Abstract
Planktonic forms of Gastrotricha have been known since the 1850s, despite the fact that they are rather uncommon and difficult to collect. They are characterized by a round sack-shaped body, an absence of furcal adhesive tubes, and a different distribution of the locomotory ciliation compared to epibenthic and periphytic gastrotrichs. Today, planktonic gastrotrichs are classified into the three taxa—Dasydytidae, Neogosseidae, and Undula—but their origin and whether they share a recent common ancestor remain largely unknown. A long-held view is that planktonic taxa are derived from benthic ancestors related to Chaetonotus (Zonochaeta), but the hypothesis has never been properly tested. Here, in order to elucidate the phylogeny and origin of planktonic Gastrotricha, we provide the first molecular data on the very rare genera Kijanebalola and Neogossea, both members of the family Neogosseidae. We use Bayesian and maximum likelihood phylogenetics to analyze sequences of 18S rDNA, 28S rDNA, and COI mtDNA spanning 71 taxa in total. We find high support for a common origin of planktonic gastrotrichs, with monophyly of both Dasydytidae and Neogosseidae. Planktonic forms have evolved from epibenthic or periphytic ancestors, and the closest extant clade comprises members of Chaetonotus (Zonochaeta) + Chaetonotus heteracanthus Remane, 1927. These results further imply that the motile spines and underlying muscle patterns that control them in species of Dasydytidae are adaptations to the planktonic environment that evolved independently of those in other species of Gastrotricha.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastrotricha is a small phylum of aquatic acoelomate animals with approximately 850 species (see Balsamo et al. 2009, 2013, 2014; Hummon and Todaro 2010; Kieneke and Schmidt-Rhaesa 2014; Todaro et al. 2014; and references therein). The group is a common component of the meiofauna and is hypothesized to act as an important link between the microbial loop and larger invertebrate predators (Balsamo and Todaro 2002). The phylum is divided into the two orders Chaetonotida and Macrodasyida. Chaetonotida, present in both freshwater and marine habitats, are generally tenpin-shaped with adhesive tubes confined to the posterior end and with the cuticle generally sculptured into various arrangements of scales and spines. Macrodasyida, with few exceptions, are entirely marine and vermiform, with adhesive tubes not confined to the posterior end, and with a smooth or sculptured cuticule.
Freshwater gastrotrichs within Chaetonotida are very small, ranging from 60 to 770 μm in total body length. Most species are not only epibenthic, periphytic, or interstitial but some also have a planktonic lifestyle. The first records of planktonic gastrotrichs were those of Dasydytes goniathrix Gosse, 1851 and Dasydytes antenniger Gosse, 1851 (now Neogossea antennigera). In the years leading up to the twentieth century, several findings of new planktonic gastrotrichs were published, e.g., Chitonodytes longisetosus (Metschnikoff, 1865), Haltidytes saltitans (Stokes, 1887), Stylochaeta fusiformis (Spencer, 1890), and Setopus bisetosus (Thompson, 1891). Zelinka (1889) separated N. antennigera (Gosse, 1851) from the rest of the planktonic gastrotrichs based on the cephalic tentacles. Von Daday (1905) erected the families Dasydytidae and Gosseidae (now Neogosseidae) and gave diagnostic characters for each group. The very rare Kijanebalola was described from an Ugandan lake by Beauchamp (1932) and regarded as a close relative to Neogossea. A leap in the knowledge of planktonic forms was made during the 1980s by Kisielewski (1991), who found several new and very interesting planktonic gastrotrichs from an evolutionary point of view, among them Undula, Ornamentula, and Kijanebalola. Common features of the planktonic species are the absence of the furcal adhesive tubes and a rearrangement of the locomotory ciliation. In planktonic taxa, the ciliation includes discrete tufts along the trunk region and at least a band of long strong propelling cilia, which more or less completely encircles the head. Today, planktonic gastrotrichs are classified into three taxa: (i) the monotypic Undula, forming the subfamily Undulinae, which is provisionally considered a sister group of the subfamily Chaetonotinae (Kisielewski 1991); (ii) Dasydytidae, which is characterized by long and movable spines (Kisielewski 1991; Kieneke and Ostmann 2012); and (iii) Neogosseidae, characterized by long posterior spines, either distributed in an unpaired median group (Kijanebalola) or in a paired lateral group (Neogossea) and a pair of club-shaped cephalic tentacles (Todaro et al. 2013).
The origin and evolution of planktonic gastrotrichs remain largely unknown (Hochberg and Litvaitis 2000; Kieneke et al. 2008a). However, the long-held hypothesis that at least some planktonic forms may have been derived from benthic ancestors (e.g., related to Chaetonotus (Zonochaeta) Remane, 1927; see Kisielewski 1991) has gained support in a recent phylogenetic study based on molecular data (Kånneby et al. 2013). In the latter study, the authors showed that the planktonic Dasydytidae is nested within a non-monophyletic Chaetonotidae. However, the non-monophyly of Chaetonotidae had been known for quite some time prior to that study, based on morphological data (e.g., Hochberg and Litvaitis 2000; Kieneke et al. 2008a). It should be emphasized that the systematics of the entire order Chaetonotida, and especially within the largest group Chaetonotidae, is unstable. This is mainly due to the classification’s heavy reliance on cuticular structures and ornamentation, characters that are extremely variable and thereby inconsistent on higher levels of classification (e.g., genus, family etc.) (see Kånneby et al. 2013). A phylogenetic approach based on molecular data may prove to be helpful in the process of re-systematization of chaetonotidan taxa, similar to what is happening in the systematization of the Macrodasyida (Todaro et al. 2012, 2014) However, several groups of chaetonotidans have not yet been sampled for molecular data; this includes Dichaeturidae, Neogosseidae, and Proichthydiidae, mainly because of lack of material. It should be emphasized that members of these groups are very uncommon, with some species only reported from their respective, often remote, type localities; as a consequence, information on these animals is generally very poor, and their origin and phylogenetic alliances remain obscure.
In this study, in order to shed some light on the hypothesized relationship between Dasydytidae and Neogosseidae suggested by previous authors, we have obtained specimens and sequences of 18S rDNA, 28S rDNA, and COI mtDNA for the uncommon planktonic genera Kijanebalola and Neogossea, comprising Neogosseidae (Table 1). We also hope, if possible, to shed light on the origin of planktonic Gastrotricha. Although efforts were made to find Undula paraënsis Kisielewski, 1991, at its type localities in Brazil, these attempts were fruitless.
Materials and methods
Collection and documentation
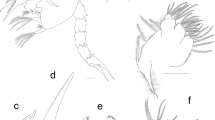
Kijanebalola devestiva Todaro et al. 2013 (Fig. 1a) and Neogossea acanthocolla Kisielewski, 1991 (Fig. 1b) were collected in February 2013 from a freshwater pond in the iSimangaliso Wetland Park (Kwazulu-Natal, South Africa); for details on specimen sampling, recording, and storage prior to DNA processing, refer to Todaro et al. (2013). Individuals of Neogossea antennigera (Fig. 1c, d) were collected with a plankton net with a mesh size of 25 μm at Bryan Country Club Lake, Bryan, Texas, USA (30° 38′ 32.18″ N; 96° 21′ 44.05″ W) on two occasions, May 23, 2012 and June 26, 2012. Collected samples contained Ceratophyllum spp. and Lemna spp. It is also noteworthy that the water of the lake as well as ambient sediment contain arsenic levels that exceed the human health criterion and are not optimal for aquatic life (Clark et al. 1998). Subsamples were treated with a 1 % MgCl2 solution and subsequently studied under an Olympus LMS225R dissecting microscope. Gastrotrichs were picked out with a micropipette, mounted on a slide and documented alive with an Olympus BH-2 microscope equipped with DIC optics and a Canon EOS Rebel T1i digital camera or a Nikon Eclipse 80i DIC microscope equipped with a Nikon Digital Sight DS-Fi1 digital camera. For molecular studies, specimens were recovered from the slide and put in 95–100 % EtOH and stored at −18 to −20 °C until further treatment. The specimens of Neogosseidae used in the phylogeny have been photographed, and the photos have been deposited as collection 855412 in Morphbank (http://www.morphbank.net/855412) (Table 1). Also refer to collection 799280 in Morphbank (http://www.morphbank.net/799280) for photographs of the other specimens used in our analysis (Table 1).
Species of Neogosseidae included in this study. a Kijanebalola devestiva, habitus of adult specimen. Note the rounded posterior end with single median group of spines. b Neogossea acanthocolla, habitus of adult specimen. Note the truncate posterior end with a pair of tufts. c Neogossea antennigera, anterior two thirds of body of adult specimen. d N. antennigera, posterior end of body of adult specimen. Note the truncate posterior end with a pair of tufts. Scale bars: a and b, 50 μm; c and d, 25 μm
Phylogenetic analyses
DNA extraction, amplification, and sequencing of 18S rDNA, 28S rDNA, and COI mtDNA follow the protocol presented in Kånneby et al. (2012). The new sequences were analyzed together with most of the sequences obtained by Kånneby et al. (2013). However, to keep the dataset more manageable, only a single sequence per morphological species per gene was included, with the exception of Neogosseidae where, in some instances, two sequences from the same morphological species were used (Table 1).
The nuclear genes were aligned with MUSCLE (Edgar 2004) implemented in Geneious v. 7.1.8 (Kearse et al. 2012) using the default settings. COI was aligned in the same way as the nuclear genes using the translation align option, ensuring that gaps in the alignment correspond to the translated amino acid sequences. The concatenated dataset (18S rDNA, 28S rDNA, and COI mtDNA) was analyzed with MrBayes v.3.2.3 (Ronquist et al. 2012) on the CIPRES Science Gateway v. 3.3 (Miller et al. 2010) under the mixed model setting with gamma distributed rate variation across sites and an estimated proportion of invariable sites. The gamma shape parameter, the substitution rates for the GTR-model, the proportion of invariable sites, and the character state frequencies were all unlinked. The dataset was partitioned according to each nuclear gene and by codon position for COI. To ensure sufficient mixing and a reliable sample from the posterior distribution, the dataset was analyzed with 8 MCMC chains for each run and a heating parameter of 0.1. The analysis started from a random starting tree and was run for 40 million generations. Convergence of the two runs was ascertained by checking the log likelihood graphs, the average standard deviation of split frequencies, and the potential scale reduction factor (PSRF+) (Gelman and Rubin 1992). After a burn-in of five million generations, chains were sampled every 1000th generation. Musellifer delamarei (Renaud-Mornant, 1968) within Muselliferidae, a group hypothesized to be the sister group of all other Paucitubulatina (e.g., Hochberg and Litvaitis 2000; Todaro et al. 2006; Leasi and Todaro 2008; Kånneby et al. 2014), was used as outgroup. The alignments of the concatenated dataset and the resulting consensus trees from the Bayesian and the maximum likelihood analyses have been deposited in TreeBASE at http://purl.org/phylo/treebase/phylows/study/TB2:S17533.
Maximum likelihood (ML) analyses were performed with RaxML GUI v. 1.3 (Stamatakis 2006; Silvestro and Michalak 2012) on the concatenated dataset as well as datasets of individual genes to check for congruence with the Bayesian analysis.
Results
The final alignment of the concatenated dataset yielded 7109 positions, with 1830, 4612, and 667 positions for 18S rDNA, 28S rDNA, and COI mtDNA, respectively.
In general, the concatenated phylogenies based on both the Bayesian and the maximum likelihood alignments follow the results obtained by Kånneby et al. (2013). Given the included data, none of the currently recognized subgenera appear monophyletic except for Chaetonotus (Zonochaeta). Lepidochaetus, considered a subgenus within Chaetonotus by Balsamo et al. (2009), is also monophyletic. The hypothesized reinvasion of certain Chaetonotidae (Halichaetonotus and marine Heterolepidoderma in a sister group relation to the freshwater taxon Chaetonotus schultzei Metschnikoff, 1865) from a limnic environment to a marine environment by Kånneby et al. (2013) also gains high support in this study (pp = 0.98) (Fig. 2).
Majority rule consensus tree based on the Bayesian analysis of the concatenated dataset. Numbers at nodes represent posterior probability. Asterisks indicate a bootstrap support value above 75, obtained from the maximum likelihood analysis of the concatenated dataset. The star indicates the clade where a permanent planktonic lifestyle has evolved. The clades containing members of Muselliferidae (outgroup) and Xenotrichulidae have been collapsed for esthetic reasons (see Table 1 for full set of taxa)
For individual gene trees, there are no major conflicts when taking into account bootstrap support values above 70. Deeper nodes within the Chaetonotidae + Dasydytidae + Neogosseidae clade generally have very low bootstrap support (results not shown).
With regard to the main aims of the current study, the Bayesian analysis gives high support for monophyly of the planktonic gastrotrichs (Dasydytidae + Neogosseidae) in our study (pp = 1). Within this clade, both Dasydytidae (pp = 0.99) and Neogosseidae (pp = 1) are monophyletic (Fig. 2). For Neogosseidae, both Kijanebalola (K. devestiva, n = 2) and Neogossea (N. acanthocolla, n = 1 + N. antennigera, n = 2) are monophyletic with high bootstrap support (pp = 1). N. acanthocolla and N. antennigera are sister taxa. Within Dasydytidae, two major clades can be discerned: Haltidytes squamosus + Ornamentula paraënsis and Dasydytes + Stylochaeta (Fig. 2). Within the latter clade, Stylochaeta fusiformis and Stylochaeta scirtetica are in a sister group relation to Dasydytes (Prodasydytes) elongatus Kisielewski, 1991 and Dasydytes (P.) papaveroi Kisielewski, 1991 (pp = 1). The subgenus Dasydytes (Prodasydytes) is non-monophyletic, because of the sister group relationship of Dasydytes (P.) carvalhoae Kisielewski, 1991 to D. (P.) elongatus + D. (P.) papaveroi and Stylochaeta (pp = 1).
The sister group of the planktonic gastrotrichs (Dasydytidae + Neogosseidae) appears as Chaetonotus (Zonochaeta) spp. + Chaetonotus heteracanthus Remane, 1927 (pp = 0.99). Polymerurus is the sister group of the latter clade and the planktonic gastrotrichs (Fig. 2).
The maximum likelihood analysis of the concatenated dataset supports monophyly of planktonic Gastrotricha (bs = 90). However, Dasydytidae appear non-monophyletic, although with very low support (bs = 55), since Stylochaeta + Dasydytes is in a sister group relationship to Neogosseidae. Haltidytes squamosus Kisielewski, 1991 and Ornamentula paraënsis Kisielewski, 1991 form the sister group of Stylochaeta + Dasydytes. Further, the support for Chaetonotus (Zonochaeta) as the sister clade of planktonic gastrotrichs is also very low (bs = 45). The deeper nodes of the maximum likelihood phylogeny generally have very low support.
Discussion
Inside the primarily benthic phylum Gastrotricha, the occurrence of planktonic taxa is in many respects of particular interest. For instance, within an evolutionary framework, these animals may prove to be excellent for studies dealing with the origin and evolution of adaptations to the planktonic environment. Knowledge of the co-location of pelagic dwellers along the gastrotrich evolutionary tree would benefit studies dealing with, for example, ancestral character patterns and evolution of traits such as movable spines and muscle systems arrangement (Kieneke and Ostmann 2012).
Unfortunately, the origin and phylogenetic alliances of planktonic Gastrotricha has not been studied to any great extent. Kisielewski (1991) considered Dasydytidae and Neogsseidae as two separate lineages and discussed their possible origin from a benthic ancestor within Chaetonotidae. This hypothesized ancestor would have developed a sack-shaped body by reduction of the caudal lobes and adhesive tubes. The ventral ciliation would also have been modified into tufts and bands encircling the head. However, whether the two groups arose from a common ancestor or not was left open.
Based on morphological observations, both Remane (1927) and Kisielewski (1991) agree on a close relationship between Chaetonotus (Zonochaeta) and Dasydytidae (and possibly Neogosseidae). In fact, members of Chaetonotus (Zonochaeta) and Dasydytidae both possess movable spines of similar structure and function, e.g., strongly cuticularised, scaleless spines with a notched apex, used for locomotion and/or defense (Schwank 1990; Kisielewski 1991; Kieneke and Ostmann 2012). In his 1991 authoritative paper on Brazilian freshwater Gastrotricha, Kisielewski described the subgenus Dasydytes (Prodasydytes), whose members he considered to have retained the plesiomorphic character states among the planktonic Gastrotricha. Comparing morphology, it is evident that Dasydytes (P.) carvalhoae, Dasydytes (P.) elongatus, and Dasydytes (P.) papaveroi exhibit several similarities with members of the putative Dasydytidae sister taxon, Chaetonotus (Zonochaeta). These traits are: (i) long and specialized spines with denticles and bifurcated tips, (ii) similar shape of scales, and (iii) long parafurcal spines. In addition, dasydytids possess long ventrolateral and/or dorsolateral motile spines. To be more specific, dasydytids, by virtue of a peculiar complex system made up of serially arranged oblique muscles and segmental longitudinal muscles, can quickly raise their spines, which causes the animal to leap through the water, e.g., in response to a predator or other threat (Kieneke et al. 2008a; Kieneke and Ostmann 2012). On the other hand, species of Chaetonotus (Zonochaeta) are further characterized by a peculiar transverse row of spines, commonly known as the girdle, across the trunk. These spines can also be raised by muscle action. Consequently, the combination of movable spines and the associated musculature can be seen as potentially homologous characters shared by the dasydytids and species of Chaetonotus (Zonochaeta) (e.g., Kisielewski 1991).
The first cladistic analysis of Gastrotricha, based on 81 morphological characters, showed a sister group relationship between Dasydytidae and Neogosseidae in close alliance to Proichthydidae and Dichaeturidae (Hochberg and Litvaitis 2000). A subsequent, more inclusive analysis based on 135 morphological characters found a monophyletic Neogosseidae nested within Dasydytidae, although with low statistical support at nodes (Kieneke et al. 2008b). In the latter work, planktonic Gastrotricha appeared as a subset of Chaetonotidae, with low statistical support at nodes, although not in a close relationship with Chaetonotus (Zonochaeta). In the recent phylogenetic analysis, based on three molecular markers, Kånneby et al. (2013) found a sister group relation between Dasydytidae (Dasydytes, Haltidytes, Ornamentula, and Stylochaeta) and a clade containing Chaetonotus (Zonochaeta) and thereby somewhat confirming the early hypothesis by Remane (1927) and Kisielewski (1991).
The current study supports the general phylogenetic scenario obtained by Kånneby et al. (2013) and provides further evidence, on a molecular basis, for a common origin of Dasydytidae and Neogosseidae. The major findings of our study are: (i) Dasydytidae and Neogosseidae both appear as monophyletic groups; (ii) Dasydytidae and Neogosseidae are in a sister group relation; and (iii) the planktonic lineage evolved from a benthic or periphytic ancestor within Chaetonotidae. Strong nodal support and congruence with previous phylogenies (e.g., Kånneby et al. (2013) for the general scenario and Hochberg and Litvaitis (2000) for the sister group relationship between Dasydytidae and Neogosseidae) make our findings very likely.
Neogosseidae is monophyletic, and the clade has high support in our analyses. Neogosseidae can be separated from Dasydytidae morphologically by the presence of a pair of club-shaped cephalic tentacles in neogosseids. Within Neogosseidae, Kijanebalola and Neogossea (Fig. 1) are sister groups in our phylogeny and can be separated morphologically from each other based on distinct autapomorphic traits of the posterior end, which appears truncate and provided with a pair of tufts in Neogossea, and rounded with a median group of spines in Kijanebalola (Todaro et al. 2013).
Dasydytidae also appears monophyletic in our analysis (Fig. 2). Morphologically, the presence of groups of motile spines along the trunk that aid ciliary swimming can be considered an autapomorphy for the family (e.g., Kieneke and Ostmann 2012). Likewise, the peculiar system of somatic oblique and segmented lateral muscles associated with the cuticular movable spines may at least provisionally be considered an additional autapomorphy of Dasydytidae (Kieneke and Ostmann 2012). Within Dasydytidae, two major clades can be distinguished (Fig. 2). The first contains two species, Haltidytes squamosus and Ornamentula paraënsis. The second contains five species, D. (P.) elongatus, D. (P.) carvalhoae, D. (P.) papaveroi, Stylochaeta fusformis, and Stylochaeta scirtetica. The grouping of Haltidytes + Ornamentula in one clade and Dasydytes in another provides support to the in-group evolutionary scenario envisioned by Kisielewski (1991) on the basis of the structure and organization of the cuticular apparatus (scales and spines) but is in contrast to the phylogenetic hypothesis put forward by Kieneke and Ostmann (2012); see position of Ornamentula and Stylochaeta in Fig. 10) based mainly on characters of the muscular system. All the sampled species in our phylogeny belong to the subgenus Dasydytes (Prodasydytes), which is, according to the results, non-monophyletic due to the early divergence of D. (P.) carvalhoae along the Dasydytes + Stylochaeta branch. By virtue of the highly uniform anatomy of species of Dasydytes, and the strong difference compared to species of Stylochaeta, we consider this result quite unlikely and perhaps an artifact due to contamination.
Our phylogeny supports the existence of a monophyletic planktonic clade, as shown by Hochberg and Litvaitis (2000) and Kieneke et al. (2008b). However, in contrast to the latter study, Neogosseidae appear to be the sister taxon of Dasydytidae and not nested within it.
In the present study, the planktonic clade appears as an offshoot of the Chaetonotidae as repeatedly indicated in the literature, for example, by Kisielewski (1981). Due to lack of material, we did not include specimens of Dichaeturidae nor Proichthydidae, and because of this, the hypothesis by Hochberg and Litvaitis (2000), where the planktonic taxa are most closely related to these groups, cannot be properly tested. It should be emphasized that Dichaeturidae and Proichthydidae are very rare, that the original descriptions are poor, and that both these conditions could make the very existence of these taxa as independent evolutionary lines open to doubt. For instance, the recent and most accurate description of a third species of Dichaeturidae (Suzuki et al. 2013) reports characteristics that could well fit for a taxon belonging to the current Chaetonotidae. However, a taxonomic revision of Dichaeturidae and Proichthydidae lies beyond the scope of the present paper; yet in agreement with Kisielewski (1991), we consider our finding very likely. According to our results, the evolution of the genuine planktonic lifestyle in Chaetonotida occurred only once (Dasydytidae + Neogosseidae) and probably from a benthic/periphytic ancestor.
From a morphological point of view, a lineage originally affiliated with the Chaetonotidae and evolving to the split between Dasydytidae and Neogosseidae would have undergone several morphological adaptations to cope with the change from a benthic or periphytic lifestyle to a permanent planktonic lifestyle. Both Dasydytidae and Neogosseidae lack a furca and adhesive tubes and have a rounded posterior end. There are also reductions and rearrangements of the locomotory ciliature: while benthic chaetonotids commonly have ventral longitudinal bands of cilia, planktonic taxa possess transverse bands or tufts of cilia along the trunk and around the head, which may increase speed and maneuverability when swimming.
These dramatic changes would also have involved the ancestors that the Dasydytidae + Neogosseidae clade share with the sister group revealed by the Bayesian analysis in our study, e.g., Chaetonotus (Zonochaeta) spp. + Chaetonotus heteracanthus. The maximum likelihood analysis does not show high support for this sister group relation, suggesting Polymerurus as a possible alternative sister group. However, we note that the most relevant autapomorphic trait of Polymerurus is the extraordinarily long furca, which makes it hard to unite taxa lacking a furca (planktonic gastrotrichs) with taxa possessing a particularly long furca. On the other hand, the presence of strong scaleless spines could testify in favor for a close relationship between Dasydytidae + Neogosseidae and Chaetonotus (Zonochaeta) spp. + C. heteracanthus. In this scenario, we acknowledge the possible independent origin of the musculature, associated with the movable spines, in dasydytids and Chaetonotus (Zonochaeta), respectively. Future morpho-functional studies on the musculature of species of Chaetonotus (Zonochaeta) could support or disprove this hypothesis.
To summarize, the phylogenetic hypothesis generated by this study gives strong support for a common origin of Dasydytidae and Neogosseidae and also a hypothesized common origin of planktonic Gastrotricha. It also appears very plausible that the planktonic clade has evolved from a benthic or periphytic ancestor by morphological adaptations to a life in the water column. Morphology and molecular data prefers Chaetonotus (Zonochaeta) spp. (and C. heteracanthus) as the sister group of planktonic Gastrotricha. The close relationship of these groups to Polymerurus is somewhat confusing and should be evaluated in future studies.
References
Balsamo, M. (1978). Prime ricerche sui Gastrotrichi dulciacquicoli italiani. Atti della Societa Toscana di Scienze Naturali Memorie Serie B, 84, 87–150. 1977.
Balsamo, M. (1983). Gastrotrichi. Guide per il riconoscimento delle specie animali delle acque interne italiane. Italy: Consiglio Nazionale delle Ricerche.
Balsamo, M., & Fregni, E. (1995). Gastrotrichs from interstitial fresh water, with a description of four new species. Hydrobiologia, 302, 163–175.
Balsamo, M., Guidi, L. & d’Hondt, J. L. (2013). Phylum Gastrotricha. In Z. Q. Zhang, (Ed.), Animal biodiversity: An outline of higher-level classification and survey of taxonomic richness (Addenda 2013). Zootaxa, 3703, 79–82.
Balsamo, M., & Todaro, M. A. (1987). Aspidiophorus polystictos, a new marine species (Gastrotricha, Chaetonotida) and its life cycle. Bollettino di Zoologia, 54, 147–153.
Balsamo, M., & Todaro, M. A. (1995). Gastrotrichi del Trentino: le Viotte del Monte Bondone. Studi Trentini di Scienze Naturali–Acta Biologica, 70, 9–22.
Balsamo, M., & Todaro, M. A. (2002). Gastrotricha. In S. D. Rundle, A. L. Robertson, & J. M. Schmid-Araya (Eds.), Freshwater meiofauna: biology and ecology (pp. 45–61). Leiden: Backhuys Publishers.
Balsamo, M., d’Hondt, J. L., Pierboni, L., & Grilli, P. (2009). Taxonomic and nomenclatural notes on freshwater Gastrotricha. Zootaxa, 2158, 1–19.
Balsamo, M., Grilli, P., Guidi, L., & d’Hondt, J. L. (2014). Gastrotricha: Biology, ecology and systematics, families Dasydytidae, Dichaeturidae, Neogosseidae, Proichthydiidae. In H. J. F. Dumont (Ed.), Identification guides to the plankton and benthos of inland waters (24th ed.). Weikersheim: Backhuys Publishers. 187 pp.
Beauchamp, P. (1932). Scientific results of the Cambridge Expedition to the East African Lakes. Journal of the Linnean Society of London, Zoology, 38, 241–248.
Brehm, V. (1917). Ergebnisse einiger im Franzensbader Moor unternommener Exkursionen. Archives of Hydrobiology, 11, 306–323.
Brunson, R. B. (1950). An introduction to the taxonomy of the Gastrotricha with a study of eighteen species from Michigan. Transactions of the American Microscopical Society, 69, 325–352.
Clark, D. R., Cantu, R., Cowman, D. F., & Maxson, D. J. (1998). Uptake of arsenic and metals by tadpoles at an historically contaminated Texas site. Ecotoxicology, 7, 61–67.
Dujardin, F. (1841). Histoire Naturelle des Zoophytes Infusoires. Paris: Libraire Encyclopedique de Roret.
Edgar, R. C. (2004). MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research, 32, 1792–1797.
Ehrenberg, C. G. (1838). Die Infusionstierchen als vollkommene Organismen. Ein Blick in des tiefere organische Leben der Natur. Leipzig: Leopold Voss.
Gelman, A., & Rubin, D. B. (1992). Inference from iterative simulation using multiple sequences. Statistical Science, 7, 457–511.
Gerlach, S. A. (1953). Gastrotrichen aus dem Küstengrundwasser des Mittelmeeres. Zoologischer Anzeiger, 150, 203–211.
Gosse, P. H. (1851). A catalogue of Rotifera found in Britain with descriptions of five new genera and thirty-two new species. Annals and Magazine of Natural History, 2, 197–203.
Greuter, A. (1917). Beträge zur Systematik der Gastrotrichen in der Schweiz. Revue Suisse de Zoologie, 25, 35–76.
Grünspan, T. (1908). Beiträge zur Systematik der Gastrotrichen. Zoologischer Jahrbücher Abteilung für Systematik, 26, 214–256.
Hochberg, R., & Litvaitis, M. K. (2000). Phylogeny of the Gastrotricha: a morphology-based framework of gastrotrich relationships. Biological Bulletin, 198, 299–305.
Hummon, W. D. (1974). Gastrotricha from Beaufort, North Carolina, U.S.A. Cahiers de Biologie Marine, 15, 431–446.
Hummon, W. D., & Todaro, M. A. (2010). Analytic taxonomy and notes on marine, brackish water and estuarine Gastrotricha. Zootaxa, 2392, 1–32.
Kånneby, T., Todaro, M. A., & Jondelius, U. (2009). One new species and records of Ichthydium Ehrenberg, 1830 (Gastrotricha: Chaetonotida) from Sweden with a key to the genus. Zootaxa, 2278, 26–46.
Kånneby, T., Todaro, M. A., & Jondelius, U. (2012). A phylogenetic approach to species delimitation in freshwater Gastrotricha from Sweden. Hydrobiologia, 683, 185–202.
Kånneby, T., Todaro, M. A., & Jondelius, U. (2013). Phylogeny of Chaetonotidae and other Paucitubulatina (Gastrotricha: Chaetonotida) and the colonization of aquatic ecosystems. Zoologica Scripta, 42, 88–105.
Kånneby, T., Atherton, S., & Hochberg, R. (2014). Two new species of Musellifer (Gastrotricha: Chaetonotida) from Florida and Tobago and the systematic placement of the genus within Paucitubulatina. Marine Biology Research, 10, 983–995.
Kearse, M., Moir, R., Wilson, A., Stones-Havas, S., Cheung, M., Sturrock, S., Buxton, S., Cooper, A., Markowitz, S., Duran, C., Thierer, T., Ashton, B., Mentjies, P., & Drummond, A. (2012). Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics, 28(12), 1647–1649.
Kieneke, A., & Ostmann, A. (2012). Structure, function and evolution of somatic musculature in Dasydytidae (Paucitubulatina, Gastrotricha). Zoomorphology, 131, 95–114.
Kieneke, A., & Schmidt-Rhaesa, A. (2014). Gastrotricha. In A. Schmidt-Rhaesa (Ed.), Handbook of zoology (Gastrotricha and Gnathifera, Vol. 3, pp. 1–134). Berlin, Boston: De Gruyter.
Kieneke, A., Martínez Arbizu, P., & Riemann, O. (2008a). Body musculature of Stylochaeta scirtetica Brunson, 1950 and Dasydytes (Setodytes) tongiorgii (Balsamo, 1982) (Gastrotricha: Dasydytidae): a functional approach. Zoologischer Anzeiger, 247, 147–158.
Kieneke, A., Riemann, O., & Ahlrichs, W. H. (2008b). Novel implications for the basal internal relationships of Gastrotrich revealed by an analysis of morphological characters. Zoologica Scripta, 37, 429–460.
Kisielewski, J. (1981). Gastrotricha from raised and transitional peat bogs in Poland (Monografie Fauny Polski, Vol. 11). Warszawa: Polska Akademia Nauk.
Kisielewski, J. (1986). Taxonomic notes on freshwater gastrotrichs of the genus Aspidiophorus Voigt (Gastrotricha, Chaetonotoidea) with description of four new species. Fragmenta Faunistica, 30, 139–156.
Kisielewski, J. (1987). Two new interesting genera of Gastrotricha (Macrodasyida and Chaetonotida) from the Brazilian freshwater psammon. Hydrobiologia, 153, 23–30.
Kisielewski, J. (1991). Inland-water Gastrotricha from Brazil. Annales Zoologici Warszawa, 43(Supplement 2), 1–168.
Leasi, F., & Todaro, M. A. (2008). The muscular system of Musellifer delamarei (Renaud-Mornant, 1968) and other chaetonotidans with implications for the phylogeny and systematization of the Paucitubulatina (Gastrotricha). Biological Journal of the Linnean Society, 94, 379–398.
Marcolongo, I. (1910). Primo contributo allo studio dei Gastrotrichi del lago-stagno craterico di Astroni. Monitore Zoologico Italiano, 21, 315–318.
Metschnikoff, E. (1865). Über einige wenig bekannte niedere Thierformen. Zeitschrift für Wissenschaftliche Zoologie, 15, 450–463.
Miller, M. A., Pfeiffer, W. & Schwartz, T. (2010). “Creating the CIPRES Science Gateway for inference of large phylogenetic trees” in Proceedings of the Gateway Computing Environments Workshop (GCE) (pp. 1–8), 14 Nov. 2010, New Orleans, LA.
Preobrajenskaja, E. N. (1926). Zur Verbreitung der Gastrotrichen in den Gewässern der Umgebung von Kossino. Arbeiten der Biologischen Station zu Kossino (Moskau), 4, 1–14.
Remane, A. (1927). Beiträge zur Systematik der Süsswassergastrotrichen. Zoologischer Jahrbücher Abteilung für Systematik, Oekologie, und Geographie der Tiere, 53, 269–320.
Remane, A. (1934). Die Gastrotrichen des Küstengrundwassers von Schilksee. Schriften des Naturwissenschaftliches Vereins für Schleswig-Holstein, 20, 473–478.
Renaud-Mornant, J. (1968). Présence du genre Polymerurus en milieu marine, description de deux espèces nouvelles (Gastrotricha, Chaetonotidae). Pubblicazioni della Stazione Zoologica di Napoli, 36, 141–151.
Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D. L., Darling, A., Höhna, S., Larget, B., Liu, L., Suchard, M. A., & Huelsenbeck, J. P. (2012). MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology, 61, 539–542.
Schrom, H. (1972). Nordadriatische Gastrotrichen. Helgoländer wissenschaftlicher Meeresuntersuchungen, 23, 286–351.
Schwank, P. (1990). Gastrotricha. In A. Brauer, J. Schwoerbel & P. Zwick (Eds.) Süsswasserfauna von Mitteleuropa. Band 3, Heft 1–2: Gastrotricha und Nemertini (252 pp.). Stuttgart, Jena, New York: Gustav Fischer Verlag.
Silvestro, D., & Michalak, I. (2012). raxmlGUI: a graphical front-end for RaxML. Organisms, Diversity and Evolution, 12, 335–337.
Spencer, T. (1890). On a new rotifer. Journal of the Quekett Microscopical Club, Series 2, 4, 59 + pl. 5.
Stamatakis, A. (2006). RaxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics, 22, 2688–2690.
Stokes, A. C. (1887). Observations on a new Dasydytes and a new Chaetonotus. The Microscope (Detroit), 7, 261–265.
Suzuki, T. G., Maeda, M., & Furuya, H. (2013). Two new Japanese species of Gastrotricha (Chaetonotida, Chaetonotidae, Lepidodermella and Dichaeturidae, Dichaetura), with comments on the diversity of gastrotrichs in rice paddies. Zootaxa, 3691(2), 229–239.
Thompson, P. G. (1891). A new species of Dasydytes, order Gastrotricha. Science Gossip, 319, 160–162.
Todaro, M. A. (1992). Contribution to the study of the Mediterranean meiofauna: Gastrotricha from the Island of Ponza. Bollettino di Zoologia, 59, 321–333.
Todaro, M. A., Telford, M. J., Lockyer, A. E., & Littlewood, D. T. J. (2006). Interrelationships of the Gastrotricha and their place among the Metazoa inferred from 18S rRNA genes. Zoologica Scripta, 35, 251–259.
Todaro, M. A., Kånneby, T., Dal Zotto, M., & Jondelius, U. (2011). Phylogeny of Thaumastodermatidae (Gastrotricha: Macrodasyida) inferred from nuclear and mitochondrial sequence data. PLoS One, 6(3), e17892.
Todaro, M. A., Dal Zotto, M., Jondelius, U., Hochberg, R., Hummon, W. D., Kånneby, T., & Rocha, C. E. F. (2012). Gastrotricha: a marine sister for a freshwater puzzle. PLoS One, 7(2), e31740.
Todaro, M. A., Perissinotto, R., & Bownes, S. J. (2013). Neogosseidae (Gastrotricha, Chaetonotida) from the iSimangaliso Wetland Park, Kwazulu-Natal, South Africa. ZooKeys, 315, 77–94.
Todaro, M. A., Leasi, F., & Hochberg, R. (2014). A new species, genus and family of marine Gastrotricha from Jamaica, with a phylogenetic analysis of Macrodasyida based on molecular data. Systematics and Biodiversity, 12, 473–488.
Valkanov, A. (1937). Rotatorien und Gastrotrichen der Umgebung von Plönsbiotopen. Forschungsberichte aus der Biologishen Station zu Plön, 11, 1–178.
Voigt, M. (1901). Mittheilungen aus der Biologischen Station zu Plön, Holstein. Über einige bisher unbekannte Süsswasserorganismen. Zoologischer Anzeiger, 24, 191–195.
Voigt, M. (1902). Drei neue Chaetonotus-Arten aus Plöner Gewässern. Zoologischer Anzeiger, 25, 116–118.
von Daday, E. (1905). Untersuchungen über die Süsswasser Mikrofauna Paraguays. Zoologica, 44, 1–374.
Wilke, U. (1954). Mediterrane Gastrotrichen. Zoologischer Jahrbücher Abteilung für Systematik (Jena), 82, 497–550.
Zelinka, C. (1889). Die Gastrotrichen. Eine monographische Darstellung ihrer Anatomie, Biologie und Systematik. Zeitschrift für Wissenschaftliche Zoologie, 49, 209–384.
Acknowledgments
The authors wish to thank Dr. Mary K. Wicksten, Department of Biology, Texas A & M University for collecting some of the samples. Dr. Ronald Griffin, Department of Agricultural Economics, Texas A & M University is greatly acknowledged for providing necessary equipment and chemicals. The authors are also thankful to Mr. James J. Kirk for improving the language and grammar of an early version of this paper. The final text benefited from the comments of two anonymous reviewers. This study was financially supported by a grant from Lennanders Stiftelse (to TK). The Swedish Taxonomy Initiative is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kånneby, T., Todaro, M.A. The phylogenetic position of Neogosseidae (Gastrotricha: Chaetonotida) and the origin of planktonic Gastrotricha. Org Divers Evol 15, 459–469 (2015). https://doi.org/10.1007/s13127-015-0223-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13127-015-0223-9