; Struck et al., 2014), which was endorsed by two subsequent, independent phylogenomic studies (Egger et al., 2015; Laumer et al., 2015a). However, Bleidorn (2019) recovered a clade comprising Nemertea and Platyhelminthes separate from the clade of Gastrotricha; thus, it is clear that further molecular and morphological work is needed to test the monophyly of the Rouphozoa. The duo-gland adhesive system, recently studied in detail for platyhelminthes (Wunderer et al., 2019), was proposed as a possible synapomorphy for both taxa (Giribet & Edgecombe, 2019). However, studies of the gastrotrich duo-gland system are much older (Tyler & Rieger, 1980; Ruppert, 1991). Consequently, detailed molecular studies of the duo-gland system in Gastrotricha and research to identify other possible synapomorphies within Rouphozoa are sorely needed.
Because of their abundance, small body size and selective micro- and meiophagous feeding behavior, gastrotrichs and microturbellarians most likely play a critical role in freshwater trophic networks and ecosystem dynamics (Palmer et al., 1997; Balsamo & Todaro, 2002; Majdi et al., 2019). However, their diversity and ecological roles in aquatic ecosystems are still insufficiently known. For freshwater gastrotrichs, the results of the few targeted studies on functional ecology appear controversial (Strayer, 1985; Hummon, 1987; Nesteruk, 1996a, 2007b; Schmid-Araya, 1997), and for freshwater microturbellarians no such studies exist to date.
In this study, we aim to give an overview of the current state of knowledge on the diversity, distribution, and ecology of freshwater rouphozoans. This includes an updated census of species in the various biogeographical regions, a summary on the importance of environmental parameters on biotic interactions and habitat preferences, spatial and temporal distribution, dispersal and trophic ecology of these two groups. We also provide recommendations to overcome methodological problems and challenges in qualitative and quantitative collection and identification of these animals, and discuss future research avenues to fill in crucial gaps in our knowledge on these important freshwater animals.
Methodologies for sampling and study
It is clear that in studies of freshwater meiofauna, Rouphozoa are frequently not considered (Fig. 1). As already noted by some authors (e.g., Traunspurger & Majdi, 2017), we hypothesize that this is because extraction methods used for these soft-bodied organisms are very different from those used for ecdysozoan taxa. This is further supported by historical studies that recovered large numbers of rouphozoan taxa using extraction methods compatible with their preservation (e.g., Strayer, 1985; Robertson et al., 2000). Finally, metabarcoding studies in a marine context routinely recover rouphozoans thought previously to be rare based solely on morphotaxonomic studies (e.g., Rzeznik-Orignac et al., 2017; Leasi et al., 2018). Accordingly, we provide up-to-date methods below for the collection, preservation, and study of rouphozoans (Tables 1, 2).
Google Scholar entries for meiofaunal studies mentioning A nematodes and/or copepods; B micro- or macroturbellarians; C gastrotrichs. Methods: Publish or Perish (Harzing, 2007) was used (3.5.20) to search Google Scholar, covering years 1985 through 2020, with the following search strings: A: (meiofauna OR meiobenth) AND (freshwater OR lake OR river OR stream) AND (copepod OR nematod) AND NOT marine; B: (meiofauna OR meiobenth) AND (freshwater OR lake OR river OR stream) AND (turbellaria OR platyhelminthes OR microturbellaria) AND NOT marine; C: (meiofauna OR meiobenth) AND (freshwater OR lake OR river OR stream) AND (gastrotrich OR gastrotricha) AND NOT marine
Sampling and extraction
Due to the patchy distribution of meiofauna, collections of many small samples during different times of the year are preferred over a single large sample (Giere, 2009). For the same reason the choice of sampling sites is also very important, and should touch all the habitats of a single biotope (Heitkamp, 1988). The main criteria for qualitative/quantitative sampling of microturbellarians and gastrotrichs are summarized in Table 1.
Individuals of both groups are more successfully studied alive in fresh samples than in preserved samples, since their body frailty and strong contractility often cause diagnostic morphological characters to be distorted after fixation (Balsamo & Todaro, 2002, Balsamo et al., 2014). In the laboratory, collected fresh samples are moved into bowls equipped with an aeration system and a lighting neon tube if also vegetation is present. The bowls are filled with additional filtered water from the sampling site (or spring water if necessary) and kept at room temperature.
A comparative summary of methods for extraction and study of freshwater microturbellarians and gastrotrichs is reported in Table 2. Extraction of animals from fresh samples implies direct observation of small amounts of sediment under a stereomicroscope and picking up single living individuals for subsequent observation and study under a compound microscope.
The extraction of all the animals from a sample is clearly critical for quantitative analyses, but regrettably, the techniques currently available are not satisfactory for gastrotrichs. A comparative study of different techniques aimed at this purpose showed that a rapid forcing of small quantities of sediment through a 130 µm sieve appears to be the most effective way for extracting chaetonotid species (Hummon, 1981; Nesteruk, 1987; Giere, 2009). Details on the methods of sampling, extraction, and study of freshwater gastrotrichs are described in Balsamo et al. (2014).
Recommended methods for extraction and examination of microturbellarians are described in Schockaert (1996). Decantation methods including agitation of sediment and substrate debris followed by sieving (63 µm screen) will dislodge many freshwater flatworms from their substrate. However, the best method for isolating freshwater microturbellarians is oxygen depletion. A layer of sediment and bottom debris are placed in a tall beaker with clear transparent walls; the beaker is then filled with water from the habitat and allowed to stand, creating a vertical cline of dissolved oxygen. Animals are thus forced out of the substrate and can then be removed from the sides of the beaker or from the surface film with a pipette.
DNA (meta)barcoding of Rouphozoa
DNA extraction and sequencing of taxonomic marker genes called DNA (meta)barcodes from bulk samples including water, aquatic sediments, and soil (eDNA), or from pooled individuals separated from the substrate, can reveal the presence of gastrotrichs and platyhelminthes in aquatic environments in percentages that would otherwise go unnoticed with traditional morphotaxonomic methods (Leray & Knowlton, 2015; Arroyo et al., 2016; Martínez et al., 2020; Fegley et al., submitted). As such, (meta)barcoding holds great promise to increase our knowledge on the diversity, ecology, and role of rouphozoans in aquatic ecosystems (Martínez et al., 2020). This approach has been reviewed recently (Schenk & Fontaneto, 2019): accordingly, we here limit ourselves to considering the promises and pitfalls of DNA-based methods for evaluation of cryptic diversity and community composition among gastrotrichs and microturbellarians, including limitations not mentioned in the paper referenced above.
Choice of amplicon
The ubiquity of MiSeq technology, with up to 300 bp paired-end reads, enables useful sequences to be recovered for most taxa from the V4/V5 region of the 18S rDNA molecule (for Rouphozoa, < 600 bp; Hugerth et al., 2014), as opposed to the V9 region (~ 120 bp; Amaral-Zettler et al., 2009) or V2/V4 (~ 400 bp; Creer et al., 2010). The greater taxonomic resolution conferred by V4/V5 is also illustrated by the fact that a recent metabarcoding trial of this amplicon on a well-studied marine beach in North Carolina, USA was able to distinguish between congeneric pairs of microturbellarian species in three cases, two of which had already been documented morphologically, and the third documented by 18S rDNA sequencing of single individuals (Fegley et al., submitted). The same study revealed the existence of numerous separate species of both taxa from two beaches in North Carolina (Online Resources 1, 2). Accordingly, at least for Rouphozoa, V4/V5 might be a better choice over the more commonly used COI barcode because of poor primer performance with platyhelminthes in general (Vanhove et al., 2013) and because COI-based species delimitation may inflate actual diversity, compared to 18S and 28S rDNA (Van Steenkiste et al., 2018). However, the development of nanopore sequencing now makes it possible to produce very long reads—4 Kb of the rDNA cluster (Krehenwinkel et al., 2019), or individually indexed reads of the full-length “Folmer” region of COI (Maestri et al., 2019; Kennedy et al., 2020). Because of the increased read length, nanopore sequencing is also far more tolerant of amplicon read-length variation than the current standard of MiSeq 300 bp paired-end sequencing—for instance, in the North Carolina study noted above, we obtained relatively few OTUs for crustaceans, as the V4/V5 region in this taxon is too long for 300 bp paired-end reads to overlap. This research area is developing rapidly, and because of portability and low cost, we urge that MinION sequencing be thoroughly tested as a routine method for biodiversity assessment of meiofauna in general.
Pitfalls
Although metabarcoding studies have the ability to reveal taxa that have not been observed with morphological taxonomy (see above), they also are liable to miss taxa that are present. For instance, Lindgren (1972) reported (“approximately”) 35 species of microturbellaria and 20 species of gastrotrichs from ISP beach, so the counts of species shown in Online Resources 1, 2 are likely an underestimate of actual species presence. More directly, a recent study on meiofaunal biodiversity along the Pacific and Atlantic coast of Panama showed that for all investigated sites, the diversity of Gastrotricha, Mollusca, Nemertea, and Xenacoelomorpha estimated by metabarcoding the V9 region of the 18S rRNA was lower than the diversity based on morphological taxonomy (Leasi et al., 2018).
DNA (meta)barcoding relies completely on meticulously curated DNA reference databases that link sequences to species identified based on morphological characters. DNA extractions of tiny animals such as rouphozoans are routinely performed on full individuals, thereby rendering physical vouchering of morphological characters of the same individual impossible. Live and transparent animals with clear diagnostic features can easily be documented digitally, but opaque animals, (pseudo)cryptic species, and species groups with uncertain taxonomic features pose more specific challenges, especially when they are rare or are co-occurring in space and time. However, DNA extractions of soft-bodied rouphozoans can be non-destructive, for instance by performing microdissections using the head for DNA extraction and the posterior part for morphological study (e.g., macrostomids in Schärer et al., 2011; Janssen et al., 2015), and could be a practical solution to incorporate “problem” individuals and species into DNA reference collections.
Promises
Recently, analysis of marine and freshwater metabarcoding data has shown its potential for DNA-based species discovery and uncovered the existence of two hitherto unknown higher-level flatworm groups in freshwater (Mitsi et al., 2019). Combined with data on abiotic and ecological data, it can provide previously unattainable insights into spatial and temporal changes in species compositions and link environmental parameters with the occurrence of specific taxa (Chariton et al., 2015). This can generate novel ecological information for taxa such as gastrotrichs and microturbellarians that are small, difficult to identify, and may only be present as resting eggs or other propagules during certain times.
However, metabarcoding and its applications in ecology are still in development and need to overcome several challenges, many of which apply to meiofauna in general and rouphozoans in particular (see Ruppert et al., 2019 for a review). DNA reference databases for gastrotrichs and microturbellarians are still poorly populated and need to be strengthened through global collaborations of taxonomic specialists. As this is an ongoing and future effort, students and researchers will need to be trained in fundamental biodiversity research, including careful identification of individuals selected for building DNA barcode databases. Other well-known issues include PCR primer bias and design, marker choice, standardization of methods, and integration with ecological data (Schenk & Fontaneto, 2019).
Methods for identification
Gastrotricha
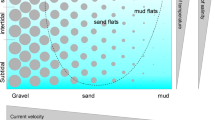
The phylum Gastrotricha currently comprises over 850 free-living species widespread in aquatic ecosystems. The division into two classes, Macrodasyoidea and Chaetonotoidea each including a single order (Macrodasyida and Chaetonotida, respectively) dates back to Remane (1925), and follows the evident differences in morphology, biology, and ecology between the two taxa (Balsamo et al., 2009, 2014, 2015; Hummon & Todaro, 2010; Kieneke & Schmidt-Rhaesa, 2015) (Fig. 2), which has also been confirmed by molecular analyses (Paps & Riutort, 2012).
Taxonomy and systematics of Gastrotricha have been traditionally founded on morphological characters, which still represent the basis to systematize species and superspecific taxa (Hochberg & Litvaitis, 2000; Kieneke et al., 2008). Diagnostic characters are the general body shape, the morphology of the body cuticle and cuticular elements, the shape and length of the caudal appendages, the arrangement of the ventral ciliation, and the structure of the pharynx. Current taxonomy also makes use of molecular techniques, and has introduced over time several changes and integrations to the traditional classification (e.g., Kånneby et al., 2013; Todaro et al., 2012, 2015). These suggest that genera including both marine and freshwater species (i.e., Chaetonotus, Aspidiophorus, Heterolepidoderma) never form monophyletic clades, but rather cluster according to habitat. It is clear that the intraphylum phylogeny is not yet resolved as are deep ingroup phylogenetic relationships; therefore, a stabilization of gastrotrich taxonomy, especially of Chaetonotida, has not yet been reached.
Details on the anatomy and biology of freshwater gastrotrichs are reported in Balsamo et al. (2014) and Kieneke & Schmidt-Rhaesa (2015). A general key to gastrotrich families and genera was recently published by Todaro et al. (2019). Keys to the freshwater gastrotrich fauna also exist (see Balsamo et al., 2014 for a references’ summary), but they are generally limited to selected taxa or to limited geographic ranges such as the Neotropics (Garraffoni & Araújo, 2010), the Nearctic (Kånneby, 2016), and the Palearctic (Balsamo et al., 2019). The Gastrotricha Portal (http://www.gastrotricha.unimore.it) and the World Register of Marine Species (WoRMS, 2020a) contains lists of marine and freshwater species, but does not provide identification keys.
Platyhelminthes
The free-living members of the phylum Platyhelminthes comprise ~ 6500 species, of which ~ 1500 species occur in freshwater or limnoterrestrial environments when also including the macrofaunal triclads. Freshwater microturbellarians can be found in 7 flatworm groups: Catenulida, Macrostomorpha, Prorhynchida, Proseriata, Rhabdocoela, Prolecithophora, and Bothrioplanida. Given the phylogenetic relationships among and within these 7 major flatworm groups, incursions of the freshwater environment almost certainly happened multiple times from different marine and/or brackish water ancestors (Schockaert et al., 2008; Laumer et al., 2015b). Conversely, returns to brackish water and marine environments have also happened (Van Steenkiste et al., 2013).
It is possible to key most platyhelminthes to family level based on morphological characters alone (e.g., Cannon, 1986; Smith et al., 2020). Useful characters are the presence/absence of a statocyst, the construction of the pharynx, the structure of the female gonad, and the morphology of the male reproductive system (Fig. 3). The basic anatomy of Platyhelminthes, including microturbellaria, is covered in detail elsewhere (e.g., Rieger et al., 1991).
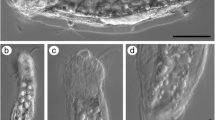
Clades of microturbellaria with pharynx simplex and homocellular female gonads (yolk contained in oocytes). A–D Catenulida: ACatenula confusa, showing anterior statocyst (st), mouth (mo), and best-developed fission plane (arrow). Scale = 200 µm; B Anterior end of Catenula lemnae, a species with consecutive well-developed fission planes (arrows). CStenostomum cf. virginianum, a genus with a well-developed pharynx simplex (ph). Scale = 200 µm; D Enlargement of C, to show multilobed brain (br), refractile bodies (arrows), and mouth; E–F Macrostomorpha: EMacrostomum sp., with anterior pigmented eyes and pharynx simplex close behind (ph), paired testes (te), and ovaries (ov). Scale = 500 µm (approximate); FMicrostomum sp. with anterior pharynx simplex (ph), three fission planes (fp) in different stages of development, and kleptocnids (arrows). Scale = 250 µm (approximate). Clades with complex pharynges and heterocellular female gonads (separate yolk cells and oocytes); G Prorhynchida: Geocentrophora cf. applanata with complex pharynx (ph) opening anteriorly, median germovitellarium marked by nuclei of germocytes (arrows), and light-colored testes follicles (te) associated with lateral branches of the digestive tract. Scale = 500 µm (approximate). H–I Rhabdocoela, Kalyptorhynchia: HOpisthocystis cf. goettei, with anterior cone-shaped muscular proboscis (pr), and median rosulate (wreath-shaped) muscular pharynx (ph). Scale = 500 µm (approximate); I enlarged view of mid-body region of H, showing pharynx, paired testes, and paired germaria (ge); J–M Rhabdocoela, Limnotyphloplanida: J Dalyelliidae; cf. Microdalyellia rossi, showing anterior doliiform (barrel-shaped) pharynx and mature egg (eg). Scale = 500 µm (approximate); K–L Typhloplanidae: K Typhloplaniid showing lateral rope-like vitellaria (vi), one of two paired testes, and posterior rosulate (wreath-shaped) pharynx with genital region shortly behind pharynx; L cf. Ascophora elegantissima overview showing paired testes, rosulate pharynx, and genital region (go). Scale = 500 µm (approximate); M enlarged view of L, showing testes, pharynx, and genital region
Although DNA taxonomy has been used to trace species radiations in Gastrotricha (Atherton, 2015), it has only been employed a few times for (cryptic) species delimitation in freshwater gastrotrichs (Kånneby et al., 2012) and microturbellarians (Larsson et al., 2008; Atherton & Jondelius, 2018, 2019). This illustrates the urgency of improving aspects of environmental high-throughput sequencing before this potentially cost-effective approach could be widely applied for species discovery, biodiversity surveys, and ecosystem assessments in aquatic ecosystems.
Well-supported intraphylum relationships among most major subtaxa (with the exception of the relative position of Rhabdocoela and Proseriata) are provided by two recent transcriptomic studies (Egger et al., 2015, Laumer et al., 2015b—Fig. 4). Recent molecular phylogenies, albeit largely based on only a few genes, have provided valuable information on relationships within the major groups, often in conflict with the traditional morphology-based taxonomy: Catenulida (Larsson & Jondelius, 2008), Macrostomorpha (Janssen et al., 2015; Atherton & Jondelius, 2019), Rhabdocoela (Willems et al., 2006; Van Steenkiste et al., 2013; Tessens et al., 2014), and Proseriata (Laumer et al., 2014; Scarpa et al., 2016). Accordingly, it has proven to be challenging to provide morphological apomorphies for many of the resulting clades. Therefore, phylogenomics based on much larger molecular datasets and advances in the study of morphological characters should be integrated to provide a more robust taxonomy for different microturbellarian groups.
A general key for freshwater microturbellarians is missing at this date, and existing keys focus on specific taxa or regions. A recent key to freshwater Platyhelminthes of the Nearctic extends to genus, and includes a species list (Noreña et al., 2015). At present, there is no genus-level key to the Palearctic, which is unfortunate, as the majority of collecting and taxonomic work has been done there (Noreña et al., 2019). The Turbellarian Taxonomic Database (Tyler et al., 2006–2016) and the World Register of Marine Species (WoRMS, 2020b) includes worldwide coverage of marine, freshwater, and limnoterrestrial Platyhelminthes, but does not provide a key.
Ecology
Studies on gastrotrich and microturbellarian autoecology and synecology are not numerous (Schwank, 1981b, 1982a; Heitkamp, 1982, 1988; Ricci & Balsamo, 2000; Kolasa, 2002; Nesteruk, 2016a, b, 2017). Abiotic and ecological factors define the qualitative and quantitative compositions of populations, whose mean densities widely vary depending on the characteristics of the habitat and seasonal dynamics, and can range from a few thousand up to 2.6 million ind/m2 for both benthic and pelagic gastrotrichs (Nesteruk, 2004a, 2009, 2011) and at least several thousand ind/m2 for microturbellarians (Kolasa, 2002); however, several studies use different units impeding a reliable comparison of values (Nesteruk, 1993).
Habitat
Various environmental parameters play an important role in defining the ecological niche of each species of freshwater rouphozoan and thus they determine their small-scale and regional diversity and distribution patterns: these parameters are summarized in Table 3.
Temperature is essential for the colonizing ability of gastrotrich populations and influences the length and intensity of reproductive activity rather than their lifespan (d’Hondt, 1971; Hummon, 1986; Balsamo & Todaro, 1988). Only a few freshwater species, mainly the epibenthic ones, are known to tolerate low oxygen concentrations, unlike some marine species that have well adapted to this particular habitat (Kraus & Colacino, 1984). Grain size, shape and sorting, as well as the amount of organic matter in the substrate determine the interstitial space available to the few interstitial species in coarse to medium-fine sands (Balsamo, 1990; Balsamo & Fregni, 1995; Nesteruk, 2007a, b). The pH can vary significantly in fresh waters; most species live in moderately acidic habitats, but some species can tolerate pH values down to 4, while others live in alkaline water up to pH 10 (Kisielewski, 1981; Nesteruk, 2004a). A few freshwater gastrotrich species are able to survive or even to live in brackish waters. Finally, all freshwater gastrotrichs are influenced by the characteristics of the water column, substrate, and aquatic vegetation.
Most freshwater chaetonotidans are epibenthic or periphytic in oxygenated habitats, and more abundant in eutrophic, standing waters (see Nesteruk, 2017 and references therein). The epibenthic community is generally more diverse and is dominated by eurytopic species of the genera Chaetonotus, Lepidodermella, Heterolepidoderma, and Ichthydium, whereas epiphytic assemblages also include semiplanktonic species of Dasydytidae and Neogosseidae (Nesteruk, 2000; Minowa & Garraffoni, 2017). Sandy sediments of lentic and running waters host all four freshwater species of Macrodasyida, but few species of Chaetonotida (see Balsamo et al., 2014). Trophic levels and zonality of water bodies also influence the diversity and density of gastrotrich populations. Water bodies with a clear zonality provide a higher habitat diversity and consequently have a richer and more abundant fauna, especially in the littoral zone (Kisielewski, 1981; Nesteruk, 2004b, 2005). Compositional differences also exist between the sublittoral and the deep zone (Nesteruk, 1996b, 2004b). Alpha-mesotrophic waters are 26–45% richer in species than waters with a lower trophic level (Nesteruk, 1996b, 2004a). The few semiplanktonic or planktonic species preferentially live in eutrophic ponds, Sphagnum bogs, and transitional peat bogs, which appear to have the highest species richness, independent from altitude, vegetation, and trophic level (Kisielewski, 1981, 1986, 1991; Balsamo, 1982; Balsamo & Todaro, 1995). In lotic habitats, gastrotrichs are mostly present where the water current is slower, such as vegetated river banks, bends of the water course, and in small streams (Kisielewski & Kisielewska, 1986; Kisielewski, 1991). A few interstitial species have been reported from sediments of springs, rivers, and streams (Ricci & Balsamo, 2000; Nesteruk, 2008; Garraffoni et al., 2017). Most gastrotrich species are able to colonize more than a single habitat and can migrate between the epibenthos, periphyton, and interstitial.
Very few studies specifically focus on the influence of abiotic variables on the occurrence and abundance of freshwater microturbellarians. Kolasa (2002) provides a brief overview on general preferences and tolerance ranges of several abiotic parameters, but only for few species tolerance ranges for temperature, oxygen, water level, oxygen, pH, and calcium are known (Heitkamp, 1982). Most species have an optimal temperature range for reproduction and population growth to occur and temperature can have a significant influence on hatching and on the generation time (Heitkamp, 1988; Sayre & Wergin, 1994; Dumont et al., 2014). Some species are stenotherm, while others are eurytherm. Microturbellarians require oxygenated layers of water and sediment. Species that live in substrates of well-oxygenated, fast-running streams are particularly sensitive to low oxygen concentrations (Kolasa, 1983). A small number of freshwater rhabdocoels are euryhaline and can also be found in brackish water habitats (Ax, 2008). However, most microturbellarians that occur in brackish water are euryhaline marine species or genuine brackish water species that do not occur in freshwater habitats. Granulometry of freshwater sediments also influences species composition and occurrence. Kolasa et al. (1987) found higher species richness and abundance in stream sediments with a grain size of 0.4–0.7 mm, compared to a low species richness and abundance for small stones or large gravel. Young (1973) found that calcium-rich and calcium-poor lakes each have their specific species of microturbellarians, but also share a number of species.
Ecological surveys of microturbellarians associated with different freshwater habitats are mostly limited to older studies from Central and Southeastern Europe (e.g., An der Lan, 1939, 1962, 1967; Mack-Fira, 1974; Kolasa, 1979; Schwank, 1981a, b, 1982a, b). More recent studies from South America and the Middle East provide valuable data on species richness and seasonal abundance of microturbellarians in permanent wetlands and temporary pools (Eitam et al., 2004; Braccini & Leal-Zanchet, 2013). Microturbellarians are found in almost all types of lentic and lotic freshwater habitats. In addition, they also occur in limnoterrestrial habitats such as mosses and forest soils (Van Steenkiste et al., 2010; Houben et al., 2015). Many species are shared between habitats, but some species are associated with specific environments. Species numbers can be high in both lentic and lotic environments with up to 94 and 57 species recorded from a single stream and lake, respectively (Kolasa, 2000). In large lakes and reservoirs, species richness and abundance are significantly higher in sediments and aquatic vegetation in the littoral zone, but some species have also been found in the limnetic zone as part of the pelagic (Dumont et al., 2014). Permanent bodies of water are usually dominated by catenulids, macrostomids, prorhynchids, and rhabdocoels associated with aquatic vegetation, plant roots, and sediment, while temporary pools typically harbor species with drought resistant resting eggs, such as typhloplanid and dalyelliid rhabdocoels (Artois et al., 2004; Eitam et al., 2004). Species compositions in lotic systems are highly variable. Mountain springs and fast flowing streams or rivers have unique hyporheic and psammophilic species or species associated with mosses and other vegetation along its course (Schwank, 1982a, b). The lower courses of river systems are inhabited by eurytopic species also found in lentic habitats. Assemblages of species are further enriched by species from habitats at the interface of lotic and lentic habitats, including limnoterrestrial, brackish water, and groundwater elements (Kolasa 1983, 2000). A very detailed review on the distribution and abundance of microturbellarians in different aquatic habitats is given by Young (2001).
Spatial and temporal dynamics of rouphozoan populations
Spatial patterns and small-scale horizontal distributions of rouphozoans are driven by abiotic and biotic factors such as the morphological features of the sediment, the heterogeneous distribution of organic matter, and bioturbation (Kisielewski, 1974–1999; Nesteruk, 1986–2017; Giere, 2009). This leads most meiofauna to aggregate in undisturbed sites or in areas richer in organic detritus, thus presenting a typical patchy distribution. Species composition can differ significantly between microhabitats, with adjoining patches of gravel, sand, plants, and organic debris having distinctive communities at the scale of centimeters.
The vertical distribution of gastrotrichs is highly related to grain size, oxygen concentration, presence and velocity of water flow, quantity of organic matter present in the interstitial water, predation pressure, as well as the tolerating abilities of different species (Palmer, 1990; Danielopol et al., 1997). The few interstitial freshwater species are mostly found in the oxygenated upper 5 cm of the sediment, in which about 46–68% of the whole gastrotrich community has been reported. Some species (about 7–10% of the total gastrotrich fauna) can migrate down to 10–15 cm deep (Nesteruk, 1991). Only a few individuals have been found at 30–40 cm deep in lotic gravel habitats where wide interstices allow the penetration of oxygen (Schmid-Araya, 1997).
Temporal patterns of gastrotrich populations and influencing factors are not well known, especially in freshwater environments. Nesteruk (1986, 2007a, 2017) reported decreased densities of some freshwater gastrotrich populations during summer and winter, probably related to the seasonal changes in oxygen concentration, water temperature, and food availability. Periods of drought and freezing in temperate zones strongly influence both the abundance and the structure of communities. In tropical zones, gastrotrichs are present and even abundant in lentic waters throughout the year, with higher abundances during the rainy season. This change in abundance is probably linked to the sediment processes and recirculation of organic matter (Kisielewski, 1991; Zébazé Togouet et al., 2007; Strayer et al., 2010).
Very few studies present data on the vertical distribution of freshwater microturbellarians in the water column and in sediments. Although some lentic microturbellarians have been found in substrates at considerable depths of 20 m or more, most studies show that the largest numbers of species and individuals were found in the shallow waters up to 1 m of the littoral zone and then decline with depth. This decline in species richness and abundance is more pronounced in eutrophic lakes than in oligotrophic lakes (Young, 2001; Kolasa, 2002). Some pelagic species of Mesostoma follow the diurnal vertical migration of their prey in the water column, rising to the surface at night to feed on cladocerans and copepods (Rocha et al., 1990). Psammic stream-dwelling microturbellarians are most abundant at 20–40 cm deep inside gravel (Schmid-Araya, 1997). Species richness and abundance are, however, mostly a function of the presence of varied microhabitats. Studies on seasonal abundances of freshwater microturbellarians give a mixed image. In Europe and Southern Brazil, different species have different seasonal abundance peaks influencing community compositions throughout the year (Young, 2001; Braccini & Leal-Zanchet, 2013). Seasonal occurrence and abundance of microturbellarians also seem to vary according to geographic location and are most likely linked to the influence of temperature, food availability, droughts and floods, and several other abiotic and biotic variables. The scarcity of studies available on these temporal dynamics highlights the need for more research in different parts of the world.
Trophic and biotic interactions
Very few studies on gastrotrichs deal with their trophic ecology, interactions within or among species, competition with and predation by other organisms, or their symbionts and parasites. Only a few qualitative experimental studies on mixed cultures of freshwater species have been done (d’Hondt, 1967; Bennett, 1975, 1979). The primary food seems to be bacteria and the particulate organic matter on the sediment surface, in interstitial spaces, and on the microbial biofilm covering the substrate. Microalgae and other protists probably supply some nutrients, but may not be essential (Packard, 1936; Brunson, 1949). As the interstitial environment is dominated by viscous forces, all prey capture devices must be adapted to overcome the functional challenge of feeding at very low Reynolds numbers. Food uptake and transport are therefore dependent on two important factors: the entrance to the pharynx (mouth) and conductance of the pharyngeal pump. Among meiofauna, only two taxa rely exclusively on suction for prey capture, nematodes and gastrotrichs (Ruppert, 1982). Both taxa have near-identical foreguts (e.g., terminal mouth, myoepithelial pharynx, triradiate lumen), yet differ in pharyngeal ultrastructure. Nematodes have strictly monosarcomeral pharynges that generate strong but slow contraction. Consequently, nematodes evolved to feed on different prey through selection on buccal size, armature, muscle supply, and pharynx shape (Munn & Munn, 2010). Alternatively, gastrotrichs have 1–12 sarcomeres/myofilament/species (Ruppert, 1982). More sarcomeres should translate into greater speed of contraction but with lower force; hence, different lineages have made an evolutionary tradeoff of force for speed (or speed for force), depending on ancestry. An exploration of these tradeoffs should be carried out by combining molecular diet analysis of selected species whose diet is already partially known (either by diagnostic PCR or by parallel sequencing—see Rubbmark et al., 2019, for comparative review) with a careful examination of pharynx structure by transmission electron microscopy and confocal laser scanning microscopy. We predict that species with monosarcomeral pharynges will be biofilm feeders, whereas species with multisarcomeral pharynges will feed primarily on eukaryotes. These studies should determine if gastrotrichs form feeding guilds akin to those in nematodes (Hochberg, pers. comm.).
Both freshwater and marine gastrotrichs seem to have chemotactic abilities to discriminate between different bacterial strains (Gray & Johnson, 1970). Sporadic observations in laboratory cultures did not show apparent reciprocal interactions with conspecific individuals (Banchetti & Ricci, 1998). Gastrotricha certainly compete with other meiofaunal organisms in feeding on bacteria, protists, biofilm, and organic detritus. Large protists, cnidarians, flatworms, polychaetes, and larvae of Diptera have been reported as natural predators of Gastrotricha (Strayer & Hummon, 1991; d’Hondt, pers. comm.). The heliozoon Actinophrys sol Ehrenberg, 1830 and the amoebozoan Amoeba spumosa Grüber, 1885 were directly observed feeding on freshwater chaetonotids, both solitarily and cooperatively in samples collected in nature and kept under laboratory conditions (Brunson, 1949; Bovee & Cordell, 1971). Escape mechanisms of Gastrotricha lie in sudden whole body contractions and rapid direction changes in locomotion. Most chaetonotidans, and especially epibenthic or semiplanktonic species, also have cuticular scales and/or long, sometimes movable spines, and protective cephalic plates that act as mechanical barriers against predators.
Individuals of freshwater Chaetonotida have been observed containing putative sporozoans in their trunk or euglenoids in their intestine, but it is not clear if these are food items, endosymbionts or parasites (Remane, 1936; Manylov, 1999; Kisielewska et al., 2015). Nothing is known about possible epibiotic associations between Gastrotricha and other taxa, like those observed in other small aquatic micrometazoa (i.e., Rotifera, Nematoda) (Bulut & Saler, 2017).
The diet of microturbellarians ranges from ciliary-assisted feeding on bacteria and algae (Catenulida) to (obligate?) diatomivory (some Macrostomorpha and Rhabdocoela) and carnivory on other meiofauna and the larvae of macroinvertebrates (see Watzin, 1983, 1986; Giere, 2009). Catenulids have a distensible mouth to engulf food and transport it to the pharynx simplex through large cilia around the mouth opening. A few species in the catenulid genus Paracatenula are mouthless and maintain symbiotic bacteria in the gut (Dirks et al., 2011, 2012). Other microturbellarians use their muscular pharynx for the capture and uptake of prey items. The pharynx can be distended to capture and ingest prey as a whole (Stenostomidae, Dalyelliidae) or protruded to breach the body wall of larger prey and suck up prey fluids and tissues (Typhloplanidae, Proseriata). Kalyptorhynchs use their anterior proboscis to capture and possibly envenomate prey and immobilize it while positioning their pharynx. Some flatworms, such as prorhynchids and Gyratrix hermaphroditus Ehrenberg, 1831, use their stylet to stab prey.
Feeding strategies of freshwater microturbellarians include mucus trapping, active searching, ambush predation, the use of toxins, and group foraging (Young, 2001; De Roeck et al., 2005; Dumont et al., 2014), but comprehensive data on diet composition and prey selection are very limited compared to marine and brackish water microturbellarians (Watzin, 1985; Reise, 1988; Menn & Armonies, 1999). Diagnostic PCR was used to reconstruct the diet in individual marine flatworm species (Maghsoud et al., 2014; Fig. 5), and could also prove valuable for freshwater microturbellarians. One recent study shows acquired prey selection of toxic and non-toxic ciliates by the catenulid Stenostomum sphagnetorum Luther, 1960; this behavior was lost after asexual reproduction (Buonanno, 2011). Freshwater microturbellarians can reach high densities and studies have shown that predation by species of Mesostoma and Phaenocora can influence the population dynamics of zooplankton or benthic communities seasonally (Young, 1977; De Roeck et al., 2005; Dumont et al., 2014). Larger microturbellarians that feed on mosquito larvae have therefore been proposed as biological control agents (Tranchida et al., 2009). Feeding guilds based in part on pharyngeal structure have been hypothesized in flatworms (e.g., Bilio, 1967; Straarup, 1970; Table 4). Species of Macrostomum may be specialist feeders on diatoms or, alternatively, take any relatively slow-moving prey small enough to swallow, including juvenile mussels and cladocerans (Delp, 2002). Proseriates with a ventrally directed plicate pharynx and rhabdocoels with a bulbous rosulate pharynx often use that to suck out prey contents (Jennings, 1974b; own observations). Rhabdocoels with an anterior barrel-shaped (doliiform) pharynx often suddenly dilate the pharynx, suck in, and swallow fast-moving prey whole (Bilio, 1967). Rapidly contracting radial muscles could play a role in overcoming viscous forces and quickly sucking in smaller prey. This mechanism is used to capture swimming prey by some members of the genus Stenostomum (Nuttycombe & Waters, 1935) and confocal microscopy of the head region in Stenostomum virginianum Nuttycombe, 1931 shows pseudostriation of the radial musculature in the pharynx—an arrangement that is predicted to increase contraction velocity (Smith & Davis, unpublished). Interestingly, pseudostriation has also been observed in the pharyngeal radial muscles of a Prolecithophoran (Rieger et al., 1991). Additionally, congenerics occurring in the same biotope (e.g., Catenula lemnae Duges, 1832 and Catenula confusa Nuttycombe, 1956) might have different diets that are reflected in the structure of their pharynges—for instance, size-selection between unicellular algae vs bacteria. In summary, one would expect to find both convergent and divergent adaptations across the different pharynx types—adaptations that depend in part on prey mobility, and in part on prey size (e.g., sucking out the body contents of oligochaetes and amphipods vs swallowing smaller prey whole). However, there appear to be no published studies directed at understanding the biomechanics of the pharynx in microturbellarians.

Adapted from Maghsoud et al. (2014)
Partial results of PCR amplifications for two primer sets directed against nematodes applied to DNA isolates from single platyhelminth individuals. GenBank accession numbers and percent sequence identities are listed for each prey species identified by Blastn. NEM nematode; ACOEL acoelomorph, TURB turbellarian.
Microturbellarians are also eaten by other invertebrates, small vertebrates, and even protists. Defensive behavior such as the release of mucous to escape from predatory ciliates has been observed (Buonanno, 2009) and rhabdites have long been suggested to be defensive, whether or not their primary role is mucus production for ciliary gliding (Rieger et al., 1991). Both intra- and interspecific predation by other microturbellarians have also been recorded (Young, 2001; own observations). Although the extent and impact of predation on microturbellarian populations have not been assessed in detail, predator exclusion did not produce the expected increase in platyhelminthes, suggesting that predation does not regulate flatworm populations except under specialized circumstances (Reise, 1979; Giere, 2009).
Freshwater microturbellarians, and then predominantly rhabdocoels, can be both ectosymbionts on other freshwater animals and hosts for other organisms. Temnocephalids are small freshwater epibionts on macroinvertebrates and turtles. They prey on other co-symbiotic organisms and feed opportunistically on particles of the host’s food. The dalyelliid Varsoviella kozminskii Gieysztor & Wiszniewski, 1947 lives on the gills of freshwater gammarids (Gieysztor & Wiszniewski, 1947). A number of freshwater species in the genera Castrada, Dalyellia, Gieysztoria, Phaenocora, and Typhloplana harbor endosymbiotic chlorophytes. Little is known about this symbiosis, but studies on Phaenocora typhlops (Vejdovsky, 1880), Dalyellia viridis (Shaw, 1791), and Typhloplana viridata (Abildgaard, 1789) suggest that worms could benefit from the photosynthate and oxygen produced by the algae (Young, 2001 and references therein). Kleptoplasty, a form of endosymbiosis where only the algal plastids are sequestered and retained, has recently been observed in marine and brackish water rhabdocoels (Van Steenkiste et al., 2019). Species of the genus Microstomum often retain nematocysts from digested Hydra tentacles as kleptocnids (Fig. 3f, arrows). Parasites of freshwater microturbellarians have occasionally been recorded in older taxonomic literature, but very few studies characterize the observed parasites in detail. Most of these parasites are protists, including apicomplexans, microsporidians, ciliates, and euglenozoans. Only a few records mention metazoan parasites such as nematodes or neodermatan flatworms (for details, see Young, 2001 and references therein). It is noteworthy that the last comprehensive review of microturbellarians as parasites and hosts was published over 100 years ago (von Graff, 1903), and less comprehensive modern summaries are available (Jennings, 1971, 1974a, 1977).
Life strategies
Gastrotrichs have various reproductive modalities. While marine Macrodasyida are hermaphrodite with cross-fertilization, freshwater Chaetonotida generally reproduce by thelytokous parthenogenesis. As a consequence, freshwater populations can start from any single individual. Many freshwater species can also produce resting eggs that can withstand environmental adverse conditions and act as dispersal propagules. The factors triggering the production and the hatching of the resting eggs are not yet known. Parthenogenesis, resting eggs, and short life cycles allow gastrotrichs to survive extreme variations in environmental conditions (e.g., droughts, floods) and colonize challenging habitats such as lotic sediments (Ricci & Balsamo, 2000), caves (Vandel, 1964; Renaud-Mornant, 1986; Kolicka et al., 2017), high mountain biotopes (Baumann, 1910; Tonolli & Tonolli, 1951; Gadea, 1988), hot springs (De Guerne, 1888), and deep crater lakes (Barrois, 1896; R. Schabetsberger, unpublished data). In addition, individuals might also be able to survive critical conditions by migrating deeper into the sediment (Nesteruk, 2007c).
Laboratory tests have evidenced the existence of a long postparthenogenic phase with production of aberrant spermatozoa in Chaetonotida. This suggests a possible amphimictic reproduction, and thus the existence of two successive reproductive modalities in a single lifespan. Such a biphasic reproduction strategy would allow for a quick increase in population numbers through apomictic parthenogenesis followed by the introduction of genetic variation through cross-fertilization (Balsamo, 1992; Hummon & Hummon, 1992).
Microturbellarians are hermaphrodites and display both sexual (cross- and self-fertilization) and asexual (paratomy) modes of reproduction (Kolasa, 2000). Catenulids and some macrostomids (e.g., Microstomum) reproduce asexually, although sexual reproduction can also occur. Most other freshwater microturbellarians reproduce by internal cross-fertilization, either by mutual copulation or sometimes by hypodermic impregnation. Self-fertilization is rare and has only been observed in a few species (Young, 2001).
Life histories of freshwater microturbellarians are not well understood and only known for a handful of species from temperate regions (Cox & Young, 1974; Heitkamp, 1988). Microturbellarians can produce both subitaneous (non-resting) eggs for rapid population growth during their active phase and dormant resting eggs/cocoons enclosed by a thicker, more resistant shell at the end of their active phase to overcome periods of high/low temperature, water level changes, or desiccation (Young, 2001). Life cycles are conditioned by seasonal cycles and droughts or flooding events. As such, many species have flexible life cycles depending on geographic location and habitat. Annual species are active year-round and restricted to permanent water bodies. Reproduction appears in one or more generations throughout the year, often during a specific season and influenced by temperature, food, and the presence of water. Seasonal species only appear in one or more seasons which often overlap with periods of vegetation growth and/or phyto- and zooplankton blooms.
A comparative summary of modes of reproduction of freshwater gastrotrichs and microturbellarians is reported in Table 5.
Global diversity and distribution
The majority of freshwater gastrotrichs are Chaetonotida, with about 350 species in 5 families and 24 genera (72% of total chaetonotidan species). Only four species of Macrodasyida, in one family and one genus (except one species incertae sedis), occur in fresh waters (Kisielewski, 1987; Kånneby & Kirk, 2017; Garraffoni et al., 2019). Diversity of freshwater Gastrotricha in different geographic areas is not as well known as that of marine species, and available data are quite heterogeneous. Most research has been carried out in Europe and the Americas. Data on geographic distribution are usually limited to the sampling sites, especially in older literature, and occasionally include some ecological data (see Balsamo et al., 2014 for previous references). This insufficient knowledge is a direct consequence of technical problems that are common to all soft-bodied meiofaunal animals and concern their collecting and handling, but also of the particular focus of most studies on the epibenthic and periphytic species from standing water bodies. Moreover, the taxonomy—especially of the order Chaetonotida—is still unstable because of the intraspecific variability of many species, the scarcity of diagnostic data in old descriptions, and the increasing evidence of the existence of cryptic species in widespread nominal species (Kieneke et al., 2012; Kånneby et al., 2012, 2013). In Europe, the continent studied most thoroughly, about 250 species have been identified and some countries have been the object of regional ‘faunas’ (Balsamo, 1983; Balsamo & Tongiorgi, 1995; Balsamo et al., 2014 for global references). Of course, the effect of sampling effort should be considered in advancing possible scenarios of the global diversity and distribution of the phylum, also because large areas in most other parts of the world have not been explored yet (Balsamo et al., 2008, 2014; Fontaneto et al., 2012). Three out of four species of freshwater Macrodasyida (fam. Redudasyidae) are reported from the Americas (Fig. 6), while the fourth species (Marinellina flagellata Ruttner-Kolisko, 1955, incertae sedis) is known from Austria (Ruttner-Kolisko, 1955; Schmid-Araya & Schmid, 1995). As for Chaetonotida, three of the five freshwater families, Dasydytidae, Neogosseidae, and especially Chaetonotidae, appear to be cosmopolitan, and most genera and species have been recorded in at least two continents, especially in tropical areas (Figs. 7, 8). Representatives of the rare family Dichaeturidae have occasionally been found in a few European localities and a single Japanese site. Each of the two species of the family Proichthydiidae has only been recorded once in their respective type localities in South America and Asia (see Balsamo et al., 2014 for detailed references). About half of the freshwater genera have an intercontinental distribution; about 1/3 of the European species and 1/3–1/2 of the South American species appear to be cosmopolitan. Tropical areas generally have a high diversity of genera and species. Brazil in particular has many endemic genera, some of which are only known from a single site in Amazonia (e.g., Undula in the chaetonotid subfamily Undulinae). There are also numerous other records of species from only one country and often from only one site, but knowledge on gastrotrich diversity in surrounding countries and regions is not sufficient to define these species as endemic (Balsamo et al., 2014: Garraffoni & Balsamo, 2017).
An update of the situation reported in Balsamo et al. (2008) highlights the increase in the number of new freshwater species of gastrotrichs recently described, mainly from the Palearctic, but also from the Neotropic and Nearctic (Balsamo et a. 2019; Todaro et al., 2019) (Table 6a; Fig. 9A). This increase is not only related to an increased sampling effort, but also to investigations in environments not yet explored such as Arctic waters and artificial water bodies (greenhouses) (Kolicka et al., 2018; Kolicka, 2019 and references therein).
Current numbers of freshwater species of A Gastrotricha and B microturbellarians in different biogeographic regions of the world (black numbers and circles), including numbers of endemic species per region (light gray numbers and circles) and numbers of species shared between regions (dark gray numbers and lines). PA Palearctic, NA Nearctic, NT Neotropical, AT Afrotropical, OL Oriental, AU Australian, PAC Pacific, ANT Antarctic
Global species numbers in the different groups of freshwater microturbellarians amount to the following numbers: Catenulida (95 species), Macrostomorpha (118 species), Prorhynchida (31 species), Proseriata (12 species), Rhabdocoela (739 species), Prolecithophora (20 species), and Bothrioplanida (2 species) (Table 6b). The majority of freshwater species belong to three groups within the rhabdocoel clade Limnotyphloplanida: Temnocephalida (160 species), Dalyelliidae (174 species), and Typhloplanidae (271 species). Knowledge on the diversity and distribution of freshwater microturbellarians in different parts of the world is relatively scarce and, as for most other freshwater meiofauna, reflects the historical efforts and geographical work area of taxonomists rather than actual microturbellarian diversity and distribution. Table 6b and Fig. 9B summarize species numbers for microturbellarians in each biogeographic zone. These numbers are the most current update since the census of freshwater turbellarians in Schockaert et al. (2008). Increased species numbers and distribution records for the Palearctic can largely be attributed to increased taxon sampling of catenulids (Larsson & Willems, 2010), macrostomids (Rogozin, 2012), rhabdocoels (Rogozin, 2011, 2017; Van Steenkiste et al., 2011b; Korgina, 2014; Timoshkin et al., 2014; Houben et al., 2015), and proseriates (Timoshkin et al., 2010), and to the recognition of cryptic species within Microstomum (Atherton & Jondelius, 2018). Species numbers and records in the Nearctic have increased slightly due to recent surveys of rhabdocoels in Canada and the USA (Van Steenkiste et al., 2011a; Houben et al., 2014). The largest increase in species numbers and records can be found in the Neotropical, Oriental, and Australian regions thanks to recent studies describing and recording several dozens of rhabdocoels and macrostomids in South America (e.g., Adami et al., 2012; Martínez-Aquino et al., 2014; Braccini et al., 2016), Southern China (Sun et al., 2015; Lin et al., 2017), and India and Australia (e.g., Van Steenkiste et al., 2012). Only a marginal increase or status quo in species records are shown for the Afrotropical, Pacific, and Antarctic regions, where almost no or very few studies on microturbellarians have been conducted in the past decades. While global species numbers have increased with over 16% in the last 12 years, biodiversity surveys of microturbellarians in some of the world’s largest and most diverse freshwater systems, such as the Pantanal or the basins of the Amazon, Congo, and Ganges–Brahmaputra rivers, are still very limited or non-existent. Several freshwater habitats, such as limnoterrestrial habitats are seldom sampled and could contain a hidden reservoir of microturbellarian diversity (Van Steenkiste et al., 2010; Houben et al., 2015). Phreatic aquifers or peat swamp forests remain unexplored altogether. As such, our freshwater microturbellarian census clearly shows a large potential for species discovery in freshwater habitats around the world. Even in the most intensely sampled biogeographical regions, including the Palearctic, Nearctic, and Neotropics, vast areas and many habitats are still to be surveyed.
As a result of the paucity of data on species diversity in many regions, biogeographic patterns for freshwater microturbellarians are hard to infer. While the majority of species have so far only been recorded from one biogeographic region (“endemic species” in Table 6b), some nominal species of catenulids (e.g., Stenostomum leucops (Duges, 1828)), macrostomids (e.g., Microstomum lineare (Müller, 1773)), and rhabdocoels (e.g., Gyratrix hermaphroditus) seem to have cosmopolitan distributions. Others are widespread, but confined to one or two biogeographic regions. For instance, several nominal species of dalyelliids (e.g., Microdalyellia armigera (Schmidt, 1861), Gieysztoria cuspidata (Schmidt, 1861), Castrella truncata (Abildgaard, 1789)) have a Holarctic distribution. One hypothesis is that the widespread distribution of micro-organisms could be the result of long-distance dispersal by long-term resistant dormant stages and the ability to colonize and reproduce quickly (Fontaneto, 2019). In addition, some of these widespread nominal species could be complexes of closely related species, the so-called (pseudo)cryptic species. This has been demonstrated in both marine (Scarpa et al., 2016; Van Steenkiste et al., 2018) and freshwater (Atherton & Jondelius, 2018) microturbellarians, where several nominal species are now considered complexes of different species.
On a superspecific level, distribution patterns of freshwater microturbellarians are even harder to untangle. Most genera have representatives in different biogeographical regions. Some genera or species groups seem confined to certain biogeographical areas and their distribution could possibly be explained by a combination of geological events and dispersal.
Perspectives
The majority of studies on rouphozoans are mostly conducted by researchers in Europe and the Americas. Programs for taxonomic capacity building in developing countries could benefit biodiversity surveys of freshwater meiofauna in the vastly undersampled but biodiverse freshwater ecosystems of Africa, Southeast Asia, and the Americas. These regions might be of crucial importance for a more realistic biodiversity estimation of microturbellarian and gastrotrich species diversity, but are at risk because of rapid habitat destruction and climate change. Wide-ranging European research programs on the freshwater animal biodiversity have been carried out in the past years (2000–2008) leading to the compilation of European and global databases of the known biodiversity at the time (Fauna.Europaea Project, see de Jong et al., 2014; FADA Freshwater Animal Diversity Assessment Project, see Balsamo et al., 2008). Increased species discovery should be a concerted effort with expanding and updating databases that consolidate existing and new taxonomic and biogeographic data. An important first step would be the development of regularly updated identification keys for freshwater rouphozoans. This could be part of a broader effort on freshwater meiofauna analogous to current efforts for marine meiofauna (Schmidt-Rhaesa, 2020). To accelerate biodiversity surveys of rouphozoans, protocols for animal collection, vouchering, DNA extraction, DNA barcode marker selection, amplification, and sequencing should be adjusted to the upcoming and promising third-generation sequencing techniques (e.g., Nanopore).
A large impediment for future research on taxonomy, biogeography, and phylogeny of Rouphozoa (and all other Metazoa for that matter) is the implementation of the Nagoya Protocol (NP). Since October 2014, NP regulates all access to, and benefit sharing of, genetic resources worldwide. The protocol was designed to ensure fair use of countries’ genetic resources, including the use of traditional knowledge. However, as logical and fair such legislation might seem, many concerns have been uttered (Deplazes-Zemp et al., 2018, and references therein). Whereas the NP and resulting legislation is needed to counter biopiracy and ensure that countries are not robbed of their economically valuable biological and genetic resources, it has devastating side effects on (descriptive) fundamental research. Without any doubt, the NP will significantly slow down taxonomic and other biodiversity studies just in an era in which such projects are much needed. For instance, in our daily work on microturbellarians, specimens are exchanged between researchers on a very regular base, in several cases involving colleagues from developing countries with whom we try to build up a structural collaboration. Because of the regulations of the NP, such exchange of material, indispensable for fruitful joint scientific activities, is hampered. The administrative workload will discourage international collaboration between researchers and will cause (and is already causing) a bias towards research in countries that did not ratify the NP. Moreover, for many biologists and institutes, it is not entirely clear (yet) what procedures should be followed in practice. We can only hope that the regulations of the NP will be revised in the future to ensure that at least the much-needed fundamental, non-profit research can continue smoothly.
Dispersal abilities of freshwater gastrotrichs and microturbellarians and the relationship between dispersal and distribution have not been specifically investigated so far. The small size of gastrotrichs and microturbellarians and the absence of planktonic stages limit active dispersal of live individuals to short distances. Wind, running water, and more mobile animal vectors have all been proposed as passive long-distance dispersal vectors for long-term desiccation-resistant eggs or cocoons of rouphozoans (Gerlach, 1977; Hagerman & Rieger, 1980; Young, 2001; Vanschoenwinkel et al., 2008, 2009; Viana et al., 2016). Human-mediated dispersal (aquaculture, ballast waters, etc.) of gastrotrichs, microturbellarians, and many other aquatic micro-invertebrates is likely, but has not yet been the subject of specific studies (Artois et al., 2011). Future studies focusing on the spatial connectivity and gene flow of freshwater gastrotrich and microturbellarian populations are highly needed to support these assumptions. Cerca et al. (2018) have stressed the importance of including ecological and life-history traits, evolutionary history and cryptic speciation, metapopulation dynamics, as well as considering vicariant events and (ancient) dispersal routes on different geographic and temporal scales to explain current-day distribution of marine meiofauna. These are all important considerations to also elucidate recent distribution patterns of freshwater gastrotrichs and microturbellarians.
The task of untangling hidden diversity, spatial connectivity, and trophic networks in Rouphozoa will certainly fall to molecular methods. Metagenetic, genomic, and transcriptomic data—when combined and integrated with morphological and ecological data—can also provide new insights into additional properties and patterns such as niche differentiation, differential gene expression, genome duplication or reduction, character evolution, reproduction modes and traits related to sexual selection, origins of symbiotic interactions, co-evolution and host specificity, nutritional strategies, and life cycle modifications. The current lack of such integrated studies impedes our understanding on evolutionary processes within rouphozoans. Many closely related species of freshwater gastrotrichs and microturbellarians occur in sympatry suggesting some kind of ecological differentiation. However, non-ecological speciation in allopatry at some point in the past has been proposed for present-day sympatric organisms (Czekanski-Moir & Rundell, 2019). Species flocks of rhabdocoels in Lake Baikal are the product of spectacular speciation events, but the mechanisms behind these radiations are not known. Revealing these underlying processes remains challenging and will require holistic multi-evidence approaches employing new techniques in high-resolution microscopy and high-throughput sequencing.
Finally, there is still a valuable role for functional morphology, specifically, studies with a biomechanical approach. From the original analysis of the role of connective tissue in soft-bodied worms (Clark & Cowey, 1958), and subsequent refinements applied to soft-tissue extensible structures more generally (Kier, 2010), biomechanical studies in Rouphozoa are rare: proboscis function in Cheliplana (Uyeno & Kier, 2010) and in Schizorhynchia more generally (Smith et al., 2015), and dynamics of duo-gland adhesion in marine microturbellaria (Wunderer et al., 2019). Accordingly, additional studies directed at a better understanding of rouphozoan biomechanics would provide a much richer context for the evolutionary and ecological work proposed above.
References
Adami, M., C. Damborenea & J. R. Ronderos, 2012. A new limnic species of Macrostomum (Platyhelminthes: Macrostomida) from Argentina and its muscle arrangement labeled with phalloidin. Zoologischer Anzeiger 251: 197–205.
Amaral-Zettler, L. A., E. A. McCliment, H. W. Ducklow & S. M. Huse, 2009. A method for studying protistan diversity using massively parallel sequencing of V9 hypervariable regions of small–subunit ribosomal RNA genes. PLoS ONE 4: e6372.
An der Lan, H., 1939. Zur rhabdocoelen Turbellarienfauna des Ochridasees (Balkan). Sitzungsberichten der Akademie der Wissenschaften in Wien: Mathematisch Naturwissenschaftliche Klasse, Abteilung I 148: 195–254.
An der Lan, H., 1962. Zur Turbellarien-Fauna der Donau. Archiv für Hydrobiologie. Supplement 27: 3–27.
An der Lan, H., 1967. Zur Turbellarien-Fauna des hyporheischen Interstitials. Archiv für Protistenkunde 33: 63–72.
Arroyo, A. S., D. López-Escardó, C. De Vargas & I. Ruiz-Trillo, 2016. Hidden diversity of Acoelomorpha revealed through metabarcoding. Biology letters 12: 20160674.
Artois, T., D. Fontaneto, W. D. Hummon, S. J. McInnes, M. A. Todaro, M. V. Sørensen & A. Zullini, 2011. Ubiquity of microscopic animals? Evidence from the morphological approach in species identification. In Fontaneto, D. (ed), Biogeography of Microscopic Organisms: Is Everything Small Everywhere? The Systematics Association Press: 244–283.
Artois, T., W. Willems, E. De Roeck, M. Jocqué & L. Brendonck, 2004. Freshwater Rhabdocoela (Platyhelminthes) from ephemeral rock pools from Botswana, with the description of four new species and one new genus. Zoological Science 21: 1063–1072.
Atherton, S., 2015. Cryptic speciation and the evolution of asexuality in marine gastrotricha. Doctoral dissertation. University of Massachusetts, Lowell.
Atherton, S. & U. Jondelius, 2018. Wide distributions and cryptic diversity within a Microstomum (Platyhelminthes) species complex. Zoologica Scripta 47: 486–498.
Atherton, S. & U. Jondelius, 2019. A taxonomic review and revisions of Microstomidae (Platyhelminthes: Macrostomorpha). PLoS ONE 14: e0212073.
Ax, P., 2008. Plathelminthes aus Brackgewässern der Nordhalbkugel. Franz Steiner Verlag, Stuttgart.
Balsamo, M., 1982. Three new gastrotrichs from a Tuscan-Emilian Apennine lake. Bollettino di Zoologia 49: 287–295.
Balsamo, M., 1983. Gastrotrichi (Gastrotricha). Guide per il riconoscimento delle specie animali delle acque interne italiane. Consiglio Nazionale delle Ricerche, Roma, AQ/1/199, 20: 1–92.
Balsamo, M., 1990. Gastrotrichs from Lakes Bolsena, Chiusi and Montepulciano (Central Italy), with the description of four new species. Bollettino di Zoologia 57: 165–178.
Balsamo, M., 1992. Hermaphroditism and parthenogenesis in lower Bilateria: Gnathostomulida and Gastrotricha. In Dallai, R. (ed.), Sex: Origin and Evolution. Mucchi, Modena: 309–327.
Balsamo, M. & E. Fregni, 1995. Gastrotrichs from interstitial fresh water, with a description of four new species. Hydrobiologia 302: 163–175.
Balsamo, M. & M. A. Todaro, 1988. Life history traits of two chaetonotids (Gastrotricha) under different experimental conditions. Invertebrate Reproduction and Development 14: 161–176.
Balsamo, M. & M. A. Todaro, 1995. Gastrotrichi del Trentino: le Viotte del Monte Bondone. Studi Trentini di Scienze Naturali. Acta Biologica 70: 9–22.
Balsamo, M. & M. A. Todaro, 2002. Gastrotricha. In Rundle, S. D., A. L. Robertson & J. M. Schmid-Araya (eds), Freshwater Meiofauna: Biology and Ecology. Backhuys Publishers, Leiden: 45–61.
Balsamo, M. & P. Tongiorgi, 1995. Gastrotricha. In Minelli, A., S. Ruffo & S. La Posta (eds), Checklist Delle Specie Della Fauna Italiana. Calderini, Bologna: 1–11.
Balsamo, M., J. L. Hondt d’, J. Kisielewski & L. Pierboni, 2008. Global diversity of gastrotrichs (Gastrotricha) in fresh waters. Hydrobiologia 595: 85–91.
Balsamo, M., J. L. Hondt d’, L. Pierboni & P. Grilli, 2009. Taxonomical and nomenclatural notes on freshwater Gastrotricha. Zootaxa 2158: 1–19.
Balsamo, M., P. Grilli, L. Guidi & J. L. Hondt d', 2014. Gastrotricha: Biology, Ecology and Systematics. Families Dasydytidae, Dichaeturidae, Neogosseidae, Proichthydiidae. Backhuys Publishers, Leiden: 1–187.
Balsamo, M., J. L. Hondt d', J. Kisielewski, M. A. Todaro, P. Tongiorgi, L. Guidi, et al., 2015. Fauna Europaea: Gastrotricha. Biodiversity Data Journal 3: e5800.
Balsamo, M., J. L. Hondt d’ & P. Grilli, 2019. Phylum Gastrotricha. In Rogers, D. C. & J. H. Thorp (eds), Thorp and Covich’s Freshwater Invertebrates. Academic Press, London: 149–218.
Banchetti, R. & N. Ricci, 1998. The behavior of Heterolepidoderma sp. (Gastrotricha). Zoologica Scripta 15: 131–137.
Barrois, T., 1896. Recherches sur la faune des eaux douces des Açores. Mémoires de la Société des Sciences Agricoles et Arts. Société des Sciences Agricoles et Arts, Lille: 1–172.
Baumann, F., 1910. Beiträge zur Biologie der Stockhornseen. Revue Suisse de Zoologie 18: 647–675.
Bennett, L. W., 1975. Partial trophic analysis of a freshwater Gastrotrich. Bulletin of the Association of Southeastern Biologists 22: 41–42.
Bennett, L. W., 1979. Experimental analysis of the trophic ecology of Lepidodermella squammata (Gastrotricha: Chaetonotida) in mixed culture. Transactions of the American Microscopical Society 98: 254–260.
Bilio, M., 1967. Nahrungsbeziehungen der Turbellarien in Küstensalzwiesen. Helgoländer Wissenschaftliche Meeresuntersuchungen 15: 602.
Bleidorn, C., 2019. Recent progress in reconstructing lophotrochozoan (spiralian) phylogeny. Organisms Diversity and Evolution 19: 557–566.
Bovee, E. C. & D. L. Cordell, 1971. Feeding on gastrotrichs by the heliozoon Actinophrys sol. Transactions of the American Microscopical Society 90: 365–369.
Braccini, J. A. L. & A. M. Leal-Zanchet, 2013. Turbellarian assemblages in freshwater lagoons in southern Brazil. Invertebrate Biology 132: 305–314.
Braccini, J. A. L., S. V. Amaral & A. M. Leal-Zanchet, 2016. Microturbellarians (Platyhelminthes and Acoelomorpha) in Brazil: invisible organisms? Brazilian Journal of Biology 76: 476–494.
Brunson, R. B., 1949. The life history and ecology of two North American gastrotrichs. Transactions of the American Microscopical Society 68: 1–20.
Bulut, H. & S. Saler, 2017. Presence of an epibiont Epistylis sp. (Protozoa, Cilophora) on some zooplankton. Fresenius Environmental Bulletin 26: 6334–6339.
Buonanno, F., 2009. Antipredator behavior of the freshwater microturbellarian Stenostomum sphagnetorum against the predatory ciliate Dileptus margaritifer. Zoological Science 26: 443–447.
Buonanno, F., 2011. The changes in the predatory behavior of the microturbellarian Stenostomum sphagnetorum on two species of toxic-secreting ciliates of the genus Spirostomum. Biologia 66: 648–653.
Cannon, L. R. G., 1986. Turbellaria of the World. A Guide to Families and Genera. Queensland Museum, Brisbane: 1–131.
Cerca, J., G. Purschke & T. H. Struck, 2018. Marine connectivity dynamics: clarifying cosmopolitan distributions of marine interstitial invertebrates and the meiofauna paradox. Marine Biology 165: 123.
Chariton, A. A., S. Stephenson, M. J. Morgan, A. D. L. Steven, M. J. Colloff, L. N. Court & C. M. Hardy, 2015. Metabarcoding of benthic eukaryote communities predicts the ecological condition of estuaries. Environmental Pollution 203: 165–174.
Clark, R. T. & J. B. Cowey, 1958. Factors controlling the change of shape of certain nemertean and turbellarian worms. Journal of Experimental Biology 35: 731–748.
Cox, N. & J. O. Young, 1974. Some observations on two populations of Dalyellia viridis (G. Shaw) (Turbellaria: Neorhabdocoela) living in temporary habitats in England. Hydrobiologia 44: 161–176.
Creer, S., V. G. Fonseca, D. L. Porazinska, R. M. Giblin-Davis, W. Sung, D. M. Power, et al., 2010. Ultrasequencing of the meiofaunal biosphere: practice, pitfalls and promises. Molecular Ecology 19: 4–20.
Czekanski-Moir, J. E. & R. J. Rundell, 2019. The ecology of nonecological speciation and nonadaptive radiations. Trends in ecology and evolution 34: 400–415.
d’Hondt, J. L., 1967. Documents sur les Gastrotriches dulcicoles des eaux françaises. Annales Limnologiques 3: 381–397.
d’Hondt, J. L., 1971. Gastrotricha. Oceanography Marine Biology Annual Reviews 9: 141–192.
Danielopol, D. L., R. Rouch, P. Pospisil, P. Torreiter & F. Mößlacher, 1997. Ecotonal animal assemblages: their interest for groundwater studies. In Gilbert, J., J. Mathieu & F. Fournier (eds), Groundwater/Surface water ecotones. Cambridge University Press, Cambridge: 11–20.
de Jong, Y., et al., 2014. Fauna Europaea – all European animal species on the web. Biodiversity Data Journal 2: e4034. https://doi.org/10.3897/BDJ.2.e4034.
De Roeck, E. R. M., T. Artois & L. Brendonck, 2005. Consumptive and non-consumptive effects of turbellarian (Mesostoma sp.) predation on anostracans. Hydrobiologia 542: 103–111.
Delp, A. M., 2002. Flatworm predation on juvenile freshwater mussels. Doctoral dissertation. Southwest Missouri State University.
Deplazes-Zemp, A., S. Abiven, P. Schaber, M. Schaepman, G. Schaepman-Strub, B. Schmid, K. K. Shimizu & F. Altermatt, 2018. The Nagoya Protocol could backfire on the Global South. Nature Ecology and Evolution 2: 917–919.
Dirks, U., H. R. Gruber-Vodicka, N. Leisch, W. Sterrer & J. A. Ott, 2011. A new species of symbiotic flatworms, Paracatenula galateia sp. nov. (Platyhelminthes: Catenulida: Retronectidae) from Belize (Central America). Marine Biology Research 7: 769–777.
Dirks, U., H. R. Gruber-Vodicka, N. Leisch, S. Bulgheresi, B. Egger, P. Ladurner & J. A. Ott, 2012. Bacterial symbiosis maintenance in the asexually reproducing and regenerating flatworm Paracatenula galateia. PLoS ONE 7: e34709.
Dumont, H. J., A. C. Rietzler & B. P. Han, 2014. A review of typhloplanid flatworm ecology, with emphasis on pelagic species. Inland Waters 4: 257–270.
Egger, B., F. Lapraz, B. Tomiczek, S. Müller, C. Dessimoz, J. Girstmair, N. Škunca, K. A. Rawlinson, C. B. Cameron, E. Beli, M. A. Todaro, M. Gammoudi, C. Noreña & M. J. Telford, 2015. A transcriptomic-phylogenomic analysis of the evolutionary relationships of flatworms. Current Biology 25: 1347–1353.
Eitam, A., C. Noreña & L. Blaustein, 2004. Microturbellarian species richness and community similarity among temporary pools: relationships with habitat properties. Biodiversity and Conservation 13: 2107–2117.
F.A.D.A. Freshwater Animal Diversity Assessment. http://fada.biodiversity.be/
Fegley, S. R., J. P. S Smith III., D. Johnson, A. Schirmer, J. Jones-Boggs, A. Edmonds & J. Bursey, 2020. (Submitted ~ 4/10). Nourished, exposed beaches exhibit altered sediment structure and meiofaunal communities. Diversity X: x–xx
Fontaneto, D., 2019. Long-distance passive dispersal in microscopic aquatic animals. Movement Ecology 7: 1–10.
Fontaneto, D., A. M. Barbosa, H. Segers & M. Pautasso, 2012. The ‘rotiferologist’ effect and other global correlates of species richness in monogonont rotifers. Ecography 35: 174–182.
Gadea, K., 1988. Nuevos datas on Chaetonotus zelinkai. Miscellanea Zoologica (Barcelona) 12: 357–360.
García-Criado, F., & C. Trigal. 2005. Comparison of several techniques for sampling macroinvertebrates in different habitats of a North Iberian pond. Hydrobiologia 545: 103–115.
Garraffoni, A. R. S. & T. Q. Araújo, 2010. Chave de identificação de Gastrotricha de águas continentais e marinhas do Brasil. Papéis Avulsos Zoologicos (São Paulo) 50: 535–552.
Garraffoni, A. R. & M. Balsamo, 2017. Is the ubiquitous distribution real for marine gastrotrichs? Detection of areas of endemism using Parsimony Analysis of Endemicity (PAE). Proceedings of the Biological Society of Washington 130: 198–211.
Garraffoni, A. R. S., T. Q. Araújo, A. P. Lourenço, L. Guidi & M. Balsamo, 2017. A new genus and new species of freshwater Chaetonotidae (Gastrotricha: Chaetonotida) from Brazil with phylogenetic position inferred from nuclear and mitochondrial DNA sequences. Systematics and Biodiversity 15: 49–62.
Garraffoni, A. R. S., T. Q. Araújo, A. P. Lourenço, L. Guidi & M. Balsamo, 2019. Integrative taxonomy of a new Redudasys species (Gastrotricha: Macrodasyida) sheds light on the invasion of fresh water habitats by macrodasyids. Scientific Reports 9: 2067.
Gastrotricha World Portal: http://www.gastrotricha.unimore.it. Accessed 24 Jan 2020
Gerlach, S., 1977. Means of meiofauna dispersal. Mikrofauna Meeresbodens 61: 89–103.
Giere, O., 2009. Meiobenthology. The Microscopic Fauna in Aquatic Sediments. Springer, Berlin: 1–527.
Gieysztor, M. & J. Wiszniewski, 1947. Sur un Turbellarié vivant sur les branchies de Gammarus ischnus G. O. Sars (Rhabdocoela, Dalyelliidae). Annales Musei Zoologici Polonici 14: 1–5.
Giribet, G. & G. D. Edgecombe, 2019. Perspectives in animal phylogeny and evolution: a decade later. In Fusco, G. (ed.), Perspectives on Evolutionary and Developmental Biology. Padova University Press, Padova: 167–178.
Gray, J. S. & R. M. Johnson, 1970. The bacteria of a sandy beach as an ecological factor affecting the interstitial gastrotrich Turbanella hyalina Schultze. Journal of Experimental Marine Biology and Ecology 4: 119–133.
Guerne, J. De, 1888. Campagnes scientifiques du Yacht monégasque l’Hirondelle, troisième année, Vol. 26. Gauthier-Villars, Paris: 72–78.
Hagerman, G. M. & R. M. Rieger, 1980. Dispersal of benthic meiofauna by wave and current action in Bogue Sound, North Carolina, USA. Marine Ecology 2: 245–270.
Harzing, A.W., 2007. Publish or Perish, available from https://harzing.com/resources/publish-or-perish.
Heitkamp, U., 1982. Untersuchungen zur Biologie, Ökologie und Systematik limnischer Turbellarien periodischer und perennierender Kleingewässer Südniedersachsens. Archiv für Hydrobiologie Supplement 64: 65–188.
Heitkamp, U., 1988. Life-cycles of microturbellarians of pools and their strategies of adaptation to their habitats. In Ax, P., U. Ehlers & B. Sopott-Ehlers (eds), Free-Living and Symbiotic Plathelminthes, Vol. 36. Fortschritte der Zoologie, Neue Folge: 449–456.
Hochberg, R. & M. K. Litvaitis, 2000. Phylogeny of Gastrotricha: a morphology-based framework of gastrotrich relationships. Biological Bulletin 198: 299–305.
Houben, A. M., N. Van Steenkiste & T. J. Artois, 2014. Revision of Phaenocora Ehrenberg, 1836 (Rhabditophora, Typhloplanidae, Phaenocorinae) with the description of two new species. Zootaxa 3889: 301–354.
Houben, A. M., P. Schwank, W. Proesmans, W. Bert & T. J. Artois, 2015. Notes on some enigmatic taxa of limnoterrestrial rhabdocoels, with the description of two new species. Zootaxa 4040: 83–92.
Hugerth, L. W., E. E. Muller, Y. O. Hu, L. A. Lebrun, H. Roume, D. Lundin, et al., 2014. Systematic design of 18S rRNA gene primers for determining eukaryotic diversity in microbial consortia. PLoS ONE 9: e95567.
Hummon, W. D., 1981. Extraction by sieving: a biased procedure in studies of stream meiobenthos. Transactions of the American Microscopical Society 100: 278–284.
Hummon, M. R., 1986. Reproduction and sexual development in a freshwater gastrotrich. 4. Life history traits and the possibility of sexual reproduction. Transactions of the American Microscopical Society 105: 97–109.
Hummon, W. D., 1987. Meiobenthos of the Mississippi headwaters. In Bertolani, R. (ed.), Biology of Tardigrades. Selected Symposia and Monographs U.Z.I. Mucchi, Modena: 125–140.
Hummon, M. R. & W. D. Hummon, 1992. Gastrotricha. In Adiyodi, K. G. & A. G. Adiyodi (eds), Reproductive Biology of Invertebrates, Vol. V., Sexual Differentiation and Behaviour Oxford & IBH, New Delhi: 137–146.
Hummon, W. D. & M. A. Todaro, 2010. Analytic taxonomy and notes on marine, brackish-water and estuarine Gastrotricha. Zootaxa 2392: 1–32.
Janssen, T., D. B. Vizoso, G. Schulte, D. T. J. Littlewood, A. Waeschenbach & L. Schärer, 2015. The first multi-gene phylogeny of the Macrostomorpha sheds light on the evolution of sexual and asexual reproduction in basal Platyhelminthes. Molecular Phylogenetics and Evolution 92: 82–107.
Jennings, J. B., 1971. Parasitism and commensalism in the Turbellaria. Advances in Parasitology 9: 1–32.
Jennings, J. B., 1974a. Symbioses in the Turbellaria and their implications in studies on the evolution of parasitism. In Vernberg, W. B. (ed.), Symbiosis in the Sea. University of South Carolina Press, Columbia, SC: 127–160.
Jennings, J. B., 1974b. Digestive physiology of the Turbellaria. In Riser, N. W. & M. P. Morse (eds), Biology of the Turbellaria. McGraw-Hill, New York: 173–197.
Jennings, J. B., 1977. Nutritional and respiratory pathways to parasitism exemplified in the Turbellaria. International Journal for Parasitology 27: 679–691.
Kånneby, T., 2016. Phylum Gastrotricha. In Thorp, J. & D. C. Roger (eds), Ecology and General Biology. Thorp and Covich’s Freshwater Invertebrates. Academic Press, Amsterdam: 115–130.
Kånneby, T. & J. J. Kirk, 2017. A new species of Redudasys (Gastrotricha: Macrodasyida: Redudasyidae) from the United States. Proceedings of the Biological Society of Washington 130: 128–139.
Kånneby, T., M. A. Todaro & U. Jondelius, 2012. A phylogenetic approach to species delimitation in freshwater Gastrotricha from Sweden. Hydrobiologia 683: 185–202.
Kånneby, T., M. A. Todaro & U. Jondelius, 2013. Phylogeny of Chaetonotidae and other Paucitubulatina (Gastrotricha: Chaetonotida) and the colonization of aquatic ecosystems. Zoologica Scripta 42: 88–105.
Kennedy, S. R., S. Prost, I. Overcast, A. J. Rominger, R. G. Gillespie & H. Krehenwinkel, 2020. High-throughput sequencing for community analysis: the promise of DNA barcoding to uncover diversity, relatedness, abundances and interactions in spider communities. Development Genes and Evolution. https://doi.org/10.1007/s00427-020-00652-x.
Kieneke, A. & A. Schmidt-Rhaesa, 2015. Gastrotricha. In Schmidt-Rhaesa, A. (ed.), Handbook of Zoology. De Gruyter, Berlin: 1–126.
Kieneke, A., O. Riemann & W. H. Ahlrichs, 2008. Novel implications for the basal internal relationships of Gastrotricha revealed by an analysis of morphological characters. Zoologica Scripta 37: 429–460.
Kieneke, A., P. M. Arbizu & D. Fontaneto, 2012. Spatially structured populations with a low level of cryptic diversity in European marine Gastrotricha. Molecular Ecology 21: 1239–1254.
Kier, W. M., 2010. The functional morphology of the tentacle musculature of Nautilus pompilius. In Saunders, W. B. & N. H. Landman (eds), Nautilus. Topics in Geobiology. Springer, Dordrecht.
Kisielewska, G., M. Kolicka & K. Zawierucha, 2015. Prey or parasite? The first observations of live Euglenida in the intestine of Gastrotricha. European Journal of Protistology 51: 138–141.
Kisielewski, J., 1974. Nowe dla fauny Polski gatunki Brzuchorzeskow (Gastrotricha). Slodkowodnych. Badania Fizjograficzne nad Polska Zachodnia 27: 103–111.
Kisielewski, J., 1981. Gastrotricha from raised and transitional peat bogs in Poland. Monografie Fauny Polska 11: 1–142.
Kisielewski, J., 1986. Freshwater Gastrotricha of Poland. VII. Gastrotricha of extremely eutrophicated water bodies. Fragmenta Faunistica 30: 267–295.
Kisielewski, J., 1987. Two new interesting genera of Gastrotricha (Macrodasyida and Chaetonotida) from the Brazilian freshwater psammon. Hydrobiologia 153: 23–30.
Kisielewski, J., 1990. Origin and phylogenetic significance of freshwater psammic Gastrotricha. Stygologia 5: 87–92.
Kisielewski, J., 1991. Inland-water Gastrotricha from Brazil. Annales Zoologici 43: 1–168.
Kisielewski, J., 1999. A preliminary study of the inland-water Gastrotricha of Israel. Israel Journal of Zoology 45: 135–157.
Kisielewski, J. & G. Kisielewska, 1986. Freshwater Gastrotricha of Poland. I. Gastrotricha from the Tatra and Karkonosze Mountains. Fragmenta Faunistica 30: 158–295.
Kolasa, J., 1979. Ecological and faunistical characteristics of Turbellaria in the eutrophic Lake Zbechy. Acta Hydrobiologica 21: 435–459.
Kolasa, J., 1983. Formation of the turbellarian fauna in an Italian submontane stream. Acta Zoologica Cracoviensia 26: 297–354.
Kolasa, J., 2000. The biology and ecology of lotic microturbellarians. Freshwater Biology 44: 5–14.
Kolasa, J., 2002. Microturbellaria. In Rundle, S., A. L. Robertson & J. M. Schmid-Araya (eds), Freshwater Meiofauna: Biology and Ecology. Backhuys Publishers, Leiden: 1–14.
Kolasa, J. & S. Tyler, 2009. Flatworms: Turbellarians and Nemertea. In Thorp, J. H. & A. P. Covich (eds), Ecology and Classification of North American Freshwater Invertebrates. Academic Press, Boston: 143–161.
Kolasa, J., D. Strayer & E. Bannon-O’Donnell, 1987. Microturbellarians from interstitial waters, streams, and springs in Southeastern New York. Journal of the North American Benthological Society 6: 125–132.
Kolicka, M., 2019. Gastrotricha – not only in sediments: new epiphytic species of Chaetonotida from the Jubilee Greenhouse of the Botanical Garden in Kraków. European Journal of Taxonomy 511: 1–100.
Kolicka, M., M. Dabert, J. Dabert, T. Kånneby & J. Kisielewski, 2016. Bifidochaetus, a new Arctic genus of freshwater Chaetonotida (Gastrotricha) from Spitsbergen revealed by an integrative taxonomic approach. Invertebrate Systematics 30(4): 398–419.
Kolicka, M., P. Gadawski & M. Dabert, 2017. A new species of freshwater Chaetonotidae (Gastrotricha, Chaetonotida) from Obodska Cave (Montenegro) based on morphological and molecular characters. European Journal of Taxonomy 354: 1–30.
Kolicka, M., L. Kotwicki & M. Dabert, 2018. Diversity of Gastrotricha on Spitsbergen (Svalbard Archipelago, Arctic) with a description of seven new species. Annales Zoologici 68(4): 609–739.
Korgina, E. M., 2014. A new species of the turbellarian worms, Phaenocora gagarini Korgina sp. n. (Turbellaria, Typhloplanidae), from the upper Volga river basin (Yaroslavl province, Russia). Zoologicheskii Zhurnal 93: 610–614.
Kraus, D. W. & J. M. Colacino, 1984. The oxygen consumption rates of three gastrotrichs. Comparative Biochemical Physiology 79: 691–693.
Krehenwinkel, H., A. Pomerantz, J. B. Henderson, S. R. Kennedy, J. Y. Lim, V. Swamy, et al., 2019. Nanopore sequencing of long ribosomal DNA amplicons enables portable and simple biodiversity assessments with high phylogenetic resolution across broad taxonomic scale. GigaScience 8(5): giz006.
Larsson, K. & U. Jondelius, 2008. Phylogeny of Catenulida and support for Platyhelminthes. Organisms Diversity and Evolution 8: 378–387.
Larsson, K. & W. Willems, 2010. Report on freshwater Catenulida (Platyhelminthes) from Sweden with the description of four new species. Zootaxa 2396: 1–18.
Larsson, K., A. Ahmadzadeh & U. Jondelius, 2008. DNA taxonomy of Swedish Catenulida (Platyhelminthes) and a phylogenetic framework for catenulid classification. Organisms, Diversity, and Evolution 8: 399–412.
Laumer, C. E., G. Giribet & M. Curini-Galletti, 2014. Prosogynopora riseri, gen. et sp. nov., a phylogenetically problematic lithophoran proseriate (Platyhelminthes: Rhabditophora) with inverted genital pores from the New England coast. Invertebrate Systematics 28: 309–325.
Laumer, C. E., N. Bekkouche, A. Kerbl, F. Goetz, R. C. Neves, M. V. Sørensen, R. M. Kristensen, A. Hejnol, C. W. Dunn, G. Giribet & K. Worsaae, 2015a. Spiralian phylogeny informs the evolution of microscopic lineages. Current Biology 25: 2000–2006.
Laumer, C. E., A. Hejnol & G. Giribet, 2015b. Nuclear genomic signals of the ‘microturbellarian’ roots of platyhelminth evolutionary innovation. eLife 4: e05503.
Leasi, F., J. L. Sevigny, E. M. Laflamme, T. Artois, M. Curini-Galletti, A. de Jesus Navarrete & K. M. Jörger, 2018. Biodiversity estimates and ecological interpretations of meiofaunal communities are biased by the taxonomic approach. Communications Biology 1: 1–12.
Leray, M. & N. Knowlton, 2015. DNA barcoding and metabarcoding of standardized samples reveal patterns of marine benthic diversity. Proceedings of the National Academy of Sciences 112: 2076–2081.
Lin, Y. T., W. T. Feng, F. Xin, L. Zhang, Y. Zhang & A. T. Wang, 2017. Two new species and the molecular phylogeny of eight species of Macrostomum (Platyhelminthes: Macrostomorpha) from southern China. Zootaxa 4337: 423–435.
Lindgren E. W., 1972. Systematics and Ecology of North Carolina Marine Sandy-beach Harpacticoida (Copepoda: Crustacea). PhD Dissertation, University of North Carolina at Chapel Hill.
Mack-Fira, V., 1974. The Turbellarian fauna of the Romanian littoral waters of the Black Sea and its annexes. In Riser, N. W. & M. P. Morse (eds), Biology of the Turbellaria. McGraw-Hill, New York: 248–290.
Maestri, S., E. Cosentino, M. Paterno, H. Freitag, J. M. Garces, L. Marcolungo, et al., 2019. A rapid and accurate MinION-based workflow for tracking species biodiversity in the field. Genes 10: 468.
Maghsoud, H., A. Weiss, J. P. S. Smith, M. K. Litvaitis & S. R. Fegley, 2014. Diagnostic PCR can be used to illuminate meiofaunal diets and trophic relationships. Invertebrate Biology 133: 121–127.
Majdi, N., J. M. Schmid-Araya & W. Traunspurger, 2019. Examining the diet of meiofauna: a critical review of methodologies. Hydrobiologia. https://doi.org/10.1007/s10750-019-04150-8.
Manylov, O. G., 1999. First finding of a microsporidian parasite in the gastrotrich, Turbanella lutheri (Gastrotricha: Macrodasyida). Protistology 1: 17–19.
Martens, P. M. & E. R. Schockaert, 1986. The importance of turbellarians in the marine meiobenthos: a review. Hydrobiologia 132: 295–303.
Martínez, A., E. M. Eckert, T. Artoi, G. Careddu, M. Casu, M. Curini-Galletti, V. Gazale, S. Gobert, V. I. Ivanenko, U. Jondelius, M. Marzano, G. Pesole, A. Zanello, M. A. Todaro & D. Fontaneto, 2020. Human access impacts biodiversity of microscopic animals in sandy beaches. Communications Biology 3: 175.
Martínez-Aquino, A., F. Brusa, C. Damborenea & D. Gibson, 2014. Checklist of freshwater sym-biotic temnocephalans (Platyhelminthes, Rhabditophora, Temnocephalida) from the Neo-tropics. Zoosystematics and Evolution 90: 147–162.
Menn, I. & W. Armonies, 1999. Predatory Promesostoma species (Plathelminthes, Rhabdocoela) in the Wadden Sea. Journal of Sea Research 41: 309–320.
Minowa, A. K. & A. R. Garraffoni, 2017. A new species of Haltidytes Remane, 1936 (Gastrotricha: Chaetonotida: Dasydytidae) from an urban lagoon in Brazil with a phylogenetic reconstruction of the genus based on morphological data. Zoologischer Anzeiger 269: 100–109.
Mitsi, K., A. S. Arroyo & I. Ruiz-Trillo, 2019. A global metabarcoding analysis expands molecular diversity of Platyhelminthes and reveals novel early-branching clades. Biology Letters 15: 20190182.
Munn, E. A. & P. D. Munn, 2010. Feeding and digestion. In Lee, D. L. (ed.), The Biology of Nematodes. CRC Press, London: 211–232.
Nesteruk, T., 1986. Freshwater Gastrotricha of Poland. IV. Gastrotricha from fish ponds in the vicinity of Siedlce. Fragmenta Faunistica 30: 215–233.
Nesteruk, T., 1987. Assessing the efficiency of three methods of extracting fresh water Gastrotricha from bottom silt. Acta Hydrobiologica 29: 219–226.
Nesteruk, T., 1991. Vertical distribution of Gastrotricha in organic bottom sediment of inland water bodies. Acta Biologica 33: 253–264.
Nesteruk, T., 1993. A comparison of values of freshwater gastrotricha densities determined by various methods. Acta Hydrobiologica Krakow 4: 321–328.
Nesteruk, T., 1996a. Density and biomass of gastrotricha in sediments of different types of standing water. Hydrobiologia 24: 205–208.
Nesteruk, T., 1996b. Species composition and dominance structure of gastrotrich (Gastrotricha) assemblages in water bodies of different trophic status. Hydrobiologia 339: 141–148.
Nesteruk, T., 1998. Changes in density and species composition of Gastrotricha in stored samples. Acta Hydrobiologica 40: 39–42.
Nesteruk, T., 2000. Epiphytic Gastrotricha – species composition and dominance. Acta Hydrobiologica 42: 53–57.
Nesteruk, T., 2004a. Benthic and epiphytic fauna of Gastrotricha in littoral of mesotrophic lake in Leczna-Wlodawa Lakeland, Poland. Fragmenta Faunistica 47: 1–6.
Nesteruk, T., 2004b. Lake zonality influence on species diversity formation of Gastrotricha. Acta Agrophysica 4: 441–447.
Nesteruk, T., 2005. Ecotone zone as a form of protection and enrichment of biological diversity. Teka Komisji Ochrony i Kształtowania Środowiska Przyrodniczego 2: 108–114.
Nesteruk, T., 2006. Gastrotricha against the background of chosen groups of zoobenthos in farm ponds. Teka Komisji Ochrony i Kształtowania Środowiska Przyrodniczego 3: 141–146.
Nesteruk, T., 2007a. A study on ecology of freshwater Gastrotricha. Rozprawy Naukowe Akademii Wychowania Fizycznego we Wrocławiu. 92. Widawnictwo Akademii Podlaskiej Siedlce: 1–119.
Nesteruk, T., 2007b. Diversity and abundance of Gastrotricha in the psammon of mesotrophic lake. Polish Journal of Ecology 55: 833–839.
Nesteruk, T., 2007c. Recolonization of two dried peat-hags by gastrotrich fauna. Teka Komisji Ochrony i Kształtowania Środowiska Przyrodniczego 4: 192–197.
Nesteruk, T., 2008. Assessment of the diversity and density of gastrotrich fauna (Gastrotricha) in bottom sediments of running and standing waters. Teka Komisji Ochrony i Kształtowania Środowiska Przyrodniczego 5: 136–143.
Nesteruk, T., 2009. Gastrotrich fauna of Elodeids and bottom sediments in a eutrophic lake. Teka Komisji Ochrony i Kształtowania Środowiska Przyrodniczego 6: 206–215.
Nesteruk, T., 2011. Comparison of gastrotrich fauna on elodeids and in bottom sediments of lakes of different trophic status (the region Polesie Lubelskie, Eastern Poland). Oceanological and Hydrobiological Studies 40: 13–21.
Nesteruk, T., 2016a. Quantitative assessment of epiphytic and benthic meioinvertebrate fauna in various types of standing water. Polish Journal of Environmental Studies 25: 1661–1668.
Nesteruk, T., 2016b. Species composition and density of Gastrotricha occurring on two species of macrophytes in a mesotrophic lake. Teka Komisji Ochrony I Kształtowania Środowiska Przyrodniczego 13: 33–40.
Nesteruk, T., 2017. Seasonal changes in the diversity and abundance of epiphytic Gastrotricha. Proceedings of the Biological Society of Washington 130: 212–222.
Noreña, C., C. Damborenea & F. Brusa, 2015. Phylum Platyhelminthes. In Thorp, J. & D. C. Rogers (eds), Ecology and General Biology. Thorp and Covich’s Freshwater Invertebrates. Academic Press, Cambridge: 181–203.
Noreña, C., A. Porifiriev & O. Timoshkin, 2019. Phylum Platyhelminthes. In Thorp, J. & D. C. Rogers (eds), Keys to the Palearctic Fauna, Volume IV. Thorp and Covich’s Freshwater Invertebrates. Academic Press, Cambridge: 113–144.
Nuttycombe, J. W. & A. J. Waters, 1935. Feeding habits and pharyngeal structure in Stenostomum. The Biological Bulletin 69: 439–446.
Packard, C. E., 1936. Observations on the Gastrotricha indigenous to New Hampshire. Transactions of the American Microscopical Society 55: 422–427.
Palmer, M. A., 1990. Temporal and spatial dynamics of meiofauna within the hyporheic zone of Goose Creek, Virginia. Journal of North American Benthological Society 9: 17–25.
Palmer, M. A., A. P. Covich, B. J. Finlay, J. Gibert, K. D. Hyde, R. K. Johnson, T. Kairesalo, S. Lake, C. R. Lovell, R. J. Naiman, C. Ricci, F. Sabater & D. Strayer, 1997. Biodiversity and ecosystem processes in freshwater sediments. Ambio 26: 571–577.
Paps, J. & M. Riutort, 2012. Molecular phylogeny of the phylum Gastrotricha: new data bring together molecules and morphology. Molecular Phylogenetics and Evolution 63: 208–212.
Reise, K., 1979. Moderate predation on meiofauna by the macrobenthos of the Wadden Sea. Helgoländer Wissenschaftliche Meeresuntersuchungen 32: 453–465.
Reise, K., 1988. Plathelminth diversity in littoral sediments around the island of Sylt in the North Sea. In Ax, P. & B. Sopott-Ehlers (eds), Free-Living and Symbiotic Plathelminthes. Fischer, Stuttgart: 469–480.
Remane, A., 1925. Organisation und systematische Stellung der aberranten Gastrotrichen. Verhandlungen der Deutschen Zoologischen Gesellshaft 30: 121–128.
Remane, A., 1936. Gastrotricha. In Bronns, H. G. (ed.), Klassen und Ordnungen des Tierreichs. Akademie Verlags, Leipzig: 1–142.
Renaud-Mornant, J., 1986. Gastrotricha. In Botosaneanu, L. (ed.), Stygofauna Mundi. A Faunistic, Distributional, and Ecological Synthesis of the World Fauna Inhabiting Subterranean Waters (Including the Marine Interstitial). Brill E.J., Leiden: 84–109.
Ricci, C. & M. Balsamo, 2000. The biology and ecology of lotic rotifers and gastrotrichs. Freshwater Biology 44: 15–28.
Rieger, R. M., S. Tyler, J. P. S. Smith III & G. E. Rieger, 1991. Platyhelminthes: Turbellaria. In Harrison, F. (ed.), Microscopic Anatomy of Invertebrates, Vol. 3., Platyhelminthes and Nemertinea Wiley-Liss, New York: 7–140.
Robertson, A. L., S. D. Rundle & J. M. Schmid-Araya, 2000. An introduction to a special issue on lotic meiofauna. Freshwater Biology 44(1): 1–3.
Rocha, O., T. Matsumura-Tundisi, J. Galizia Tundisi & C. Padovesi Fonseca, 1990. Predation on and by pelagic Turbellaria in some lakes in Brazil. Hydrobiologia 198: 91–101.
Rogozin, A. G., 2011. On the use of correspondence analysis in systematics of the family Stenostomidae (Catenulida, Turbellaria). Zoologichesky Zhurnal 90: 746–755.
Rogozin, A. G., 2012. New and rare species of Turbellaria Archoophora (Catenulida and Macrostomida) for the Russian fauna. Zoologicheskiy Zhurnal 91: 643–647.
Rogozin, A. G., 2017. New and rare Typhloplanoid species (Turbellaria, Neorhabdocoela) for fauna of the Urals and Russia. Biology Bulletin 44: 643–647.
Rubbmark, O. R., D. Sint, S. Cupic & M. Traugott, 2019. When to use NGS or diagnostic PCR in diet analysis. Molecular Ecology Resources 19: 388–399.
Ruppert, E. E., 1982. Comparative ultrastructure of the gastrotrich pharynx and the evolution of myoepithelial foreguts in Aschelminthes. Zoomorphology 99: 181–220.
Ruppert, E. E., 1991. Gastrotricha. In Harrison, F. W. & E. E. Ruppert (eds), Microscopic Anatomy of Invertebrates. Aschelminthes. Wiley-Liss, New York: 44–109.
Ruppert, K. M., R. J. Kline & M. S. Rahman, 2019. Past, present, and future perspectives of environmental DNA (eDNA) metabarcoding: A systematic review in methods, monitoring, and applications of global eDNA. Global Ecology and Conservation 17: e00547.
Ruttner-Kolisko, A., 1955. Rheomorpha neiswestnovae und Marinellina flagellata, zwei Phylogenetisch interessante Würmtypen aus dem Süsswasserpsammon. Österreichische Zoologische Zeitschrift 6: 55–69.
Rzeznik-Orignac, J., D. Kalenitchenko, J. Mariette, J. Y. Bodiou, N. Le Bris & E. Derelle, 2017. Comparison of meiofaunal diversity by combined morphological and molecular approaches in a shallow Mediterranean sediment. Marine Biology 164: 40.
Sandlung, O. T., 1982. The drift of zooplankton and microzoobenthos in the river Strandaelva, Western Norway. Hydrobiologia 94: 33–48.
Sayre, R. M. & W. P. Wergin, 1994. Adenoplea nanus n. sp. (Turbellaria: Neorhabdocoela) introduced in Maryland, U.S.A. and predatory on soil nematodes. Transactions of the American Microscopical Society 113: 263–275.
Scarpa, F., P. Cossu, T. Lai, D. Sanna, M. Curini-Galletti & M. Casu, 2016. Meiofaunal cryptic species challenge species delimitation: the case of the Monocelis lineata (Platyhelminthes: Proseriata) species complex. Contributions to Zoology 85: 123–145.
Schärer, L., T. J. Littlewood, A. Waeschenbach, W. Yoshida & D. B. Vizoso, 2011. Mating behavior and the evolution of sperm design. Proceedings of the National Academy of Sciences of the United States of America 108: 1490–1495.
Schenk, J. & D. Fontaneto, 2019. Biodiversity analyses in freshwater meiofauna through DNA sequence data. Hydrobiologia. https://doi.org/10.1007/s10750-019-04067-2.
Schmid-Araya, J. M., 1997. Temporal and spatial dynamics of meiofaunal assemblages in the hyporheic interstitial of a gravel stream. In Gibert, J., J. Mathieu & F. Fournier (eds), Groundwater Surface Water Ecotones: Biological and Hydrological Interactions. Cambridge University Press, Cambridge: 29–36.
Schmid-Araya, J. M. & P. E. Schmid, 1995. The invertebrate species of a gravel stream. Jahresbericht Biologische. Station Lunz 15: 11–21.
Schmidt-Rhaesa, A., 2020. Guide to the Identification of Marine Meiofauna. Verlag Dr. Freiderich Pfeil, München.
Schockaert, E. R., 1996. Turbellarians. In Hall, G. (ed.), Methods for the Examination of Organismal Diversity in Soils and Sediments. CAB International, Wallingford: 211–225.
Schockaert, E. R., M. Hooge, R. Sluys, S. Schilling, S. Tyler & T. Artois, 2008. Global diversity of free living flatworms (Platyhelminthes, “Turbellaria”) in freshwater. Hydrobiologia 595: 41–48.
Schwank, P., 1981a. Turbellaria, Oligochaeta and Archiannelida from Breitenbach and other highland streams in Eastern Hesse – I. Local geographical distribution and the occurrence of the species in the various streams in relation to substrate. Archiv für Hydrobiologie Supplement 62: 1–85.
Schwank, P., 1981b. Turbellaria, Oligochaeta and Archiannelida from Breitenbach and other highland streams in Eastern Hesse – II. The systematics and autecology of species. Archiv für Hydrobiologie Supplement 62: 86–147.
Schwank, P., 1982a. Turbellaria, Oligochaeta and Archiannelida from Breitenbach and other highland streams in Eastern Hesse – III. The Taxocoenoses of Turbellaria and Oligochaeta in running waters – a synecological classification. Archiv für Hydrobiologie Supplement 62: 191–253.
Schwank, P., 1982b. Turbellaria, Oligochaeta and Archiannelida from Breitenbach and other highland streams in Eastern Hesse – IV. Fundamental principles of the distribution of Turbellaria and Oligochaeta in running waters. Archiv für Hydrobiologie Supplement 62: 254–290.
Smith III, J. P. S., M. K. Litvaitis, S. Gobert, T. Uyeno & T. Artois, 2015. Evolution and functional morphology of the proboscis in Kalyptorhynchia (Platyhelminthes). Integrative and Comparative Biology 55: 205–216.
Smith III, J. P. S., N. Van Steenkiste & T. Artois, 2020. Platyhelminthes. In Schmidt-Rhaesa, A. (ed.), Guide to the Identification of Marine Meiofauna. Verlag Dr. Freiderich Pfeil, München: 54–103.
Straarup, B. J., 1970. On the ecology of turbellarians in a sheltered brackish shallow-water bay. Ophelia 7: 185–216.
Strayer, D. L., 1985. The benthic micrometazoans of Mirror Lake, New Hampshire. Archiv für Hydrobiologie Supplement 72: 287–426.
Strayer, D. L. & W. D. Hummon, 1991. Gastrotricha. In Thorp, J. H. & A. P. Covich (eds), Ecology and Classification of North American Freshwater Invertebrates. Academic Press, New York: 181–194.
Strayer, D. L., W. D. Hummon & R. Hochberg, 2010. Gastrotricha. In Thorp, J. H. & A. P. Covich (eds), Ecology and Classification of North American Freshwater Invertebrates. Academic Press, New York: 163–172.
Struck, T. H., A. R. Wey-Fabrizius, A. Golombek, L. Hering, A. Weigert, C. Bleidorn & P. Kück, 2014. Platyzoan paraphyly based on phylogenomic data supports a noncoelomate ancestry of Spiralia. Molecular Biology and Evolution 31: 1833–1849.
Sun, T., L. Zhang, A. T. Wang & Y. Zhang, 2015. Three new species of freshwater Macrostomum (Platyhelminthes, Macrostomida) from southern China. Zootaxa 4012: 120–134.
Tessens, B., T. Janssen & T. Artois, 2014. Molecular phylogeny of Kalyptorhynchia (Rhabdocoela, Platyhelminthes) inferred from ribosomal sequence data. Zoologica Scripta 43: 519–530.
Timoshkin, O. A., A. G. Lukhnev & E. P. Zaytseva, 2010. First data on the endemic fauna of turbellaria proseriata (Platyhelminthes, Otomesostomidae) from Lake Baikal. Biology Bulletin 37: 861–875.
Timoshkin, O. A., O. V. Popova, A. G. Luknev & E. P. Zaytseva, 2014. Fauna and distribution of microturbellarians in the splash zone of Lake Baikal, with a description of two new species of the genus Opisthocystis (Platyhelminthes, Turbellaria, Kalyptorhynchia). Zoologicheskii Zhurnal 93: 412–425.
Todaro, M. A., M. Dal Zotto, U. Jondelius, R. Hochberg, W. D. Hummon, T. Kånneby & C. E. Rocha, 2012. Gastrotricha: a marine sister for a freshwater puzzle. PLoS ONE 7: e31740.
Todaro, M. A., M. Dal Zotto & F. Leasi, 2015. An integrated morphological and molecular approach to the description and systematisation of a novel genus and species of Macrodasyida (Gastrotricha). PLoS ONE 10: e0130278.
Todaro, M. A., J. A. Sibaja-Cordero, O. A. Segura-Bermúdez, G. Coto-Delgado, N. Goebel-Otárola, J. D. Barquero, et al., 2019. An introduction to the study of Gastrotricha, with a taxonomic key to families and genera of the group. Diversity. https://doi.org/10.3390/d1107011.
Tonolli, V. & L. Tonolli, 1951. Osservazioni sulla biologia ed ecologia di 170 popolamenti zooplanctonici di Laghi Italiani di Alta Quota. Memorie dell’Istituto Italiano di Idrobiologia 6: 53–136.
Tranchida, M. C., A. Maciá, F. Brusa, M. V. Micieli & J. J. García, 2009. Predation potential of three flatworm species (Platyhelminthes: Turbellaria) on mosquitoes (Diptera: Culicidae). Biological Control 49: 270–276.
Traunspurger, W. & N. N. Majdi, 2017. Meiofauna. In Hauer, F. R. & G. A. Lamberti (eds), Methods in Stream Ecology, Vol. 1., Ecosystem Structure Elsevier, Academic Press, Amsterdam: 273–295.
Tyler, S. & G. E. Rieger, 1980. Adhesive organs of the Gastrotricha. Zoomorphologie 95: 1–15.
Tyler, S., S. Schilling, M. Hooge & L.F. Bush (comp.), 2006–2016. Turbellarian taxonomic database. Version http://turbellaria.umaine.edu
Uyeno, T. A. & W. M. Kier, 2010. Morphology of the muscle articulation joint between the hooks of a flatworm (Kalyptorhynchia, Cheliplana sp.). The Biological Bulletin 218: 169–180.
Van Steenkiste, N., P. Davison & T. Artois, 2010. Bryoplana xerophila n. g. n. sp., a new limnoterrestrial microturbellarian (Platyhelminthes, Typhloplanidae, Protoplanellinae) from epilithic mosses, with notes on its ecology. Zoological Science 27: 285–291.
Van Steenkiste, N., S. Gobert, P. Davison, J. Kolasa & T. Artois, 2011a. Freshwater Dalyelliidae from the Nearctic (Platyhelminthes, Rhabdocoela): new taxa and records from Ontario, Canada and Michigan and Alabama, USA. Zootaxa 3091: 1–32.
Van Steenkiste, N., B. Tessens, K. Krznaric & T. Artois, 2011b. Dalytyphloplanida (Platyhelminthes: Rhabdocoela) from Andalusia, Spain, with the description of four new species. Zootaxa 2791: 1–29.
Van Steenkiste, N., B. Tessens, W. Willems, E. Van Mulken & T. Artois, 2012. The “Falcatae”, a new Gondwanan species group of Gieysztoria (Platyhelminthes: Dalyelliidae), with the description of five new species. Zoologischer Anzeiger 251: 344–356.
Van Steenkiste, N., B. Tessens, W. Willems, T. Backeljau, U. Jondelius & T. Artois, 2013. A comprehensive molecular phylogeny of Dalytyphloplanida (Platyhelminthes: Rhabdocoela) reveals multiple escapes from the marine environment and origins of symbiotic relationships. PLoS ONE 8: e59917.
Van Steenkiste, N. W. L., E. R. Herbert & B. S. Leander, 2018. Species diversity in the marine microturbellarian Astrotorhynchus bifidus sensu lato (Platyhelminthes: Rhabdocoela) from the Northeast Pacific Ocean. Molecular Phylogenetics and Evolution 120: 259–273.
Van Steenkiste, N. W. L., I. Stephenson, M. Herranz, F. Husnik, P. J. Keeling & B. S. Leander, 2019. A new case of kleptoplasty in animals: marine flatworms steal functional plastids from diatoms. Science Advances 5(7): eaaw4337.
Vandel, A., 1964. Biospeleology. The Biology of Cavernicolous Animals. Pergamon Press, Oxford: 1–326.
Vanhove, M. P. M., B. Tessens, C. Schoelinck, U. Jondelius, D. T. J. Littlewood, T. Artois & T. Huyse, 2013. Problematic barcoding in flatworms: a case-study on monogeneans and rhabdocoels (Platyhelminthes). ZooKeys 365: 355–379.
Vanschoenwinkel, B., A. Waterkeyn, T. Vandecaetsbeek, O. Pineau, P. Grillas & L. Brendonck, 2008. Dispersal of freshwater invertebrates by large terrestrial mammals: a case study with wild boar (Sus scrofa) in Mediterranean wetlands. Freshwater Biology 53: 2264–2273.
Vanschoenwinkel, B., S. Gielen, M. Seaman & L. Brendonck, 2009. Wind mediated dispersal of freshwater invertebrates in a rock pool metacommunity: differences in dispersal capacities and modes. Hydrobiologia 635: 363–372.
Viana, D. S., L. Santamaría & J. Figuerola, 2016. Migratory birds as global dispersal vectors. Trends in Ecology & Evolution 31: 763–775.
von Graff, L., 1903. Die Turbellarien als Parasiten und Wirte. Leuschner & Lubensy’s Universitats-Buchhandlung, Graz.
Watzin, M. C., 1983. The effects of meiofauna on settling macrofauna: meiofauna may structure macrofaunal communities. Oecologia 59: 163–166.
Watzin, M. C., 1985. Interactions among temorary and permanent meiofauna: observations on the feeding and behavior of selected taxa. The Biological Bulletin 169: 397–416.
Watzin, M. C., 1986. Larval settlement into marine soft-sediment systems: interactions with the meiofauna. Journal of Experimental Marine Biology and Ecology 98: 65–113.
Willems, W. R., A. Wallberg, U. Jondelius, D. T. J. Littlewood, T. Backeljau, E. R. Schockaert & T. J. Artois, 2006. Filling a gap in the phylogeny of flatworms: relationships within the Rhabdocoela (Platyhelminthes), inferred from 18S ribosomal DNA sequences. Zoologica Scripta 35: 1–17.
WoRMS (2020a). Gastrotricha.. Accessed at: http://marinespecies.org/aphia.php?p=taxdetails&id=2078 on 2020-03.28
WoRMS (2020b). Platyhelminthes. Accessed at: http://www.marinespecies.org/aphia.php?p=taxdetails&id=793 on 2020-03-21.
Wunderer, J., B. Lengerer, R. Pjeta, P. Bertemes, L. Kremser, H. Lindner, T. Ederth, M. W. Hess, D. Stock, W. Salvenmoser & P. Ladurner, 2019. A mechanism for temporary bioadhesion. Proceedings of the National Academy of Sciences of the United States of America 116: 4297–4306.
Young, J. O., 1973. The occurrence of microturbellaria in some British lakes of diverse chemical content. Archiv für Hydrobiologie 72: 202–224.
Young, J. O., 1977. An ecological study of Phaenocora unipunctata (Oersted) (Turbellaria Rhabdocoela): population dynamics. Acta Zoologica Fennica 154: 105–118.
Young, J. O., 2001. Keys to the freshwater microturbellarians of Britain and Ireland, with notes on their ecology. Freshwater Biological Association, Ambleside, Cumbria: 1–142.
Zébazé Togouet, S. H., T. Njine, N. Kemka, M. Nola, S. Foto Menbohan, W. Koste, C. Boutin & R. Hochberg, 2007. Spatio-temporal changes in the abundance of the populations of the gastrotrich community in a shallow lake of tropical Africa. Limnologica 37: 311–322.
Acknowledgements
Julian P.S. Smith III was supported by grant P20GM103499 (SC INBRE) from the National Institute of General Medical Sciences, National Institutes of Health; Niels Van Steenkiste and Brian Leander were supported by grants from the National Science and Engineering Research Council of Canada (2019-03986) and the Hakai Institute; Maria Balsamo and Loretta Guidi were supported by Scientific Research grants from the Italian Ministry of University (MIUR, 2019). The authors are grateful to Dr. Rick Hochberg for the free and open sharing of his ideas concerning feeding guilds in gastrotrichs, and to Dr. Seth Tyler for pointing us to historical literature on microturbellaria as parasites and hosts.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guest editors: Nabil Majdi, Jenny M. Schmid-Araya & Walter Traunspurger / Patterns and Processes of Meiofauna in Freshwater Ecosystems.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10750_2020_4287_MOESM1_ESM.xls
Online resource 1. Results of a recent metabarcoding trial of the amplicon V4/V5 on gastrotrichs (Fegley et al., submitted). (XLS 31 kb)
10750_2020_4287_MOESM2_ESM.xlsx
Online resource 2. Results of a recent metabarcoding trial of the amplicon V4/V5 on microturbellarians (Fegley et al., submitted). (XLSX 15 kb)
Rights and permissions
About this article
Cite this article
Balsamo, M., Artois, T., Smith, J.P.S. et al. The curious and neglected soft-bodied meiofauna: Rouphozoa (Gastrotricha and Platyhelminthes). Hydrobiologia 847, 2613–2644 (2020). https://doi.org/10.1007/s10750-020-04287-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-020-04287-x