Abstract
Current evidence suggests regular overland transport of different freshwater invertebrates by wind, mainly over short distances. Yet, very little is known about the mechanism and scale of this process or about differences in wind dispersal dynamics and capacities among taxa and propagule types. We investigated wind dispersal of freshwater invertebrates in a cluster of temporary rock pools (spatial scale: 9,000 m2) in South Africa. Dispersing propagules and propagule bank fragments (i.e. aggregates of sediments and propagules) were intercepted during 1 month using a combination of windsocks (1.5 m above ground level) and sticky traps (ground level). The potential movement of propagule bank fragments (i.e. aggregates of propagules and sediments) was also simulated by tracking inter-pool movements of differently sized artificial substrate fragments similar to dry propagule bank fragments. We detected differences in the composition of dispersing communities intercepted at different altitudes (ground level and at 1.5 m). Comparison of dispersal distance distributions also revealed significant differences among taxa. Overall, larger propagule types (e.g. adult ostracods and oribatid mites) dominantly travelled near ground level while small resting eggs and cryptobiotic life stages of copepods were most frequently collected at higher altitudes (1.5 m) and dispersed over the longest distances. Finally, not only dispersal of single propagules but also ground level transport of propagule bank fragments was shown to contribute to local dispersal dynamics in temporary aquatic habitats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most non-insect invertebrates of pools, ponds and lakes disperse passively as small, dormant propagules or other cryptobiotic life stages. Therefore, diapause is often considered essential for the dispersal of many freshwater organisms (Panov et al., 2004; Panov & Cáceres, 2007). Although wide distribution patterns and patterns of genetic differentiation of aquatic invertebrates have sometimes been (partially) attributed to wind dispersal (Mayr, 1963; Chaplin & Ayre, 1997), direct evidence in support of wind dispersal of aquatic invertebrates is lacking. Compared to the directional dispersal associated with water connections (Michels et al., 2001; Vanschoenwinkel et al., 2008a) and animal vectors which repeatedly visit water bodies (e.g. amphibians: Bohonak & Whiteman, 1999; waterfowl: Green & Figuerola, 2005; Frisch et al., 2007; aquatic insects: Van De Meutter et al., 2008; mammals: Vanschoenwinkel et al., 2008c) propagules that disperse by wind have a low chance of landing in a suitable habitat patch due to the dominance of the terrestrial environment in natural landscapes. Hence, successful wind dispersal events are considered very rare, particularly over long distances. On the other hand, several studies indicate that wind dispersal can be important over short distances. Caceres & Soluk (2002) and Cohen & Shurin (2003) showed successful colonisation of mesocosms by several zooplankton species in the absence of animal vectors. Direct dispersal measurements in temporary pool clusters using glue traps (Vanschoenwinkel et al., 2008b) and windsocks (Vanschoenwinkel et al., 2008a) subscribe that wind dispersal from dry propagule banks can be frequent over short temporal and at small spatial scales and may be common for a diverse group of invertebrate taxa. Still, freshwater invertebrates seem to have different dispersal fluxes. In a 1-year field study using windsocks, Jenkins & Underwood (1998) only caught bdelloid rotifers and no other zooplankton. Brendonck & Riddoch (1999) in a 3-day study using glue traps around a set of temporary pools, only caught eight anostracan cysts, despite the presence of diverse invertebrate communities in these pools (Jocqué et al., 2006). Colonisation experiments of new water bodies also revealed interspecific differences in arrival time (Jenkins & Buikema, 1998; Louette & De Meester 2005) which may reflect differences in dispersal capacities.
The wind dispersal mode that is implicitly considered in the literature is what we call ‘pick up and deposit’ transport: loose propagules are picked up by wind to a certain altitude and are deposited elsewhere, possibly in another water body. Initial pick up in combination with turbulent updrafts is thought to facilitate the transport of propagules to higher air layers which presumably mediate long distance transport (Nathan et al., 2005). Secondly, Brendonck & Riddoch (1999) suggested that propagules might also travel near ground level by means of rolling and saltational movements, i.e. repetitive pick up and deposit events of propagules near ground level. A third possibility is that the propagule bank can break up into fragments upon drying and that these fragments may also be transported by wind, potentially facilitating the joint dispersal of large numbers of propagules of multiple taxa.
We have studied passive aerial dispersal of freshwater invertebrates in a cluster of temporary rock pools situated on a rocky outcrop in central South Africa by intercepting dispersing propagules and propagule bank fragments using a combination of mounted windsocks (1.5 m above ground level) and sticky traps (ground level). Temporal and spatial wind dispersal patterns have been documented in previous studies (Vanschoenwinkel et al., 2008a, b), however, differences in dispersal capacities among different taxa have thus far not been investigated. In this study, we compared the magnitude of local wind dispersal rates for different invertebrate taxa and interpreted differences in species composition between samples collected via windsocks and sticky traps to assess the relative importance of different dispersal modes for different propagule types. We hypothesised that the studied taxa differ in their ability to disperse via wind and that smaller propagules are more likely to be picked up by wind and travel greater distances. Compared to windsocks, sticky traps also sample movement of propagules near ground level. Hence, we expected that larger propagules will be more abundantly represented in the sticky traps than in the windsock samples. Dispersal distance distributions were reconstructed to compare mean and maximum dispersal distances among taxa. Finally, we also investigated the potential for dispersal of propagule bank fragments experimentally by tracking the movement of artificial substrate fragments between dry pool basins.
Materials and methods
Study site
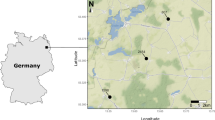
The study site consists of a cluster of 36 temporary rock pools situated at the summit of the Korannaberg mountain (Free State Province, South Africa) (1,700 m a.s.l.; 28°51′13′S; 27°13′51′E) (Fig. 1). The site comprises an area of approximately 9,000 m². The region lies within a predominantly summer rainfall area with an annual precipitation of between 600 and 800 mm. For a detailed description of the study site and the invertebrate communities we refer to Vanschoenwinkel et al. (2007). Previous research has shown that local passive dispersal dynamics on the study site are intense and are mainly mediated by wind and water, the latter only being important for certain pools that form temporary connections after heavy rains (Vanschoenwinkel et al., 2008a, b).
a Location of the Korannaberg mountain in South Africa and b Lay out of different rock pools (white) and position of sticky traps and windsocks on the study site. All pools are located on a single rock ledge (outline) at the mountain summit. Orientation of the study site is indicated using a north arrow
Sampling procedure
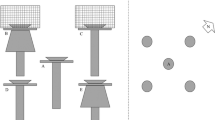
The current article makes use of two published datasets (Vanschoenwinkel et al., 2008a, b) of wind dispersing propagules collected in the Korannaberg pool cluster. Collections were made both at ground level (n = 63) using sticky traps and at a height of 1.5 m using windsocks (n = 9) during 1 month in the dry season.
Position of windsocks and sticky traps on the study site is illustrated in Fig. 1. For a more detailed overview of the sampling procedure and identification of taxa we refer to Vanschoenwinkel et al. (2008a) for the windsock and to Vanschoenwinkel et al. (2008b) for the sticky trap approach. To investigate whether entire propagule bank fragments are dispersed by wind, all soil and sediment particles that were caught on the sticky traps were dissected under a stereo microscope and their contents investigated for invertebrate propagules.
Anemometer data were collected by the South African weather service at the weather station of Ficksburg (60 km east of the study site) and included average and maximal wind speed (m s−1), wind direction (degrees), % calms (the percentage time with zero wind speed). The effects of wind speed and wind direction and % calms on local wind dispersal dynamics are discussed in Vanschoenwinkel et al. (2008a).
Data analysis
Direct dispersal measurements
We compared the total list of intercepted wind dispersing taxa to a list of the passive dispersing taxa known from active communities in the 36 rock pools, which were sampled intensively (four times in one season) in a previous study (Vanschoenwinkel et al., 2007). We calculated the percentage of taxa known from active communities which were found in wind dispersal samples, the percentage of taxa known from active communities and which were not found in wind dispersal samples as well as a percentage of taxa which could not be reliably linked to known propagule types. We remark that due to problems with identification of propagules resolution of identification was lower for propagules than for adults in active communities. We did not relate relative abundances of taxa in the dispersing community to relative abundances in active communities as these do not reliably reflect the relative abundance of different corresponding propagules in the propagule bank (Brendonck & De Meester, 2003). Differences in magnitude of observed dispersal rates among taxa can be a consequence of variation in size of their corresponding propagule banks (density dependent dispersal; Amarasekare, 2004). However, as detailed information about the relative composition of propagule banks is unavailable for our study site, we could not simply use measured dispersal rates to infer taxon-specific dispersal capacity. Instead, we compared differences in composition of dispersing communities intercepted at different heights to assess differences in dispersal mode and ability. We tested for differences in relative abundance of different taxa in windsocks and sticky traps using non parametric Mann–Whitney U tests since the normality assumption of parametric ANOVA was unfulfilled. In this analysis sticky traps or windsocks that did not collect propagules were excluded. We did not compare absolute dispersal rates due to differences in sampling intensity for windsocks (n = 9) and sticky traps (n = 63), respectively. The effect of distance to source populations on dispersal rates of different taxa was investigated by comparing dispersal distance distributions and testing differences in mean dispersal distances using non parametric Kruskal–Wallis tests since dispersal distance distributions were highly skewed. Dispersal distances were calculated as the distance between the location where a propagule was intercepted and the nearest pool from which the taxon was recorded in active populations (Vanschoenwinkel et al., 2007). This analysis was only performed for the sticky traps as the low number of windsocks (n = 9) did not allow to reliably reconstruct dispersal kernels. For both aforementioned analyses P values were Bonferroni corrected. To confirm whether entire propagule bank fragments can be transported by wind, any sediment fragments collected in wind dispersal samples (windsocks and sticky traps) were dissected to retrieve and identify propagules. Caeculid mites (Acari) were excluded from all analyses because they are presumably also capable of active overland dispersal. Nematoda were excluded because their small size and transparency made them virtually impossible to detect on the sticky traps.
Simulation experiment
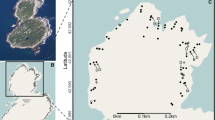
On 17 August 2006, we placed 10 large (10 × 10 × 3 cm), 20 medium (5 × 5 × 3 cm) and 40 small (3 × 3 × 3 cm) light weight wood fibre blocks (Softboard® isolation material), simulating actual propagule bank fragments, in the dry basin of 10 source pools (Fig. 2). From hereon, we will refer to these as artificial substrate fragments. Both size and weight of the different fragments (5, 10 and 60 g, respectively) were comparable with actual dry propagule bank fragments (Vanschoenwinkel, unpublished data). Fragments from each source pool were marked with a different colour. One week after the start of the experiment the final position of each fragment was marked on a detailed digital GIS map (ArcGIS 8.2; ESRI, 2002) and the distances to the source pool was measured. We also calculated the fraction of fragments that were blown from each source pool and the fraction that were successfully transported to another basin. Relations between fragment size and the fraction of fragments blown from each pool basin, the fraction of fragments dispersing to other pool basins and dispersal distance were investigated using non parametric Kruskal–Wallis tests. As we expected that basin depth may inhibit dispersal of propagule bank fragments, we distinguished two types of source pools: shallow (max depth < 200 mm) and deep pools (max depth > 200 mm) and investigated the relationship between basin depth, the fraction of fragments that were blown from each pool basin, the fraction of fragments dispersing to another pool basin, and dispersal distance using Mann–Whitney U tests as model assumptions of parametric ANOVA were unfulfilled. All analyses were performed using Statistica version 8.1 (StatSoft Inc., 2007, Tulsa, Oklahoma).
Results
Not all rock pool invertebrates were frequently dispersed by wind. During 1 month a total of 4,312 wind dispersing propagules (windsocks: 850; sticky traps: 3,462) were intercepted. Captured taxa included both resting eggs and cryptobiotic juvenile and adult life stages. Taxa that dominantly contributed to the intercepted dispersing community were the cryptobiotic aquatic mite Aquanothrus montanus (24%), cryptobiotic adults of the harpacticoid copepod Bryocamptus sp. (18%), resting eggs of calanoid copepods (12%) and ostracods (12%). Branchiopod crustaceans (Cladocera, Anostraca and Spinicaudata) accounted for 24% of the dispersing community (Table 1).
Most passively dispersing species (73%) that were previously collected from the pools as adults were also retrieved in the dispersing community. Exceptions were the cladoceran Moina micrura, the gastropod Bulinus tropicus and a number of taxa for which the dispersal stage is unknown such as cyclopoid copepods. The relative contribution of different taxa to the intercepted wind dispersing community differed between sticky traps and windsocks. Calanoida resting eggs (24 vs. 9%) and cryptobiotic Harpacticoida (Bryocamptus sp.) were relatively more abundant in windsocks (46 vs. 12%) while adult ostracods (14 vs. 3%) and cryptobiotic mites (A. montanus) (31 vs. 1%) were relatively more abundant in sticky trap samples (Table 1). Simocephalus vetulus and Caeculidae sp. were exclusively found on the sticky traps. A detailed overview of test statistics and corresponding P values is provided in Table 1.
A general trend of dispersal rates decreasing with distance to potential source populations was observed for most taxa with dispersal rates usually already dropping dramatically within 10 m from a nearest source. (Fig. 3). Mean dispersal distance significantly differed among taxa (Kruskal–Wallis; H = 461.6, P < 0.001). Calanoida resting eggs dispersed significantly further than the mite A. montanus (Kruskal–Wallis test: pairwise comparisons; P = 0.02) while these two taxa in turn dispersed further than most other taxa (Kruskal–Wallis test: pairwise comparisons; all P < 0.001). Bryozoan statoblasts (Plumatella sp.), Chydorus sphaericus and Chydoridae spp. dispersed over significantly shorter distances than most other taxa (Fig. 3). No clear differences in maximum dispersal distances were observed except for three rare taxa from which very few propagules were intercepted: M. propinqua (n = 8), S. vetulus (n = 16) and L. striatoconcha (n = 28) (Fig. 3).
Fourteen propagule bank fragments containing on average 29 (SD = 50; range: 1–176) viable propagules (Table 1) were intercepted dispersing over an average distance of 6 m (SD = 3.6 m). No such fragments were recovered from windsock samples.
Mean (±1 standard error) dispersal distances of taxa caught on sticky traps. Full circles represent maximum dispersal distances. Alona = Alona costata, A mont = Aquanothrus montanus, B trid = Branchipodopsis spp., Bryo = Bryocamptus sp., Cal (eggs) = Calanoida sp., Chyd = Chydorus sphaericus, Chyd sp = Chydoridae sp. Lept = Lepthesteria striatoconcha, Macr = Macrothrix propinqua, Ost = Ostracoda sp., Plum = Plumatella sp., Simo = Simocephalus vetulus
14.5% of artificial propagule fragments were blown from their source pool and 3.2% successfully dispersed to another pool basin (average dispersal distance: 10 m; SD = 7 m; maximum dispersal distance: 24 m). No significant relation was found between fragment size and the fraction of fragments blown from each pool basin (Kruskal–Wallis test, H = 1.36, P > 0.05) or between fragment size and the fraction of fragments dispersing to another pool (Kruskal–Wallis test, H = 1.37, P > 0.05). Fragments from shallow pool basins did not disperse more frequently (Mann–Whitney U = 13, Z = −0.63, P > 0.52) but were transported over greater distances than fragments from deeper pool basins (Mann–Whitney U = 173, Z = −2.1, P = 0.03). No significant difference was found between dispersal distances of small, medium and large fragments, respectively (Kruskal–Wallis test, H = 1.37, P > 0.05). Post dispersal positions of individual fragments are visualised in Fig. 2. Movements of fragments predominantly ended in vegetation patches.
Discussion
Link between propagule properties and dispersal capacity
Contrary to the well-studied aerial dispersal capacity of seeds and fruits (e.g. Willson & Traveset, 2000; Minami & Azuma, 2003) there is no comparable information about the importance of morphological properties for aerial dispersal of freshwater invertebrate propagules (Pajunen, 1986; Brendonck & Riddoch, 1999). In botanical literature it is commonly stated that larger seeds or fruits are less dispersible by wind because of their greater mass (Salisbury, 1975; Meyer & Carlson, 2001). Weights of different propagules types could not be determined in this study due to their small size and the interference with the glue from the sticky traps. Therefore, we could not directly relate this structural variable to dispersal capacity. Still, it is conspicuous that the largest propagules in our study: Bryozoan statoblasts (Plumatella sp.; 700–800 μm), oribatid mites (A. montanus; 700–900 μm) and especially caeculid mites (Caeculidae sp.; 1,400–1,600 μm) and Simocephalus vetulus ephippia (700–1,100 μm) were systematically underrepresented or absent (Caeculidae sp. and S. vetulus) in windsock samples. Bryozoan statoblasts also dispersed over the shortest distances (Fig. 3). High observed dispersal capacity of calanoid copepod eggs was not unexpected as these are the smallest propagules intercepted in this study (70–110 μm). Colonisation experiments support high dispersal capacity of copepods including principally cyclopoids which produce cryptobiotic life stages (Jenkins & Buikema, 1998; Caceres & Soluk, 2002; Frisch & Green, 2007). The relative dominance of the harpacticoid copepod Bryocamptus sp. in windsock samples could not be explained by size, as dispersing individuals were relatively large (~500 μm) compared to other intercepted propagules. Yet the absence of a heavy shell or a thick integument as present in most other propagules in this study (e.g. mites, ephippia and statoblasts) may render them relatively light. Our results do not entirely support the hypothesis that smaller propagules disperse more easily over longer distances, as certain larger propagules such as (adult) ostracods and oribatid mites (A. montanus) were among the taxa that dispersed over the longest distances. This apparent discrepancy is probably related to the fact that our dispersal distance analysis was based on interception of propagules at ground level in a flat open area. In such an environment heavy propagules may indeed still be able to travel reasonably long distances (>30 m) using rolling and saltational movement. However, when they are not picked up and carried to higher air layers they are less likely to contribute to long distance dispersal.
Seeds and fruits are typically released at a certain height or launched into the air by the mother plant. Plant species therefore developed a number of ‘glider’ adaptations (parachutes, kapok, samaras) to disperse over longer distances (Minami & Azuma, 2003; Greene & Quesada, 2005). Invertebrate propagules, on the other hand, differ in the important fact that they need to be lifted from ground level. Graham & Wirth (2008) showed that the structure of the surface sediment layer was important for successful pick up of anostracan eggs as eggs launched more easily from disturbed dormant egg banks. None of the dispersing propagules intercepted in our study exhibited obvious morphological properties that might promote aerial dispersal. The apparent absence of observable structural adaptations for wind dispersal in aquatic invertebrates may be due to physiological–developmental constraints. Since copepod and large branchiopod eggs are carried in an eggsac or broodpouch, morphological attributes to facilitate wind dispersal may impose a cost associated with less efficient packaging of eggs. Cladoceran ephippia, in turn, are formed within the body cavity of the female. Adult dimensions may therefore also impose constraints on the evolution of (macroscopic) adaptations to wind dispersal. Taking into account the high cost of unsuccessful dispersal, it is not unlikely that there will also be selection for features that inhibit dispersal. Production of larger, heavier propagules, for instance, may help organisms to reduce excessive losses of propagules due to wind erosion (Vanschoenwinkel et al., 2008a). Brendonck & Riddoch (1999) demonstrated that the fairy shrimp Branchipodopsis wolfi, produces two types of eggs: smooth eggs and eggs that collect debris after deposition; the latter being expected to be less dispersible by wind.
Dispersal distances and scale
The small scale of our study site was not limiting for wind dispersal and most known passively dispersing taxa found in the pools were represented in the wind dispersing community. The absence of the cladoceran M. micrura in wind dispersal samples is probably related to the rarity of this species on the study site (small populations in three pools). In case of the gastropod B. tropicus its large size (±1 cm) and heavy shell probably impeded transport by wind. Most propagules were able to reach the most isolated sticky traps. As a result, no clear differences in maximum dispersal distances were observed with the exception of L. striatoconcha, S. vetulus and M. propinqua which were characterised by lower maximal dispersal distances. These taxa, however, are uncommon at the study site (Vanschoenwinkel et al., 2007) and the limited numbers of collected propagules (<30) did not allow us to reliably reconstruct dispersal distance distributions during the short duration of this study. Although wind dispersal rates were very high over short distances, dispersal distance distributions for different taxa showed that wind dispersal rates already dropped dramatically after 2–3 m from a nearest (potential) source pool (Fig. 4). Dispersal kernels are typically characterised by very long tails which are responsible for rare long distance dispersal events. Due to the stochastic nature of wind dispersal and the limited spatial and temporal scale of experimental studies, information about such events is usually unavailable (Nathan et al., 2003). Observed dispersal distance distributions, as estimated in our study, do not capture long distance wind dispersal and it is likely that over longer distances directional transport via animal vectors will be more efficient. Still, this does not preclude the possibility of occasional successful long distance wind dispersal events. Perhaps intensive sampling of the atmosphere for propagules will shed new light on the feasibility of long distance wind dispersal.
Dispersal of propagule bank fragments
Besides dispersal of single propagules, we demonstrated that wind mediated transport of multiple propagules embedded in a sediment matrix can also contribute to local dispersal dynamics. Still, the bulk of the propagules on our study site were transported as single propagules, suggesting that this mode of transport remains quantitatively more important. Group dispersal of propagules, however, may have some advantages as joint arrival and hatching of multiple propagules in new habitats may increase chances of successful colonisation of sexually reproducing organisms as it increases the chance of encountering a mate. 3.2% of artificial fragments successfully dispersed to other pool basins, additionally subscribing the potential of ‘propagule bank dispersal’. This simulation experiment confirmed that also much larger propagule bank fragments (surface area of 100 cm²) than the ones intercepted on the sticky traps (surface area of 0.4–6 cm²), can be blown from a source pool to other pool basins, even during the relatively short time period of one week. We found no indication that light fragments dispersed more frequently than the larger, heavier ones. As this type of transport is restricted to ground level, it is expected that even minor landscape elements such as ridges and low vegetation might already form dispersal barriers. This is illustrated by the fact that most dispersing fragments in our study that did not manage to disperse to another pool basin were caught in terrestrial vegetation patches (low shrubs and grasses). We therefore expect that this dispersal mode will be only effective on very local scales in open landscapes and may be relevant for shallow aquatic systems that periodically dry. This may be the case for vernal ponds, rock pools and even larger temporary ponds and playas in arid and semi arid areas, which often occur in high local densities in sparsely vegetated landscapes (Williams, 2006). The conditions of shallow basins and frequent drying to facilitate dispersal are typically not met in permanent ponds and lakes in temperate regions, suggesting that dispersal of propagule bank fragments is highly unlikely for these systems.
References
Amarasekare, P., 2004. Spatial variation and density-dependent dispersal in competitive coexistence. Proceedings of the Royal Society of London Series B-Biological Sciences 271: 1497–1506.
Bohonak, A. J. & H. H. Whiteman, 1999. Dispersal of the fairy shrimp Branchinecta coloradensis (Anostraca): effects of hydroperiod and salamanders. Limnology and Oceanography 44: 487–493.
Brendonck, L. & L. De Meester, 2003. Egg banks in freshwater zooplankton: evolutionary and ecological archives in the sediment. Hydrobiologia 491: 65–84.
Brendonck, L. & B. J. Riddoch, 1999. Wind-borne short-range egg dispersal in anostracans (Crustacea: Branchiopoda). Biological Journal of the Linnean Society 67: 87–95.
Caceres, C. E. & D. A. Soluk, 2002. Blowing in the wind: a field test of overland dispersal and colonization by aquatic invertebrates. Oecologia 131: 402–408.
Chaplin, J. A. & D. J. Ayre, 1997. Genetic evidence of widespread dispersal in a parthenogenetic freshwater ostracod. Heredity 78: 57–67.
Cohen, G. M. & J. B. Shurin, 2003. Scale-dependence and mechanisms of dispersal in freshwater zooplankton. Oikos 103: 603–617.
Esri, 2002. ARCGIS 8.1. ESRI, Redlands, California.
Frisch, D. & A. J. Green, 2007. Copepods come in first: rapid colonization of new temporary ponds. Fundamental and Applied Limnology 168: 289–297.
Frisch, D., A. J. Green & J. Figuerola, 2007. High dispersal capacity of a broad spectrum of aquatic invertebrates via waterbirds. Aquatic Sciences 69: 568–574.
Graham, T. B. & D. Wirth, 2008. Dispersal of large branchiopod cysts: potential movement by wind from potholes on the Colorado Plateau. Hydrobiologia 600: 17–27.
Green, A. J. & J. Figuerola, 2005. Recent advances in the study of long-distance dispersal of aquatic invertebrates via birds. Diversity and Distributions 11: 149–156.
Greene, D. F. & M. Quesada, 2005. Seed size, dispersal, and aerodynamic constraints within the Bombacaceae. American Journal of Botany 92: 998–1005.
Jenkins, D. G. & A. L. Buikema, 1998. Do similar communities develop in similar sites? A test with zooplankton structure and function. Ecological Monographs 68: 421–443.
Jenkins, D. G. & M. O. Underwood, 1998. Zooplankton may not disperse readily in wind, rain, or waterfowl. Hydrobiologia 388: 15–21.
Jocqué, M., K. Martens, B. Riddoch & L. Brendonck, 2006. Faunistics of ephemeral rock pools in southeastern Botswana. Archiv für Hydrobiologie 165: 415–431.
Louette, G. & L. De Meester, 2005. High dispersal capacity of cladoceran zooplankton in newly founded communities. Ecology 86: 353–359.
Mayr, E., 1963. Animal Species and Evolution. Belknap Press of Harvard University Press, Cambridge.
Meyer, S. E. & S. L. Carlson, 2001. Achene mass variation in Ericameria nauseosus (Asteraceae) in relation to dispersal ability and seedling fitness. Functional Ecology 15: 274–281.
Michels, E., K. Cottenie, L. Neys & L. De Meester, 2001. Zooplankton on the move: first results on the quantification of dispersal of zooplankton in a set of interconnected ponds. Hydrobiologia 442: 117–126.
Minami, S. & A. Azuma, 2003. Various flying modes of wind-dispersal seeds. Journal of Theoretical Biology 225: 1–14.
Nathan, R., G. Perry, J. T. Cronin, A. E. Strand & M. L. Cain, 2003. Methods for estimating long-distance dispersal. Oikos 103: 261–273.
Nathan, R., N. Sapir, A. Trakhtenbrot, G. G. Katul, G. Bohrer, M. Otte, R. Avissar, M. B. Soons, H. S. Horn, M. Wikelski & S. A. Levin, 2005. Long-distance biological transport processes through the air: can nature’s complexity be unfolded in silico? Diversity and Distributions 11: 131–137.
Pajunen, V. I., 1986. Distributional dynamics of Daphnia species in a rock-pool environment. Annales Zoologici Fennici 23: 131–140.
Panov, V. E. & C. E. Cáceres, 2007. Role of diapause in dispersal of aquatic invertebrates. In Alekseev, V. & B. De Stasio (eds), Diapause in Aquatic Invertebrates: Role for Ecology, Physiology and Human Uses. Springer, Dordrecht.
Panov, V. E., P. I. Krylov & N. Riccardi, 2004. Role of diapause in dispersal and invasion success by aquatic invertebrates. Journal of Limnology 63(Suppl. 1): 56–69.
Salisbury, E., 1975. Survival value of modes of dispersal. Proceedings of the Royal Society of London Series B-Biological Sciences 188: 183–188.
Statsoft, 2007. STATISTICA (data analysis software system). Statsoft inc., Tulsa, Oklahoma.
Van de Meutter, F., R. Stoks & L. de Meester, 2008. Size-selective dispersal of Daphnia resting eggs by backswimmers (Notonecta maculata). Biology Letters 4: 494–496.
Vanschoenwinkel, B., C. De Vries, M. Seaman & L. Brendonck, 2007. The role of metacommunity processes in shaping invertebrate rock pool communities along a dispersal gradient. Oikos 116: 1255–1266.
Vanschoenwinkel, B., S. Gielen, M. Seaman & L. Brendonck, 2008a. Any way the wind blows – frequent wind dispersal drives species sorting in ephemeral aquatic communities. Oikos 117: 125–134.
Vanschoenwinkel, B., S. Gielen, H. Vandewaerde, M. Seaman & L. Brendonck, 2008b. Relative importance of different dispersal vectors for small aquatic invertebrates in a rock pool metacommunity. Ecography 31: 567–577.
Vanschoenwinkel, B., A. Waterkeyn, T. Vandecaetsbeek, O. Pineau, P. Grillas & L. Brendonck, 2008c. Dispersal of freshwater invertebrates by large terrestrial mammals: a case study with wild boar (Sus scrofa) in Mediterranean wetlands. Freshwater Biology 53: 2264–2273.
Williams, D. D., 2006. The Biology of Temporary Waters. Oxford University Press, London: 337 pp.
Willson, M. F. & A. Traveset, 2000. The ecology of seed dispersal. In Fenner, M. (ed.), Seeds: The Ecology of Regeneration in Plant Communities. CAB International, Wallingford: 85–110.
Acknowledgements
Bram Vanschoenwinkel was supported by the Fund for scientific research Flanders (FWO). We thank Mr. Danie Vorster, Oom Thys and Annelize Strydom, for logistic support and access to the site and Ine Beyen for help in the field. We are grateful to Ria Van Houdt and Stef Usé for their contribution to the design and manufacturing of the windsocks. This research is financially supported by project G.0118.03 of the FWO (Fund for scientific research Flanders).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: B. Oertli
Rights and permissions
About this article
Cite this article
Vanschoenwinkel, B., Gielen, S., Seaman, M. et al. Wind mediated dispersal of freshwater invertebrates in a rock pool metacommunity: differences in dispersal capacities and modes. Hydrobiologia 635, 363–372 (2009). https://doi.org/10.1007/s10750-009-9929-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-009-9929-z